Abstract
Key message
We established silver birch phenology in the Pyrenees. We highlight actual evapotranspiration as the main driver and the altitudinal effect within a north–south phenological pattern.
Abstract
Research into the phenology of trees is one of the most important issues in making reliable predictions of possible range shifts due to current climate change. Under the current climate change, warming and water stress can threaten tree performance by altering phenological events, specially at the edge of the southern especies distribution. We aimed at establishing bud burst, leaf and stem growth phenology of the Betula pendula in the Spanish Pyrenees, to determine the environmental cues triggering phenological events and to assess the effect of climate and soil water balance on annual growth cycle. The most important cues for bud burst are heat accumulation and long days that reduced the thermal time to bud burst. Leaf unfolding, the period of stem cambial activity, and the variation of the stem radial growth rates throughout the year are mainly regulated by the actual evapotranspiration. Leaf yellowing and abscission are advanced when hydric stress is pronounced shortening leaf longevity. The increase in spring temperatures brings forward the date of bud burst and the start of stem growth. However, the increase in the duration of hotter droughts causes an advance in the date of cessation of birch growth. From the southern Pyrenees to the boreal birch populations, there is a marked latitudinal gradient of phenological events, which are not linear except for the maximum stem growth rate. In the Pyrenees, the growing season is longer than in the boreal forests, but ring thickness is similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research into the phenology of the tree species aims to understand variations in the annual rhythms of trees growth and the relationship that may have with climate change, as the timing of the phenological events may be significantly altered threatening tree growth, reproduction, and survival of trees (Chuine 2010; Xu et al. 2022). In temperate and cold zones and in high mountain areas in southern Europe, trees go through an annual cycle in which a period of growth and a dormant state in winter alternate. This phenological cycle is synchronized with seasonal changes in photoperiod, temperature, and humidity (Zeitgebers) by means of internal biological clocks, genetic timers of phenological rhythms. Day length is the most reliable environmental cue, but temperature and humidity also constitute environmental timing cues (Welling et al. 2004; Dodd et al. 2005; McClung 2006; Harmer 2009; Mwimba et al. 2018; Xu et al. 2022). Temperature and water availability variability exert a significant influence on plant phenology, and therefore phenological changes reflect climate change with more marked effects in spring than in autumn (Estrella and Menzel 2006; Pudas et al. 2008; Juknys et al. 2012; Piao et al. 2019). Climate effects also drive cambium phenology, which is highly sensitive to variations in the annual climate regime as well (Fritts 1976; Campelo et al. 2021).
Birch has a free-growing pattern, growing indefinitely on long-day photoperiod. The short-day photoperiod in early autumn induces growth cessation, bud set, and leaves coloring (Junttila et al. 2003; Viherä-Aarnio et al. 2005; Cooke et al. 2012; Junttila and Hänninen 2012). It has also been described that leaf senescence can be advanced by high temperatures and reduced water availability in the summer (Li et al. 2002; Juknys et al. 2012; Mariën et al. 2021; Fernandez-Martinez et al. 2016). In autumn, lower temperatures favor acclimatization to adverse winter conditions (Sakai and Larcher 1987; Welling et al 2004; Li et al. 2005). During dormancy, internal physiological processes prevent bud development even when environmental conditions are favorable (Welling et al. 2004; Singh et al. 2017; Gil and Park 2019), birch trees become insensitive to the photoperiod, and environmental temperature regulates physiological and molecular processes of dormancy (Singh et al. 2017).
For many Nordic species, including birch, buds need to be exposed to cold temperatures for a period of time (Myking and Heide 1995; Rinne et al. 1997; Cooke et al. 2012). After this chilling requirement, bud break date is considered to depend on degree-day accumulation which drives ontogenetic development and buds burst (Heide 1993; Rousi and Heinonen 2007; Linkosalo et al. 2006; Junttila and Hänninen 2012). Other authors have shown that day length, together with temperature, plays an important role in the timing of bud burst (Heide 1993; Myking and Heide 1995; Häkkinen et al. 1998; Li et al. 2003), while others found that silver birch, like pioneer species, was insensitive to photoperiod length (Basler and Körner 2012).
There are many studies of birch phenology, but few have considered the joint phenology of the shoots and the cambium (Schmitt et al. 2004; Vitas 2011; Dox et al. 2020; Marchand et al. 2021). As has been described for deciduous pioneer species with wood diffuse porosity, among them the birch (Suzuki et al. 1996), cambium activity begins after leaf formation and ends before the leaves turn yellow. On the other hand, the growth period and the trunk growth rate increments are not constant (Schmitt et al. 2004; Vitas 2011). The accumulation of wood increments throughout the growth period follows a sigmoidal pattern (Schmitt et al. 2004; Rossi et al. 2006). The span of the growth period and the growth rate can vary greatly between individuals and from year to year. This can be due to water stress (Breda et al. 2006; Cooke et al. 2012; Mwimba et al. 2018; Dox et al. 2020), nutrient availability, particularly nitrogen (Cooke et al. 2012) or by a shortening of growth period due to an earlier leaf senescence (Chen et al. 2020; Dox et al. 2020). These authors state that the same factors trigger leaf senescence and wood formation. The differences between individuals are due to genetic differences (Viherä-Aarnio et al. 2005; Rousi and Pusenius 2005) and the size/age of the individuals (Vitas 2011). All these factors determine the physiological characteristics and the response that the tree can give to climate variability. If the phenological calendar of B. pendula is mainly regulated by the annual climate regime, we should find that the phenological dates vary between years. However, we should also find a synchronization in the phenological calendar and in the variability of trunk growth rates between individuals and between years.
In view of the above, and taking into account the latitudinal gradient, it would be expected that birch trees growing at lower altitudes, in the Pyrenees, would have a longer growing period, and therefore wider annual rings than those of the birch trees in northern Europe. But other factors come at play, such as altitude and annual precipitation distribution, which can have a significant impact on birch phenology. For Betula spp. it is well documented that bud burst is delayed in high altitude trees compared to low altitude trees (Myking and Heide 1995; Ovaska et al. 2005; Mir et al. 2016). While in the Nordic region, birch trees grow at low altitudes, the birch trees we studied in the Pyrenees are located at 1,536 m asl, where mountain climatic conditions could minimize the latitudinal effect to such an extent that the growth period and/or the growth rate could be significantly reduced. Precipitation distribution and amount could also affect the length of the growing period and/or the growth rate; in southern Pyrenees, summer droughts and high in autumn temperature can impair physiological processes (Fernàndez-Martínez et al. 2016) and lead to growth arrest due to water stress (Cooke et al. 2012; Mwimba et al. 2018; Xu et al. 2022). Other factors that differ from northern Europe, such as the length of the night and daily temperature variaton during the growing period, could also have a significant effect on growth rate.
