Abstract
Key message
Considering their drought tolerance and growth characteristics, rare native tree species are well-suited admixed species for the development of climate-stable forests in Central Europe.
Abstract
In our study, we assessed the growth and drought reaction of the four rare native tree species European hornbeam (Carpinus betulus L.), European white elm (Ulmus laevis Pall.), field maple (Acer campestre L.), and wild service tree (Sorbus torminalis (L.) Crantz). Based on tree-ring data, we (I) evaluated their species-specific growth characteristics and variability and examined the influencing site and tree characteristics on annual growth. (II) We quantified their reaction to single drought events, also depending on site and tree variables. (III) We compared our results to oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) and European beech (Fagus sylvatica L.). As they are well-known Central European tree species, there is a broad knowledge about their growth and drought response across wide geographical ranges available. Bringing the results of European beech and oak in relation with the rare native species, it allows to categorise their growth and drought reaction and to contextualise their performance. Our results show, that besides European white elm, the rare species showed an overall lower annual growth with a higher variability than European beech and oak. However, especially field maple and wild service tree were better adapted to drought than European beech and partially even recovered better than oak. Combining the aspects of growth stability and drought tolerance, we conclude that rare native tree species are well suited as admixed species in future forest stands. European hornbeam is a suitable match for European beech on wetter sites, while field maple and wild service tree are a sensible complement for the climate stable oak on drier sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, Central Europe has experienced several severe drought summers with high temperatures and low precipitation (Buras et al. 2020). Climate change projections for Central and Northern Europe predict even drier and hotter climate conditions in the future, with more frequent, severe and prolonged droughts (Cook et al. 2020; Eyring et al. 2016; Ionita and Nagavciuc 2021; Spinoni et al. 2019; Zscheischler and Seneviratne 2017). The associated consequences for forest ecosystems are substantial. Persistent climatic changes enhance the risk of growth decline, forest mortality (Allen et al. 2010, 2015; Senf et al. 2020), and diminish forest productivity (Rita et al. 2020), diversity, and carbon storage (Pilli et al. 2022). Recent droughts in Central European forests have caused canopy damages (Beloiu et al. 2022; Buras et al. 2020; Philipp et al. 2021; Sturm et al. 2022; Thonfeld et al. 2022) resulting in reduced crown size and height growth (Jacobs et al. 2021). The composition of Central European forests is expected to change in future due to different drought resistance of tree species. The introduction and promotion of alternative admixed species is a viable option to increase forest stability (Sturm et al. 2022). In this context, non-native tree species, as western hemlock (Tsuga heterophylla (Raf.) Sarg), northern red oak (Quercus rubra L.) or sweet chestnut (Castanea sativa Mill.), that are expected to be well adapted to hot summer temperatures and drought seem to be promising and recommended for an increased cultivation (Bolte et al. 2009; Hoffmann et al. 2018; Pötzelsberger et al. 2020). However, non-native species might be maladapted to colder winter temperatures and late frost events, which are also expected to increase in the future (Kreyling et al. 2015; Vitasse et al. 2019; Zohner et al. 2020). Therefore, native and drought resilient species may be a more reasonable alternative to complement present prevailing species. Other than for non-native species, they exhibit less uncertainties concerning the exposure to pests, adoption by local fauna, the potential use of their wood and their influence on other ecosystem services (Castro-Díez et al. 2019; Matevski and Schuldt 2021; Sapsford et al. 2020).