Phenological studies of birch (Betula pendula Roth.) are abundant in northern and central Europe, but not in Spain, where its geographical distribution is limited to mountainous areas and does not form extensive forests (Blanco et al. 1997). In this study, our research focuses on the phenology of birch based on detailed ground phenological records of a population located in the Spanish central Pyrenees. Specifically, our main objectives were (1) to characterize the annual phenological pattern (bud burst, leaf longevity and cambium activity) and determine the environmental cues that trigger these phenofases, and (2) to determine the stem growth at breast height and assess the effect of climate on intrannual wood increments. To achieve our objectives, we monitored weekly birch phenology at a site in the southern Pyrenees for 2 years (2000–2001). We also used additional phenological data from studies carried out in central and northern Europe, which allowed us to compare the phenological calendars and the stem growth patterns. We, thus, established and completed the latitudinal phenological gradient from the Pyrenees to northern Finland. Understanding how Betula pendula grows under the Mediterranean climate influence in the southern central Pyrenees can help us predict how European birch populations may respond to climate change under higher temperatures and more frequent droughts.
Materials and methodology
Site of study
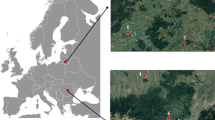
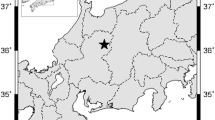
The study site is located in the southern central Pyrenees (Catalonia, NE Spain), specifically in the Forest of Arrose in Esterri d'Àneu (42º 36′ 11″ N, 1º 6′ 18″ E), at an altitude of 1,536 m, on a steep, northern oriented slope. The study site is a forest of young Scots pine trees (tree age about 60 years), tree density is 825 trees/ha and without understory. The average height and DBH of the pines are 11.48 m and 24.61 cm, rspectively. Small groups of multi-stemmed Betula pendula trees grow in the forest gaps, the age of the birches is about 30 years according to the number of rings counted on cores extracted at the DBH. To carry out our study, we selected three birch trees about 50 m apart, growing in patches. The climate of the study site is mid-mountain (see Online Resource SI_1 Fig. S1.1a) with a strong Mediterranean influence that is manifested in frequent summer droughts and a high variability in the annual climate regimes as it happened in the 2 years of study. In 2000 (see Online Resource SI_1 Fig. S1.1b), there was a strong drought in summer, while in 2001, droughts occurred in winter (February), in spring (June), and in summer (August) but rains were abundant in July that year (see on Online Resource SI_1 Fig. S1.1c). The main soil type of the area is a cambisol developed on metamorphic argillaceous limestone (IUSS Working Group WRB 2022),
Obtaining the phenological data
We recorded phenological data of the bud opening and of the fully expanded leaves, the appearance of the first yellow leaves, and the date of fall from February 2000 to September 2001. The mean sampling frequency from April to November was 7.7 ± 2.4 days (mean ± 1SD). In winter, from December to March, the sampling frequency was 25.6 ± 10.7 days (mean ± 1SD).
Measurements of the stem intra-annual increments
Changes in Betula pendula perimeter at breast height (DBH) were monitored using stainless-steel manual band dendrometers (Agricultural Electronics Co., Tucson, USA); (Keeland and Sharitz 1993; Gutiérrez et al. 2011). In February 2000, we installed band dendrometers on the dominant trees of three multi-stem trees of B. pendula at a height of 1.30 m from the trunk base. The DBH of the three selected trees (B1, B2, and B3) was 21.04, 10.11, and 22.79 cm, respectively.
The dendrometer data were converted to diametric growth, assuming the trunk was cylindrical. Cumulative growth was obtained by summing the increments in diameter between successive samplings. Daily diametric growth rates were obtained by dividing the increments produced between successive sampling days by the number of days between samplings.
Band dendrometers detect changes in the tree trunk perimeter not related to the wood formation, such as expansion and contraction of the bark and xylem, after a rain event or a prolonged drought, respectively. To minimize errors, we took six measurements per dendrometer with an electronic caliper with accuracy of 0.01 mm. We considered abnormal measurements those that could correspond to sudden changes (increase or decrease in diameter) of more than 15% of the total annual increase, and anomalous changes in diameter observed in a tree but not in others (Gutiérrez et al. 2011). Some anomalous measurements could be caused by animals or blockages in the parts of the dendrometer due to insects.
Meteorological data
The meteorological data used come from the Esterri d'Àneu meteorological station, located at an altitude of 948 m asl and about 3 km from the study site. Since the study site is located at 1,536 m asl, we made the altitudinal correction of the temperatures, taking into account a decrease of 0.6 ºC per 100 m (adiabatic gradient):
where \({T}_{E}^{^\circ }\) and hE are the temperatures and altitude of Esterri d’Àneu, and \({T}_{a}^{^\circ }\) and ha are the temperatures and altitude of the study site.
Soil water balance and evapotranspiration
Potential evapotranspiration (PET) and soil water balance variables were calculated using the Thornthwaite method based on daily data from the Esterri d'Àneu meteorological station and daily extraterrestrial radiation (Thornthwaite 1948; Allen et al. 1998, Camara et al. 2004) (see Online Resource SI_2). We estimated the field capacity (FC) considering that the soil texture was clay loam and that the average depth of the birch roots was 0.5 m (see Online Resource SI_2 Table S2.1). Soil water storage (S), changes of soil water storage (ΔS), surface runoff (R = P − (AET + ΔS), the actual evapotranspiration (AET), the potential evapotranspiration (PET), and the soil water deficit (D = PET − AET) were also estimated.
Growing degree days to growth onset
To determine the accumulated heat, growing degree days (dd), until the opening of the buds, the development of the leaves and the start of the stem diameter growth, we calculated the sum of the degree days from January 1st above the base temperature of + 5 ºC (dd5) (see Rousi and Heinonen 2007). The model used (Eq. 2) is the spring warming (SW) of Chuine et al. (2013).
where
where t0 is the date of the year, Julian days, from which degree days, \({R}_{f}\left({x}_{t}\right)\), begin to accumulate. F* is the critical sum of degree days until growth occurs on date y. xt is the average daily temperature. Tb1 is the minimum or base temperature required to accumulate degree days.
Growth dynamics of trees stem diameter at breast height
Daily growth rates and cumulative growth in diameter at breast height (DBH) were used to determine the growth dynamics of birch during the 2 years of the study. We obtained the cumulative growth by adding the increment in diameter produced between successive samples, and to characterize the pattern of cumulative growth, we have used the three-parameter Gompertz sigmoidal model (Winsor 1932). This function is characterized by its asymmetry, with an initial concave phase of accelerated growth (exponential) that decelerates from the point of inflection becoming convex. The expression of the function is
where y is the cumulative growth in diameter of the trunk (mm); x is the time, (day of the year, DOY); k is the asymptote when y = k, which remains constant over time, it is the growth produced in a period of 1 year (mm/year) corresponding to the thickness of the annual ring; e is Euler's number (the base of natural logarithms, e = 2.71828); and the parameters a and b are positive constants related to the growth rate and the displacement along the x-axis. By fitting this function, we have calculated the maximum growth rate (MGR, mm/day) and the x and y coordinates of the inflection point position, where x is the DOY value and y is the reached increment in diameter since the onset of growth for that year.
We have also characterized daily stem growth rates in diameter by differentiating Eq. (4), so that
We fitted the model using the Sigmaplot12 program (see the Gompertz function in on Online Resource SI_3 and on Online Resource SI_4 Table S4.3 for equivalences of function expressions and parameters).
Results
Birch phenology in 2000 and 2001
The calendar of the different phenological variables recorded for the birch over the 2-year period is shown in Fig. 1, Table 1, and in Online Resource SI_4 Tables S4.1 and S4.2. The date of buds opening does not coincide in the 2 study years. The advance of bud opening in 2001 compared to 2000 coincides with a faster accumulation of dd5 (shorter thermal time) in 2001, by March 25 (DOY84) 49.33 dd5 had already been accumulated, whereas by the same dates in 2000, only 43.91 dd5 had accumulated (Fig. 2 and Table 1). Low temperatures prevented sufficient dd5 until April 9, 2000 (DOY100). But the progressive reduction of night hours meant that, despite the lack of dd5 accumulation, bud opening finally occurred on April 2, 2000 (DOY93).
In 2000, all leaves were fully formed on May 5 (DOY126). The first yellow leaves appeared on October 3 (DOY277) and all leaves turned yellow by October 8 (DOY282). Unfortunately, we do not have the date of leaf unfolding for 2001. We observed the first yellow leaves on August 27 (DOY239), which is 38 days earlier than in 2000, and by September 11 (DOY254), all leaves were yellow and starting to fall, which is 28 days earlier than in 2000 (Online Resource SI_4 Table S4.1).
Growth period in diameter at trunk breast height
To determine the period of growth in diameter at breast height (DBH), we assumed that trees start growing when they have reached at least 2.5% of total final growth and that trees stop growing when the asymptotic value (Fig. 1) corresponds to the final annual ring width (Online Resource SI_1 Fig. S1.2).
In 2000, we found a significant increase in diameter from May 12 (DOY133), 7 days after total leaf formation and 40 days from bud burst. The increase in diameter continued until August 4 (DOY217) (Fig. 1, Online Resource SI_4 Table S4.2). From the beginning of August, the growth in diameter did not increase, once the maximum growth was reached, only oscillations were observed around the asymptotic size of the diameter due to the processes of hydration and dehydration of the trunk. The length of growing period in 2000 was 84 days.
In 2001, we measured significant increases in diameter from May 25 (DOY145), 61 days after bud burst. The increase in diameter continued until July 24 (DOY205). The growth period was only 60 days, 24 days shorter than in 2000. This year the increase in diameter started 12 days earlier and ended 12 days later than in the year 2001 (Fig. 1, Online Resource SI_4 Table S4.2), which was reflected into a wider growth ring (Online Resource SI_1 Fig. S1.2).
Regarding the accumulation of degree days in 2000, it was observed that the start of diameter growth occurred when 156.13 dd5 had been accumulated (DOY133). For the same dates in 2001, the accumulated degree days were only about 115.88 dd5. Growth in 2001 was delayed until the heat accumulation was 177.04 dd5 (DOY145) (Fig. 2).
Growth dynamics in diameter at breast height
The variability of the daily trunk growth rates over the year is synchronous between the trees; the Spearman correlation coefficients (mean ± 1SD) for the years 2000 and 2001 are rS = 0.785(± 0.072), p value = 0.000, n = 34, and mean rS = 0.871(± 0.029), p value = 0.0001, n = 23. The smallest tree, B2, is the one that shows the lowest correlations with the other two trees (Fig. 1).
The accumulated wood increments throughout the growth period significantly fit the sigmoid Gompertz model (Fig. 3, Online Resource SI_42 Table S4.3). The total diameter increment at the end of the annual growth cycle (asymptotic value, k) predicted by the model was 1.405 and 0.970 mm (mean of the three trees) for the years 2000 and 2001, respectively. The total observed mean annual increment was 1.362 and 1.011 mm for the years 2000 and 2001, values very similar to those predicted by the model. There are differences in the total annual increment in diameter among the three birch trees (Fig. 1, Table 2). Tree B2, the smallest, showed a lower cumulative increase in DBH, for the years 2000 and 2001, the increases were 0.531 and 0.528 mm/year, respectively. On the other hand, trees B1 and B3 with the largest initial diameters (21.04 and 22.79 cm, respectively) showed higher cumulative increase (2.075 and 1.480 mm/year in 2000, 1.675 and 0.821 mm/year in 2001) (Table 2).
Adjustment of the Gompertz model to the cumulative growth and of the first derivative of the model to the growth rates in diameter of the trees trunk at breast height (DBH). Growth data used are the average of the three trees under study. See Table 2 and Online Resource Table S4.3
The model predicts that the onset of growth in diameter in 2000, when trees had already accumulated 2.5%, occurred on May 17, 12, and 15 for birches B1, B2, and B3, respectively, the observed dates were on May 13 for all trees. In 2001, the predicted start dates were on May 22, 12, and 18 and the observed dates were on May 25 for all trees. Therefore, there is a delay in the year 2001 compared to 2000.
The mean maximum growth rates occurred on June 24 and on June 16 in 2000 and 2001, respectively, with a mean maximum growth rate of 0.029 and 0.031 mm/day (Table 2, Figs. 1 and 3). The maximum growth rate is quite different between trees, but the inter-annual values are similar. Tree B2, the smallest in DBH, is the one with the lowest maximum rate. The maximum growth rate calculated from the parameters of the Gompertz model and fitting the derivative to daily rates predicted that the maximum growth rate should occur on June 9 and 6 in 2000 and 2001, respectively, 16 and 10 days earlier than observed. Fitting the derivative to the daily rates predicts a maximum growth rate of 0.027 and 0028 mm/day for the years 2000 and 2001, which are similar to the observed rates of 0.029 and 0.031 mm/day (Table 2).