Among native species, especially European hornbeam (Carpinus betulus L.), European white elm (Ulmus laevis Pall.), field maple (Acer campestre L.), and wild service tree (Sorbus torminalis (L.) Crantz) seem to be promising options for admixture in Central European forests. The distribution range of European hornbeam is mainly determined by annual mean and winter temperature of the region (Varol et al. 2022). It tolerates annual mean temperatures up to 15 °C, which qualifies it for the use in Central European silviculture, where its future distribution range is considered to still be congruent to its present one (Koch et al. 2022). In the strictly preserved zone of Bialowieza national park, it even increased its share in the tree species composition in the last decades (Brzeziecki et al. 2018, 2020). Severe and short droughts can be withstood by European hornbeam due to a relatively stable sap flow under stress conditions (Leuzinger et al. 2005). Seedlings, however, were found to show a high drought sensitivity, but also a high capacity to recover (Beloiu et al. 2020). In addition, Scharnweber et al. (2020) found a strong growth decline of mature European hornbeams after the drought of 2019. Overall, detailed studies about the drought tolerance of adult trees of European hornbeam are currently scarce.
European white elm is a tree species of riparian forests with a current distribution range that covers climates from dry and cool to warm and moist (Collin and Bozzano 2015). It is expected to keep (Koch et al. 2022) or even expand (Thurm et al. 2018) this distribution range in central Europe under predicted climate change scenarios, especially where a decline of current dominant species is observed. At the same time, European white elm is able to maintain a high growth performance under different climatic scenarios (Thurm et al. 2018). Mild drought stress does not have an influence on morphological traits of its saplings (Black-Samuelsson et al. 2003). However, drought reactions may highly vary between different provenances (Black-Samuelsson et al. 2003; Venturas et al. 2015). Leonova et al. (2022) found that European white elm, similar to oak, can maintain a constant amount of fine root biomass under drought conditions and can even enlarge it during drought.
Field maple favours a mild climate with low autumn precipitation and occurs within the entire temperate climate zone, except high mountain areas, regions with high precipitation (e.g. the British Isles) and the Mediterranean (Coudun et al. 2006). In a previous study, field maple showed a high drought tolerance, even exceeding that of oak (Kunz et al. 2018). In a greenhouse experiment, seedlings were found to have a high drought resistance (Kunz et al. 2016), which could, however, not be confirmed under field conditions where a high mortality and low recovery was noted (Beloiu et al. 2020). In general, field maple is expected to be well adapted to future climate change scenarios (Walentowski et al. 2014).
Wild service tree is currently growing in warm and dry parts of Europe (Rasmussen and Kollmann 2004) which are regions that correspond to future climate conditions in Central Europe. This adaptation makes the wild service tree a promising tree species under climate change conditions (Walentowski et al. 2014). A recent study noticed a higher drought tolerance than European beech, but not than oak (Kunz et al. 2018). Seedlings are sensitive to drought, but recover quickly afterwards (Kunz et al. 2016).
General growth patterns of the species are widely unknown. Only for European hornbeam, a yield table for Northern Germany exists (Lockow 2009). For wild service tree, Pyttel et al. (2013) used stem analysis to determine annual increments and deduced age-height and height-diameter curves. In other studies, rare species are often aggregated in categories as “other hard wood species”. For weighing out different species against each other and choose sensible species for cultivation, it is, however, important for forest managers to assess the potential growth of the species. It is also crucial to further evaluate the potential of rare domestic tree species to cope with climate change to develop adapted management strategies for Central European forests. Hence, more information on drought response patterns of these species is urgently needed. Most studies addressing the drought sensitivity of rare species are based on experiments with seedling or young plants or simulations (e.g. Kunz et al. 2016; Thurm et al. 2018; Varol et al. 2022). Studies using data from older trees are underrepresented. However, not only drought tolerance is an important characteristic of tree species for forest managers, but also a reliable and stable growth with economic potential.