The percentage of the cumulative growth and the growth rates of the DBH allow a comparison between years and the growth of birch from northern sites in Europe. In the Pyrenees, the cumulative growth in the months of April and May was 19.71% and 10.69% in 2000 and 2001, respectively (Fig. 4). The highest growth increase was reached in June, with 44.12% and 65.73%, respectively, representing an accumulated growth of 63.83% and 76.41% in 2000 and 2001, respectively. The increase was lower in July, about 32.30% and 21.31%, respectively. Finally, the accumulated growth between August and September was only 5.81% and 2.27% in 2000 and 2001, respectively. Most of the growth is concentrated in June, which is very fast compared to the growth of B. pendula growth in Lithuania (Vitas 2011), but slower than that of the birch in northern Finland (Schmitt et al. 2004) (Fig. 4a). The comparison of the DBH growth rates (Fig. 4b) shows that the longest growth period is in Lithuania and the shortest in northern Finland; however, the ring thickness of birch trees in the southern Pyrenees is similar to that of northern Finland (Online Resource S_4 Table S4.2).
Relating intra-annual stem growth rates to climate and soil hydrological regime
Figure. 5 shows the detailed relationships between growth and hydrological variables. Mean daily DBH growth rates in 2000 show a significant positive correlation with mean temperatures averaged between sampling dates (Fig. 5a). For this year, the strongest, positive, and most significant correlation coefficients are between the mean growth rate and mean minimum temperatures. Taking the trees individually, the correlation is significant only for trees B1 and B2. For accumulated precipitation between sampling dates, the correlation is also positive, but it is not statistically significant. On the other hand, the results for the year 2001 (Fig. 5b) show that although all the correlations between growth rates and temperatures are still positive, none of them is statistically significant; as for the precipitations, only B2 tree growth rate, the smallest tree in diameter, shows a significant correlation.
a and b Pearson correlation coefficients between growth rates in diameter and climatic variables between sampling dates during the growth period. c and d Pearson correlation between monthly growth rates in diameter and the variables of the monthly water balance throughout the year. Tx, Tn, and tm stand for mean maximum, minimum, and mean temperature, respectively. P precipitation
The analysis of the relationship between the variables of the hydrological regime and the mean monthly growth rate (Fig. 5c and d, Online Resource SI_1 Fig. S1.3) shows that monthly mean temperature always had a positive effect on birch growth rates, but the correlation is only significant (p < 0.025) for the year 2000. PET also shows a positive correlation for both years, and its effect is significant (p < 0.01 and p < 0.05) for the years 2000 and 2001. In contrast, fluctuations in soil water storage always showed negative correlations with the growth rates, which were in 2001 (p < 0.025). The strongest association of all is between growth rates and monthly AET, which is positive and significant at p < 0.005 and p < 0.025 for the years 2000 and 2001, respectively.
The results of the relationships between the AET averaged between sampling dates and the growth rate in DBH are shown in Fig. 6a and b. For the year 2000, the correlation is r = 0.659, p ≤ 0.005 and for the year 2001, it is r = 0.422, p ≤ 0.045. As shown in Fig. 6c, in 2000, the growth rate of DBH started between May 5 and 12 (DOY126 and 133) when the AET exceeded 1.7 mm/day. In 2001, DBH growth started between May 19 and 25 (DOY139 and 145) when the AET exceeded 1.8 mm/day (Fig. 6d). Although it is true that during the previous week, there were values exceeding 2 mm/day, it should be noted that during the previous 15 days, the AET was very low, below 1.5 mm/day, due to low temperatures. This probably explains the delayed onset DBH growth in 2001 compared to the previous year (Fig. 2).
Figure 6c and d shows the joint evolution of the average growth rate together with daily AET and PET (see also resource SI_1 Fig. S1.4). For the year 2000, the growth rate shows a strong decrease on June 18 (DOY170) related to the decrease in the AET of the DOY162 and 163 up to 1.1 and 1.7 mm/day as a consequence the temperature decrease from 15 ºC to 6 and 8 ºC, respectively. The slow increase in temperature caused a delay in the AET, that did not recover above 3 mm/day until DOY175, which is reflected in the increased growth rate from DOY176 onwards. In DOY193, there was a further drop in temperature from 18 to 10 ºC, which caused a drop in AET to values of 2.1 mm/day, which is reflected in an abrupt drop in the growth rate in DOY207. From DOY210 up to DOY241, water deficit was strong; PET was much higher than the AET due to low rainfall and a depletion of soil water storage, which ultimately did not compensate for the water birches needed for transpiring. Growth ceased on DOY217, 7 days after the water deficit began. Between DOY241 and DOY261, episodes of soil water deficit are observed. By DOY277, the leaves started to yellow and by DOY282, the leaves were yellow and most had dropped. From the first period of water deficit, small oscillations in growth rate were observed, probably due to wood hydration associated with rainfall episodes, which occasionally increased AET. However, the effect of the bark hydration must be small, as it is only 3–5 mm thick (own measurements).
Similarly, Fig. 6d shows the joint evolution of the mean growth rate and the daily AET and PET for the year 2001 (see also Online Resource SI_4 Fig. S1.4b). In DOY175, a decrease of the growth rate to values of 0.001 mm/day, practically zero, is observed. Prior to this date, from DOY161 to DOY170, there is a period of low temperatures of around 10 ºC, when they should be around 15 ºC. The accumulation of so many days of low temperatures resulted in a drop in the AET to values of only 2.5 mm/day, well below the 3.5 mm/day that would be expected for these dates. In DOY200, there is a further drop in AET to 1.9 mm/day due to a drop in mean temperature from 16 ºC to 8 ºC, which caused the growth rate on DOY205 to drop to values of 0.006 mm/day, practically zero. In DOY214, but especially in DOY223, we find negative values of the growth rate, coinciding with the onset of the water deficit period, which lasted until DOY264. The cessation of the DBH growth rate occurred in DOY205, slightly before the onset of the water deficit. By DOY239, the leaves began to turn yellow and by DOY254, they were all yellow, just before the end of the water deficit.
Discussion
The phenology of Betula pendula has been widely studied in central and northen Europe. Our study, the first in the southen Pyrenees (Spain) where climatic conditions are quite different, completes a phenological latitudinal gradient and serves to discuss the similarities and specific response to environmental cues, climate, and soil water availability effects.
In the southern Pyrenees, bud burst occurred 4 and 12 days after the vernal equinox in 2001 and 2000, respectively. The delay in 2000 was due to lower temperatures preventing faster heat accumulation, and although heat accumulation was almost zero until DOY110, longer photoperiods activated bud opening in DOY93, 1 week before heat accumulation began to increase exponentially (Fig. 2, Online Resource SI_4 Table S4.2). It appears that when temperatures are high, bud burst occurs a few days after the equinox, but when temperatures are low, bud burst occurs more days after the equinox. Our results show that both temperature and photoperiod are sequentially involved in birch bud burst. If birch buds were only regulated by photoperiod, they would develop every year on the same dates. Our results are in agreement with those of Heide (1993) and Pletsers et al. (2015), and in general, most authors agree that temperature and photoperiod are the most important environmental cues (Myking and Heide 1995; Li et al. 2003; Linkosalo et al. 2006). Nevertheless, analyses of long data series on bud burst show good and highly significant correlations with temperature (Hänninen et al. 1995; Rousi and Pusenius 2005); however, Häkkinen et al. (1998) obtained better predictions of bud burst by including both photoperiod and temperature in their models. Light and temperature are the main environmental cues whose signals are integrated into the biological clocks (see Gil and Park 2019 and Xu et al. 2022). Biological clocks are flexible but the thresholds within which it operates need to be determined to better predict the impact of the current climate change.