To make our results for the rare species easier to access for forest managers and forest scientists, we compare them to European beech (Fagus sylvatica L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.). As these are the dominant deciduous tree species in central Europe (Meyer et al. 2020), their growth and drought reactions are already well studied and widely known. European beech shows pronounced growth declines in large parts of is distribution range as a reaction to recent drought events (Leuschner 2020; Schuldt et al. 2020). Although it has been found to have the potential to genetically adapt to increasing drought stress (Petrik et al. 2022; Pfenninger et al. 2021), a declined growth and distribution range are anticipated, especially at its drier and warmer distribution limits (Del Martinez Castillo et al. 2022; Muffler et al. 2020). Pedunculate oak and sessile oak show a higher drought tolerance than European beech (Mette et al. 2013; Meyer et al. 2020) and are expected to show stable growth even under drier and hotter conditions. However, a decrease in sap flow rates and canopy conductance were observed in response to extreme drought events (Süßel and Brüggemann 2021). These negative effects of extreme drought on the ecophysiological processes of oaks can be mitigated by mixing with other species, e.g. European beech or Scot’s pine (Steckel et al. 2020; Stimm et al. 2021). In general, oak and European beech show different drought reactions (Scharnweber et al. 2011; van der Werf et al. 2007), with higher stability of oak (Kasper et al. 2022) and also a higher resistance and resilience towards drought (Meyer et al. 2020).
In our study, we used tree-ring data to:
-
(I)
Assess the dendrometric growth characteristics of European hornbeam, European white elm, field maple, and wild service tree and the overall variability of growth, depending on site and tree variables. We hypothesise that there are species-specific growth characteristics and differences in the influence of site conditions.
-
(II)
Quantify and compare the species’ reaction to single drought events. We hypothesise that the species show differences in their resistance and resilience towards drought and their recovery after drought events. We further hypothesise that the species-specific drought reactions are influenced by the individual tree size and site-specific climate conditions.
-
(III)
Compare the growth characteristics and drought reaction of the four rare native tree species with the well-studied European beech and oak which themselves differ in their growth and reaction to drought. We hypothesise that rare species show generally a smaller annual growth than European beech and oak and that they are at least as drought tolerant as European beech.
Materials
Sites and sample tree selection
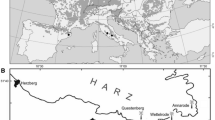
For each species, we took tree cores from trees in two stands on two different sites in South-Eastern Germany in winter 2020/2021. Except for wild service tree for which on the second site, we only took cores in one stand. For European hornbeam, we additionally sampled trees of two stands located in eastern and central Poland, respectively. In total, we sampled the selected rare species in 17 stands on 9 sites (Fig. 1). We selected stands where the species grew in large proportions to reflect mono-specific or only slightly mixed conditions. The sites per species ideally covered different site conditions. However, due to the rarity of the species the desired variation in growing conditions could not be always realised. Therefore, the two sites of field maple and European white elm did not differ strongly in site conditions. All stands were undergoing regular silvicultural treatment in the past. As all stands were even-aged, the two stands per site covered a dbh-range per species (Table 1). The two stands per site were chosen as close to each other as possible to ensure similar site conditions.
In each stand, we sampled 15–20 trees, covering the whole diameter spectrum of the stand. Trees were randomly selected, however, not standing directly next to each other. Depending on the occurrence, additional 10–15 European beech or oak trees or both were sampled (in total 105 European beeches and 107 oaks). They were chosen to grow in the direct vicinity of each stand with the sampled rare species and within a similar species composition, past treatment, age and site conditions (Table 2). When selecting oaks, we did not differentiate between sessile oak and pedunculate oak. We additionally included tree-ring data from two existing experimental plots of European beech that were in close vicinity to the plots with rare species (Schmied et al. 2023). The values of basal area included in Tables 1 and 2 were determined doing an angle count sample at each cored tree.
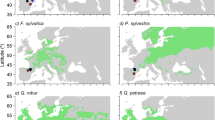
The selected sites for European beech, field maple, oak and wild service tree were located in their core distribution area in Europe. The two European hornbeam sites in Poland showed lower precipitation rates than the German sites. Both sites of European white elm were located on sites with a rather high precipitation compared to the rest of the European white elm’s distribution range (Fig. 2).