In comparison to northern Europe (Finland), birch bud burst in the southern Pyrenees occurs a month earlier and requires a higher heat accumulation (Table 1, Fig. 7). The reason for this could be that temperatures are higher in the south, and thus the need for a higher heat accumulation would prevent an advance of bud burst. In experiments where ecotypes were grown together, Rousi and Pusenius (2005) found that the buds of ecotypes from cooler regions generally opened earlier than the buds of ecotypes from warmer regions in Finland. The advancement of bud burst in the Pyrenees is due to optimal thermal values being reached earlier. Marchand et al. (2021) study at 51º12′N reported that the 50% of the buds broke out on DOY101, about 17 days later than the Pyrenees. The study by Juknys et al. (2012) at 54 ºN reported a wide range, from 24 days earlier to 23 days later than in the southern Pyrenees. While Schmitt et al. (2004) reported opening dates 66 and 87 days later than in the Pyrenees for sites between 66 ºN and 69 ºN (Fig. 7, Online Resource SI_4 Table S4.1). The progressive delay in bud burst from south to north is due to the temperature gradient, despite the higher altitude of the Pyrenean location. Pudas et al. (2008), Häkkinen et al. (1995), and Juknys et al. (2012) analyzed long data series of bud burst, their results show high inter-annual variation, but their bud burst data are aligned to the general latitudinal phenological gradient which is non-linear (Fig. 7).
For leaf unfolding, we only have data for year 2000; in this year, birch leaves were fully developed 33 days after bud burst and 7 days before the start of radial stem growth. (Figs. 1 and 2, Online Resource SI_4 Table S4.1). Contrary to bud burst, leaves unfolding is delayed in relation to central Europe (Marchand et al. 2021) and takes place earlier than in northern Finland (Schmitt et al. 2004) (Fig. 7). At 51º12′N, 50% of the leaves were fully developed on DOY106 in 2018, 5 days after bud burst (Marchand et al. 2021). Schmitt et al. (2004) found that in birch stands in northern Finland, the interval between bud burst and the start of wood formation was about 2 to 3 weeks, and the leaves were fully developed 1 week before the start girth increment. In contrast, in the plots located further north, at 69 ºN, leaf development started 3 weeks after bud burst and ended 1 week after the start of wood formation (Fig. 7). In any case, clear and non-linear latitudinal gradients of the different birch phenofases emerged from the Pyrenees to northen Finland, in contrast to the gradient described by Schmitt et al. (2004) in Finland which covers a much smaller spatial scale.
Rötzer and Chmielewski (2001) calculated the onset of leaf expansion of B. pubescens from a model that takes into account altitude, latitude, and longitude. We applied their model to our data on B. pendula, a species very close to B. pubescens, obtaining the DOY116, 10 days before the observed DOY126. The same authors make an average estimate for several species in the area of the Alps, located about 300 km further to the north, for the period 1961–1998, obtaining values of DOY105 for valleys and of DOY133 for stands at altitudes higher than 1500 m asl. The authors state that there is an advance of 2.4 days per 100 km of latitude from north to south. Extrapolating this to our site in the Pyrenees, altitude about 1500 m asl would mean that leaf formation would occur 7.2 days earlier than in the Alps, and would, therefore, be the DOY125.8, a date to the observed DOY126 that we have recorded.
Yellowing of the leaves and their progressive fall are triggered, in general, by low temperatures (Estrella and Menzel 2006; Juknys et al. 2012). But water stress can also be an important cue triggering leaf yellowing and abscission (Chen et al. 2020) and this could be the rule for the southern birch populations under the ongoing climate change as it occurred in both years of our study. Water deficit was even stronger in 2001 causing leaves yellowing and falling much earlier than in 2000, to the point that on August 27th, the first yellow leaves appeared, and all the leaves were shed by September 11th long before the arrival of the first cold weather (Figs. 1 and 7, Online Resources SI_1 and SI_4 Table S4.1). The ecophysiological study by Fernàndez-Martínez et al. (2016) in the central Pyrenees supports our results; they showed that B. pendula is highly vulnerable to hydric stress in such a way that water deficit effects on this species even caused alterations in leaf morphology and a decline in net photosynthesis due to stomatal closure.
In relation to climate change, the increased frequency of more intense and hotter droughts would make birch in the southern Pyrenees less competitive by shortening the growing period and reducing tree growth (see below) in front of nearby Mediterranean species, and would also make it more susceptible to pests and diseases (Mwimba et al. 2018). A reduction in its distribution range in the southern edge could be a fact, with some small forest stands now surviving in humid valley bottoms, ravines, and near streams probably will disappear. This situation expected for the south of the birch range would contrast with what would be expected in northern Europe where if temperatures increase but water is not limiting, evapotranspiration would be higher and the length of the growing season would be longer, leading to higher CO2 fixation and the formation of much wider rings.
Timing and drivers of birch stem growth at breast height
The stem growth period falls within the period of the leaf phenology (Figs. 1 and 7, Online Resource SI_4 Table S4.1 and S4.2). This pattern was the expected for a species with rings of diffuse porosity (Suzuki et al. 1996). However, in central (Marchand et al. 2021) and northern Europe (Schmitt et al. 2004), cambial activity starts earlier than leaf formation. Stem girth growth started when heat accumulation was high (mean dd5 = 167), when temperatures were high (mean T > 10 ºC) and AET coincided with PET in mid- to late-May (Figs. 1 and 2). The growth period in diameter was shorter in 2001 than in 2000, although budding occurred earlier in 2001, the shortening of the birch wood formation was due to the lack of water in summer. In fact, at the end of July, the water deficit combined with high summer temperatures caused a sudden drop in AET relative to PET, triggering the cessation of stem growth. This is in contrast to what happens in central and northern Europe, where there is usually no water deficit and low temperatures at the end of summer may be the most important factor. In relation to central Europe, birch wood growing period in the Pyrenees was shorter (Kairiũkštis 1963- in Vitas 2011; Vitas 2011; Dox et al. 2020), but it is much shorter in northern Finland (Schmitt et al. 2004) (Fig. 7, Online Resource SI_4 Table S4.2).
In the context of climate warming, Michelot et al. (2012) have predicted a lengthening of the growing period due to the advance of bud burst. May be this can happen in central Europe, but in southern Pyrenees, it is quite difficult an enlargement of the growing period because (1) birch bud burst dates are quite close to the equinox, (2) temperatures are already high, and (3) there is a large delay between bud burst and the start of the stem growing period. In the southern Pyrenees, clearly hotter droughts and increasingly severe water stress in summer can significantly shorten the growing period, and these climatic constraints have intensified in the last two decades.
Accumulated annual growth (ring width) in 2001 was lower than in 2000 due to a shorter growth period caused by low temperatures in spring and drought in summer (Fig. 3, Online Resource SI_1 Fig. S1.2, Online Resource SI_4 Table S4.2). However, the maximum growth rates values were the same and occurred at the same dates (Table 2). The total annual growth is much lower than in central Europe (Lithuania) (Vitas 2011), but similar to birch growth in the far north of Finland (Schmitt et al. 2004). The reason for this is that although the growing season in northern Finland is considerably shorter, the growth rates are much higher than in the Spanish Pyrenees (Fig. 4, Online Resource SI_4 Table S4.2), which may be attributed to the ‘polar day syndrome’ of northern birches (Tenkanen et al. 2022).
In the Pyrenees, the maximum stem growth rates were reached on 24 June and 16 June in 2000 and 2001, respectively (Table 2); this result is in line with Rossi et al. (2006), who proposed that in conifers from cold and temperate climates, there is a synchronization between the maximum growth rate and the length of the day, with the growth rate starting to decrease after the summer solstice. However, this is not the case for birch in central and northern Europe (Fig. 4). We think that the factor that could better explain the change in trend in the growth rate of Pyrenean birch could be the AET, which integrates the day length, the temperature, and the available water. All these factors are closely related to photosynthesis, and therefore to biomass production. This is why we find that AET correlates with diameter growth. The AET could also explain the different dates of the maximum growth rates between the 2 years of study, the results depicted in Fig. 4b point to this hypothesis.
The gradient of the maximum growth rates in stem is the only one that shows a linear trend. The altitude effect is no longer observed in this phenophase in the Pyrenees, which occurs earlier than in central Europe (Fig. 7). These results seem to indicate that maximum stem growth rate is not regulated by the photoperiod, and it is not reached on the summer solstice as reported by Rossi et al. (2006). In our opinion, the date of maximum growth rate could vary among species, years, latitudes, and climatic conditions of the site, probably because the day length determines PET, which in turn depends on temperatures.
The influence of climatic conditions on the growth of stems birch trees
B. pendula is a specie vulnerable to lack of water, but trees’ transpiration does not depend directly on precipitation, but on the storage of soil water availability. According to our results, AET is the most relevant variable to better explain the inter-annual variability of the growth rate pattern of birches in the southern Pyrenees (Figs. 5, 6). The importance of water stored in the soil would justify the low correlation we found between intranual growth rates and temperature, precipitation and even with the P-PET drought index. This is one of the drought indices commonly used; nevertheless, we have excluded it from from our study because it does not give any statistically significant results for any of the 2 years of study (Fig. 5c and d), most likely because it does not include soil water storage.
The relationship between the stem growth rates and the AET indicates that when there is no water deficit, the variations in growth rate observed throughout the year are due to temperature variability, a response similar to what would be expected in northern Europe. However, when there is a severe water deficit (AET < PET), the growth rate slows down and even stops when the water stored in the soil has been completely used by evapotranspiration.
In the southern Pyrenees, B. pendula grows close to its southern geographical distribution limit, where Mediterranean summer droughts are frequent, as they were during the 2 years of our study. The severity of climate change is well defined in the IPCC reports and its effects on tree phenology and growth have been already reported by many authors years ago (Menzel and Fabian 1999; Chmielewski and Rötzer 2001; Rötzer and Chmielewski 2001). These and other authors warn of the lengthening of spring due to a progressive advance in the onset of growth, an increase in minimum temperatures, and a more irregular annual precipitation regime with more heavy rainfall events generating greater surface runoff. If we apply this scenario to the phenology of our birches in the Pyrenees, we could envisage.
-
1.
Growth arrest in DBH well before leaf fall and onset of dormancy: The predicted hydric stress would cause an advance growth arrest. It would also cause earlier leaf fall in the south and a delay in the north. As a result, the growing period could be shortened and tree rings would be narrower and forest net primary production would be lower (Michelot et al. 2012).
-
2.
A shortening of the winter dormancy period: In the short term, this would not be a problem either in the Pyrenees at altitudes above 1,500 m asl or in Fennoscandia, where the risk of a chilling deficit is low (Myking and Heide 1995). In the Pyrenees, there will be enough days with low and negative temperatures to prepare the buds to emerge from dormancy (Welling et al. 2004), but further studies are needed given the complexity of the orography.
-
3.
Earlier bud burst advancement and risks: The increase in minimum temperatures would favor the accumulation of degree days that would advance the bud burst, increasing the risk of late frosts, which are typical of mountain areas and becoming more frequent due to climate change. It is true that birch trees can resprout after these frosts using the dormant buds in reserve. However, these events would make birches less competitive and more susceptible to parasites and diseases (Mwimba et al. 2018).
-
4.
Shortening of the growing period: The predicted increase in temperature will increase PET. The growth onset in DBH growth would also be earlier if not affected by late frosts. However, as rainfall would be more erratic and torrential, the AET would decrease due to the water deficit and this will slow down the growth rate of DBH. Higher minimum temperatures would increase the night respiration, which would also negatively affect the increase in DBH growth. Episodes of water stress would also cause a loss of leaf area, reducing the photosynthetic capacity of trees (Fernàndez-Martínez et al. 2016).
Conclusion
The phenological calendar was synchronized between the three birches we studied, although the timing of phenological events varied from year to year. The onset of bud burst is mainly regulated by the accumulation of dd5 and by the photoperiod. The onset of bud burst occurred shortly after the vernal equinox because spring temperature was high, and so the thermal period for heat accumulation was short (year 2001). But in 2000, on the other hand, spring temperature was low and the subsequent long photoperiod triggered bud burst much later after the vernal equinox (year 2000). Leaves started to yellow in late August due to water stress in 2001, and in early October due to low temperatures in 2000. Water deficit effects on birch even cause alterations in leaf morphology and a decline in net photosynthesis due to stomatal closure (Fernàndez-Martínez et al. 2016) in the central Pyrenees.
The stem growth of birch trees was included in the life span of the leaves. Cambial activity started after the leaf unfolding and ended before leaf yellowing. The onset and termination of DBH growth, as well as the variation in intra-annual growth rate, were regulated mainly by the AET, which in turn depends on temperature and the soil water availability.