Climate space diagram showing the location of sampled stands (black squares) in the species’ distribution range (Mauri et al. 2017) based on annual mean temperature and precipitation (DWD Climate Data Center 2022a). Coloured areas show the classified density distribution of species occurrences. A version containing the names of Table 1 can be found in the supplementary material
Climate data
For the sites located in Germany, we used data of monthly mean, maximum and minimum temperature and precipitation derived from a 1 × 1 km grid of the German weather service (DWD) (DWD Climate Data Center 2022a; b). For the sites in Poland, we used climate data of the Climate Research Unit (CRU) (Harris et al. 2020).
Using the temperature and precipitation data, we calculated the standardised precipitation evaporation index (SPEI) for each stand (Vicente-Serrano et al. 2010). The SPEI is a multiscalar drought index which combines precipitation and temperature data and is a well performing and frequently used index in studies which evaluate the impact of drought on forest growth (Bhuyan et al. 2017; Ionita and Nagavciuc 2021; Skiadaresis et al. 2019; Vicente-Serrano et al. 2012). For the potential evapotranspiration, that is included in the index, we were using the Hargreaves equation (Droogers and Allen 2002; Hargreaves 1994). We calculated the SPEI for periods of 3, 6, 10 and 12 months to also cover lagged climate-induced growth reactions.
To characterise the long-term climatic conditions on a site, we used the De Martonne aridity index (DMI) (Martonne 1926), based on the reference period of 1991–2020. The index is calculated as \(\mathrm{DMI}=P / (T +10)\) with P being the sum of annual precipitation and T the annual mean temperature. For a general characterisation of sites, the DMI is more suitable than the SPEI, as the SPEI is a standardised index that approaches zero for long-term means.
For the sites located in Germany, we also used the soil moisture index (SMI) (Helmholtz Centre for Environmental Research 2021; Samaniego et al. 2013; Zink et al. 2016) obtained from the Helmholtz Centre for Environmental Research (Helmholtz Centre for Environmental Research 2021; Zink et al. 2016). The SMI is an index scaled between 0 and 1 describing the soil moisture in comparison to a long-term expected value. It uses interpolated climate data and implements it into the hydrological model system mHM (Kumar et al. 2013; Samaniego et al. 2010) to simulate soil moisture at a resolution of 4 × 4 km. In this paper, we used the data of the total soil column (1.8 m) from 1951 to 2020 in a monthly resolution.
Sample and data preparation
For the collection of tree cores, we used a HAGLÖF increment corer with a diameter of 5 mm. We extracted two cores per tree at breast height (dbh, 1.30 m), one from northern and one from eastern cardinal direction. This minimised the influence of reaction wood induced by the main wind direction from SW and allowed a better representation of overall growth (Pretzsch et al. 2013; Speer 2010). Since wild service tree and European white elm are very rare species with valuable timber, we were only able to extract cores at a height of 30 cm due to forest owner preferences.
For an easy handling and measurement, we glued the increment cores on wooden boards and subsequently sanded with increasingly finer abrasive paper from 400 to 800 grit to enhance the visibility of tree-ring borders. Measurements were taken to the nearest 1/100 mm using a digital positioning table (Kutschenreiter and Johann; Digitalpositiometer, Biritz and Hatzl GmbH, Austria). Visual crossdating was performed based on common matching patterns of wide and narrow rings (Stokes and Simley 1996; Schweingruber et al. 1990; Speer 2010). Afterwards, we statistically verified crossdating using the dplR package in R (Bunn 2010).
Due to the low visibility of the tree rings of European hornbeam, we used an alternative method for measurements of this species. After sanding, the cores were photographed using the microscopic camera of a Lignostation (Rinntech) with a resolution of 1/10 mm. The resulting photographs were imported into Adobe Photoshop (version 22). Using a high pass filter with a radius of 40 pixels, a linear light blending mode and changing the displayed colour spectrum, tree-ring borders were easier to detect. More compact wood sections appeared more yellow, less compact section more blueish. The resulting photos were then imported into the Lignovision software (Rinntech, version 1.37). Here, tree rings were marked, measured, and visually crossdated.