Pyrenean birch trees showed a delay in the expected linear phenological dates for bud burst, leaf unfolding, and the start of growth in DBH with respect to the same phenological events along the European latitudinal gradient. The high altitude of the site of study in the Pyrenees could be the cause. The cessation of stem growth occurred in similar dates to birch in the far north but earlier than in central Europe.
At the southern distribution limit of its range, the increased frequency of more intense and hotter droughts would make birch less competitive with neighboring Mediterranean species, and would also make them more susceptible to pests and diseases. This could result in a reduction in its range, with the disappearance of some small forest stands that survive in wet valley bottoms and ravines. In central and northern Europe, the predicted rise in temperatures will lead to increase in PET. If this is not compensated by increased precipitation, AET is expected to decrease. This would lead to periods of water stress, which would affect CO2 fixation and therefore thinner rings. It could also shorten the growing season, as is currently the case in southern Europe.
Author contribution statement
The two authors, XCM and EGM, equally contributed to the study conception, design, material preparation, data collection and analysis. The manuscript was written by XCM and EGM.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration—guidelines for computing crop water requirements—FAO Irrigation and drainage paper 56. https://www.fao.org/3/x0490e/x0490e00.htm#Contents. Accessed 16 August 2022
Basler D, Körner C (2012) Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agric For Meteorol 165:73–81. https://doi.org/10.1016/j.agrformet.2012.06.001
Blanco E, Casado MA, Costa M et al (1997) Los Bosques Ibéricos: una interpretación geobotánica. Planeta, Madrid
Breda N, Roland H, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann for Sci 63:625–644. https://doi.org/10.1051/forest:2006042
Camara R, Martínez JR, Díaz del Olmo F (2004) Metodología y aplicaciones de los balances hídrico y bioclimático y su aplicación al estudio ecodinámico de las formaciones vegetales. Universidad de Sevilla. España. Unpublished
Campelo F, Ribas M, Gutiérrez E (2021) Plastic bimodal growth in a Mediterranean mixed-forest of Quercus ilex and Pinus halepensis. Dendrochronologia 67(125836):1–12. https://doi.org/10.1016/j.dendro.2021.125836
Chen L, Hänninen H, Rossi S et al (2020) Leaf senescence exhibits stronger climatic responses during warm than during cold autumns. Nat Clim Chang 10:777–780. https://doi.org/10.1038/s41558-020-0820-2
Chmielewski F, Rötzer T (2001) Response of tree phenology to climate change across Europe. Agric for Meteorol 108:101–112. https://doi.org/10.1016/S0168-1923(01)00233-7
Chuine I (2010) Why does phenology drive species distribution? Phil Trans r Soc B 365:3149–3160. https://doi.org/10.1098/rstb.2010.0142
Chuine I, de Cortazar-Atauri IG, Kramer K, Hänninen H (2013) Plant development models. In: Schwartz, M. (eds) Phenology: an integrative environmental science. Springer, Dordrecht, pp 275–293. https://doi.org/10.1007/978-94-007-6925-0_15
Cooke JE, Eriksson ME, Junttila O (2012) The dynamic nature of bud dormancy in trees: environmental control and molecular mechanisms. Plant Cell Environ. https://doi.org/10.1111/j.1365-3040.2012.02552.x
Dodd AN, Salathia NJ, Hall A et al (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309(5734):630–633. https://doi.org/10.1126/science.1115581
Dox I, Gričar J, Marchand LJ et al (2020) Timeline of autumn phenology in temperate deciduous trees. Tree Physiol 40(8):1001–1013. https://doi.org/10.1093/treephys/tpaa058
Estrella N, Menzel A (2006) Responses of leaf colouring in four deciduous tree species to climate and weather in Germany. Clim Res 32:253–267. https://doi.org/10.3354/cr032253
Fernàndez-Martínez J, Fransi MA, Fleck I (2016) Ecophysiological responses of Betula pendula, Pinus uncinata and Rhododendron ferrugineum in the Catalan Pyrenees to low summer rainfall. Tree Physiol 36(12):1520–1535. https://doi.org/10.1093/treephys/tpw104
Fritts H (1976) Tree rings and climate. Academic Press, London
Gil KE, Park CM (2019) Thermal adaptation and plasticity of the plant circadian clock. New Phytol 221(3):1215–1229. https://doi.org/10.1111/nph.15518
Gutiérrez E, Campelo F, Camarero JJ et al (2011) Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees 25:637–646. https://doi.org/10.1007/s00468-011-0540-3
Häkkinen R, Linkosalo T, Hari P (1995) Methods for combining phenological time series: application to bud burst in birch (Betula pendula) in Central Finland for the period 1896–1955. Tree Physiol 15:721–726. https://doi.org/10.1093/treephys/15.11.721
Häkkinen R, Linkosalo T, Hari P (1998) Effects of dormancy and environmental factors on timing of bud burst. Tree Physiol 18(10):707–712. https://doi.org/10.1093/treephys/18.10.707
Harmer S (2009) The circadian system in higher plants. Annu Rev Plant Biol 60:357–377. https://doi.org/10.1146/annurev.arplant.043008.092054
Heide OM (1993) Daylength and thermal time responses of bud burst during dormancy release in some northern deciduous trees. Physiol Plant 88:531–540. https://doi.org/10.1111/j.1399-3054.1993.tb01368.x
IUSS Working Group WRB (2022) World Reference Base for Soil Resources. International soil classification system for naming soils and creating legends for soil maps. International Union of Soil Sciences (IUSS), Vienna
Juknys R, Sujetovienė G, Žeimavičius K et al (2012) Comparison of climate warming induced changes in silver birch (Betula pendula Roth) and Lime (Tilia cordata Mill.) phenology. Baltic For 18(2):25–32. https://www.balticforestry.mi.lt/bf/
Junttila O, Hänninen H (2012) The minimum temperature for bud burst in Betula depends on the state of dormancy. Tree Physiol 32(3):337–345. https://doi.org/10.1093/treephys/tps010
Junttila O, Nilsen J, Igeland B (2003) Effect of temperature on the induction of bud dormancy in ecotypes of Betula pubescens and Betula pendula. Scand J for Res. https://doi.org/10.1080/02827581.2003.9728291
Keeland BD, Sharitz RR (1993) Accuracy of tree growth measurements using dendrometer bands. Can J for Res 23:2454–2457. https://doi.org/10.1139/x93-304
Li C, Puhakainen T, Welling A et al (2002) Cold acclimation in silver birch (Betula pendula). Development of freezing tolerance in different tissues and climatic ecotypes. Physiol Plant. https://doi.org/10.1034/j.1399-3054.2002.1160406.x
Li C, Junttila O, Ernstsen A, Heino P, Palva ET (2003) Photoperiodic control of growth, cold acclimation and dormancy development in silver birch (Betula pendula) ecotypes. Physiologia Plantarum 117:206–212. https://doi.org/10.1034/j.1399-3054.2003.00002.x
Li C, Puhakainen T, Welling A, Puhakainen T et al (2005) Differential responses of silver birch (Betula pendula) ecotypes to short-day photoperiod and low temperature. Tree Physiol 25:1563–1569. https://doi.org/10.1093/treephys/25.12.1563
Linkosalo T, Häkkinen R, Hänninen H (2006) Models of the spring phenology of boreal and temperate trees: is there something missing? Tree Physiol 26(9):1165–1172. https://doi.org/10.1093/treephys/26.9.1165
Marchand LJ, Dox I, Gričar J et al (2021) Timing of spring xylogenesis in temperate deciduous tree species relates to tree growth characteristics and previous autumn phenology. Tree Physiol 41(7):1161–1170. https://doi.org/10.1093/treephys/tpaa171
Mariën B, Dox I, de Boeck HJ et al (2021) Does drought advance the onset of autumn leaf senescence in temperate deciduous forest trees? Biogeosciences 18:3309–3330. https://doi.org/10.5194/bg-18-3309-2021
McClung C (2006) Plant circadian rhythms. Plant Cell 18:792–803. https://doi.org/10.1105/tpc.106.040980
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397(6721):659–659. https://doi.org/10.1038/17709
Michelot A, Simard S, Rathgeber C et al (2012) Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol 32(8):1033–1045. https://doi.org/10.1093/treephys/tps052
Mir NA, Masoodi TH, Mir AA et al (2016) Phenology and growth performance of Himalayan birch (Betula utilis) in Kashmir Western Himalayas along the different altitudinal gradients. Indian J Agric Sci 86(8):1086–1091
Mwimba M, Karapetyan S, Liu L et al (2018) Daily humidity oscillation regulates the circadian clock to influence plant physiology. Nat Commun 9:4290. https://doi.org/10.1038/s41467-018-06692-2
Myking T, Heide OM (1995) Dormancy release and chilling requirement of buds of latitudinal ecotypes of Betula pendula and B. Pubescens Tree Physiology 15:697–704. https://doi.org/10.1093/treephys/15.11.697
Ovaska JA, Nilsen J, Wielgolaski FE, Kauhanen H, Partanen R, Neuvonen S et al (2005) Phenology and performance of mountain birch provenances in transplant gardens: latitudinal, altitudinal and oceanity-continentality gradients. Plant ecology, herbivory, and human impact in Nordic mountain birch forests. Springer, Berlin, pp 99–115
Piao S, Liu Q, Chen A et al (2019) Plant phenology and global climate change: Current progresses and challenges. Glob Change Biol 25(6):1922–1940. https://doi.org/10.1111/gcb.14619
Pletsers A, Caffarra A, Kelleher CT, Donnelly A (2015) Chilling temperature and photoperiod influence the timing of bud burst in juvenile Betula pubescens Ehrh. and Populus tremula L. trees. Ann for Sci 72:941–953. https://doi.org/10.1007/s13595-015-0491-8
Pudas E, Leppälä M, Tolvavanen A et al (2008) Trends in phenology of Betula pubescens across the boreal zone in Finland. Int J Biometeorol 52:251–259. https://doi.org/10.1007/s00484-007-0126-3
Rinne P, Hänninen H, Kaikuranta P et al (1997) Freezing exposure releases bud dormancy in Betula pubescens and B. pendula. Plant Cell Environ 20(9):1199–1204. https://doi.org/10.1046/j.1365-3040.1997.d01-148.x
Rossi S, Deslauriers A, Anfoldillo T et al (2006) Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol 170:301–310. https://doi.org/10.1111/j.1469-8137.2006.01660.x
Rötzer T, Chmielewski F (2001) Phenological maps of Europe. Climate Res 18:249–257. https://doi.org/10.3354/cr018249
Rousi M, Heinonen J (2007) Temperature sum accumulation effects on within-population variation and long-term trends in date of bud burst of European white birch (Betula pendula). Tree Physiol 27(7):1019–1025. https://doi.org/10.1093/treephys/27.7.1019
Rousi M, Pusenius J (2005) Variations in phenology and growth of European white birch (Betula pendula) clones. Tree Physiol 25(2):201–210. https://doi.org/10.1093/treephys/25.2.201
Sakai A, Larcher W (1987) Frost survival of plants: responses and adaptation to freezing stress. Springer-Verlag, New York
Schmitt U, Jalkanen R, Eckstein D (2004). Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fennica 38(2):167–178. https://doi.org/10.14214/sf.426
Singh RK, Svystun T, AlDahmash B et al (2017) Photoperiod - and temperature mediated control of phenology in trees—a molecular perspective. New Phytol 213(2):511–524. https://doi.org/10.1111/nph.14346
Suzuki M, Yoda K, Suzuki H (1996) Phenological comparison of the onset of vessel formation between ring-porous and diffuse-porous deciduous trees in a Japanese temperate forest. IAWA J 17(4):431–444. https://doi.org/10.1163/22941932-90000641
Tenkanen A, Keinänen M, Oksanen E et al (2022) Polar day syndrome: Differences in growth, photosynthetic traits and sink-size patterns between northern and southern Finnish silver birch (Betula pendula Roth) provenances in native and non-native photoperiods. Tree Physiol 00:1–15. https://doi.org/10.1093/treephys/tpac104
Thornthwaite CW (1948) An approach toward a rational classification of climate. Geogr Rev 38(1):55–94. https://doi.org/10.2307/210739
Viherä-Aarnio A, Häkkinen R, Partanen J et al (2005) Effects of seed origin and sowing time on timing of height growth cessation of Betula pendula seedlings. Tree Physiol 25(1):101–108. https://doi.org/10.1093/treephys/25.1.101
Vitas A (2011) Seasonal growth variations of Pine, Spruce, and Birch recorded by band dendrometers in NE Lithuania. Balt for 17(2):197–203
Welling A, Rinne P, Viherä-Aarnio A et al (2004) Photoperiod and temperature differentially regulate the expression of two dehydrin genes during overwintering of birch (Betula pubescens Ehrh.). J Exp Bot 55(396):507–516. https://doi.org/10.1093/jxb/erh045
Winsor CP (1932) The Gompertz curve as a growth curve. Natl Acad Sci 18(1):1–8. https://doi.org/10.1073/pnas.18.1.1
Xu X, Yuan L, Yang X et al (2022) Circadian clock in plants: Linking timing to fitness. J Integr Plant Biol 64(4):792–811. https://doi.org/10.1111/jipb.13230
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Sonja Vospernik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castells-Montero, X., Gutiérrez-Merino, E. Phenology and stem growth dynamics of Betula pendula Roth. in the Spanish Pyrenees. Trees 37, 1593–1607 (2023). https://doi.org/10.1007/s00468-023-02444-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02444-y