During the measurement and crossdating process, we had to reject several cores due to an extremely weak visibility of tree rings that made it impossible to measure and crossdate the cores. The final number of cores per plot used in this study can be found in Tables 1 and 2.
Methods
Selection of drought years
For the selection of single drought years, we identified one single drought indicator. Requirements of this index were a reliable identification of drought, a high spatial resolution, and a high correlation with tree growth over all species and stands. Therefore, we calculated bootstrapped Pearson’s correlation between all climate variables and drought indices and our species-specific site chronologies. For all variables and indices, we used monthly values as well as seasonal means for spring (March–May), summer (June–August), autumn (September–November), winter (December–February of the following calendar year) and the vegetation period (April–September). For precipitation, we summed up the values for the corresponding season.
To calculate average site-overarching coefficients for each tree species, we transformed the correlation coefficients using Fisher’s z scores to avoid underestimations due to skewed distributions (Silver and Dunlap 1987) and averaged them. After the calculation, they were back-transformed. Chronologies of young European white elm and oak of stand OFF1 were excluded from the analysis of climate growth relationships, as well of the analysis of drought reaction due to the short time-period covered. Coefficients of correlations for all sites and species can be obtained from the supplements (Figs. S1 and S2).
Of the climate data available for this study, SMI and SPEI were the most reliable drought indices (Schwarz et al. 2020). As they combine both temperature and precipitation, they are more suitable to determine drought years than indices only considering one of the variables (Zscheischler and Seneviratne 2017). While SMI also includes soil data, SPEI was available in a higher spatial resolution. The seasonal SPEI3 for summer (SPEI3summer) showed the highest correlation and lowest inter-stand variation for all species and stands and was used in further analysis. Drought years selected with this index are also resembling future climatic conditions, where drought events will mainly happen in summer, but also in spring months (Cook et al. 2020; Ionita et al. 2020; Ionita and Nagavciuc 2021), as it includes mainly summer data but due to its 3-month frame also spring months. Furthermore, SPEI3 is a suitable index as it is targeting short-term extreme events and seasonal precipitation changes and is widely used to assess drought in forest ecosystems (Bachmair et al. 2018; Dell’Oro et al. 2020; Obladen et al. 2021; Spinoni et al. 2017).
Drought year identification was based on classifications by Slette et al. (2019). We considered all years with SPEI3summer ≤ – 1 as potential drought years. From these years, we chose the three driest years of the time covered by the chronology of each stand separately (Supplements Table S2).
Analysis of tree-ring data
Tree-ring data were detrended using a 30-year spline with a 50% frequency cutoff (Cook 1992; Cook and Peters 1997; Klesse 2021). This spline smoothes low-frequency variation in the tree-ring series which are associated with management (e.g. due to thinnings) and age trends. Inter-annual variability and high-frequency variation, however, are still preserved. The detrending procedure resulted in dimensionless ring-width indices (RWI) (see Fig. 3).
For the two cores of each tree, the mean value per year was calculated using a Tukey’s biweight robust mean. Finally, we transformed the RWI of each site into respective average chronologies. Years with measurements from less than 5 trees were truncated. In addition, we calculated the subsample signal strength (SSS) (Wigley et al. 1984) for each plot and year covered by the data. The SSS (Wigley et al. 1984) is a value quantifying the correlation between a subsample of time series and a larger sample and is an indicator for the strength of representation of the larger sample by the smaller subsample (Buras 2017). Years in a chronology with a SSS smaller than 0.85 were truncated, following the recommendations of Wigley et al. (1984). The quality of the chronologies was furthermore assessed by using common dendroecological key figures. The values can be obtained from Supplementary Table S1.
Variability of growth and influence of site and tree variables
To assess the dispersion of tree-ring series, we used the Gini coefficient, calculated from the detrended year ring widths, as proposed by Biondi and Qeadan (2008). The Gini coefficient is a frequently used index of data variability, covering all lags in a tree-ring series. To test differences between the species-specific Gini coefficients, we pooled the detrended tree-ring data for each species and calculated bootstrapped 95% confidence intervals (Dixon et al. 1987).
To assess the sensitivity of annual growth on tree and site variables, we fitted a mixed effects model for each tree species. For the evaluation of annual drought conditions, we used the SPEI3summer. The global model was fitted using the following formula:
where t referred to the year and i to the random intercept on tree level and a0, a1, a2, a3, a4, a5 and a6 were regression coefficients and \({\varepsilon }_{it}\) the normally distributed error term. The bi variable respected the nested data structure on tree level. To consider the presence of an autocorrelation between consecutive tree-ring measurements, we included an auto-regressive correlation structure (Pinheiro and Bates 2000; Venables and Ripley 2002).
The model assumptions of homoscedasticity and normality of residuals were checked visually using qq-plots and by plotting residuals vs. fitted values. The plots showed no violation of model assumptions.
The global model was then stepwise reduced using the Akaike Information Criterion (AIC). For European white elm, we additionally removed the DMI from the model, as both sites showed very similar values (36.11 and 38.68). We scaled and centred the predictive variables to enhance the comparability and comprehensibility of variables and their influence on the response variable (Schielzeth 2010). A summary of the unstandardised input variables can be found in supplementary table S3.
Analysis of drought reaction
Reaction to single drought events
For the selected drought years, we subsequently calculated indices of resistance (Rt), recovery (Rc) and resilience (Rs) according to Lloret et al. (2011) to quantify growth responses towards droughts. These indices are commonly used in dendroecological studies (Schwarz et al 2020). They are calculated as ratios between the growth in a period before (PreDr), during (Dr), and after drought (PostDr):
We chose variable pre- and post-drought periods of 2, 3 and 5 years as recommended by Schwarz et al. (2020). However, we could not find an evident difference between different periods. We decided to use a pre- and post-drought period of 2 years, as this period restricts the influence of other effects like mast years or defoliation by insects (Bottero et al. 2021; Schwarz et al. 2020) and also allowed us to include the recovery values of 2018 in our study.
To detect significant differences between the median values of the species for all Lloret indices, we used a Wilcoxon rank-sum test with Bonferroni correction and a p value of 0.05, as the assumption of normal distribution of the samples could not be accepted for all species.
As proposed by Schwarz et al. (2020), we also compared our species-specific relationship between resistance and recovery to the “line of full resilience”. This line is derived by the relation between recovery, resilience and resistance (5), when the resilience values is set to 1 (6):
The species-specific relationship follows a power-function with the following formula:
The line of full resilience shows the hypothetical recovery values a tree needs to reach to fully obtain its pre-drought growth level (full resilience) for each resistance value. By observing the deviation and progression of the species-specific curve in comparison to the line of full resilience, we can rank and summarise the growth responses to drought and assess the recovery potential of the species.
To detect differences in the relationships of recovery and resistance between the species, we linearized formula (7) by using a logarithmic transformation and included the species as a linear term:
We performed post hoc comparisons of coefficient combinations using the glht function from the multcomp package (Hothorn et al. 2015) to evaluate differences in the progression of the curve of full resilience between species.
Effect of site and tree variables on drought response
To assess the dependency of resilience, resistance and recovery on site variables and diameter, we fitted mixed effect models for every species, following the same approach as for the model of annual growth sensitivity.
This resulted in the following global models:
where t referred to the year and i to the random intercept on tree level and a0, a1, a2, a3, a4, a5 and a6 were regression coefficients and \({\varepsilon }_{it}\) the normally distributed error term. SPEI3t stands for the SPEI3 of summer months of the year t. The bi variable represented the random intercept on tree level. The model’s explanatory variables were stepwise reduced, resulting in the model with the lowest AIC.
The model assumptions of homoscedasticity and normality of residuals were again checked visually using qq-plots and by plotting residuals vs. fitted values. The plots showed no violation of model assumptions.
Statistical software
All analyses in our study were carried out using R, version 4.2.1 (R Core Team 2022). For calculating SPEI values, we used the SPEI package (Beguería and Vicente-Serrano 2017), described in Beguería et al. (2014). For detrending, the calculation of descriptive statistics and chronology building of tree-ring data, we used the R package dplR (Bunn 2010; Bunn et al. 2021) and pointRes (van der Maaten-Theunissen et al. 2015, 2021). Climate growth relationships were calculated using the treeclim package (Zang and Biondi 2015). Linear mixed effect model were fitted using lme4 (Bates et al. 2015) and lmerTest (Kuznetsova et al. 2017). For general linear hypothesis testing, we used the package multcomp (Hothorn et al. 2015), and for the calculation of the Gini coefficient, the package DescTools (Signorell 2022).
Results
Growth rates and growth variability
The basic tree-ring values for all species can be obtained from Table 3. European white elm showed the highest maximal year ring widths of all species, with also the highest standard deviation. This resulted in a strong variability of diameters at a given age (Fig. 4). Compared to the other tree species, the tree-ring widths of European hornbeam did not vary much between different sites and stands. The dbh-age curves of the trees were very similar. This applied to both the plots located in Germany and the plots located in Poland. While mean tree-ring widths of wild service tree remained under the level of European beech and oak, its maximum tree-ring width showed a higher value than oak and European beech.
The growth variability of detrended tree rings was higher for the rare species than for European beech and oak (Fig. 5). Here, European white elm showed the highest Gini coefficient, European beech the lowest.
Effect of site and tree variables on annual growth
The fit and reduction of the global model (Eq. 1) resulted in the species-specific models displayed in Supplementary Table S4.
The annual growth of all species was significantly affected by dbh, annual SPEI and, was applicable, by the DMI. For European beech, European hornbeam, field maple, wild service tree and oak we could observe significant differences in annual growth between sites with poor and better water supply. On very dry sites, the annual growth decreased for bigger trees. On better sites, however, this decrease was weaker for field maple and wild service tree or did not happen at all for European beech, European hornbeam, and oak. Here, we could even observe an increase in annual growth for bigger dbhs. For European beech and field maple also, the reaction on annual weather conditions, displayed by the SPEI, differed between sites. For these species, the increase of annual growth as a reaction to high SPEI values was higher on good sites than on very dry sites. All other species did not show a difference in their reaction between sites. However, higher SPEI values generally led to a higher annual growth. European white elm and European hornbeam showed a higher sensitivity and increase in annual growth towards wetter years for thinner trees.
Species-specific drought reaction
Resistance, resilience, and recovery towards drought
We could sort the species by their median values for the index of resistance in the following order: Field maple (0.61) < European hornbeam (0.73) < wild service tree (0.74) < oak (0.76) < European beech (0.79) < European white elm (0.92) (Fig. 6). After testing the hypothesis of equal medians, we could differ between three groups: field maple with low resistance values, oak and European hornbeam with a middle position and European white elm with the highest resistance values. European beech and wild service tree took an intermediate position between European hornbeam and European white elm.
Index values of the Lloret indices of recovery, resilience and resistance for European beech, European hornbeam, European white elm, field maple, oak, and wild service tree. Significant differences (p < 0.05) between the medians of the index value per species, determined by a Wilcoxon rank-sum test with Bonferroni correction, are displayed with letters
The median recovery values were ranked as follows: European white elm (1.05) < oak (1.13) < European hornbeam and wild service tree (1.18) < European beech (1.20) < field maple (1.63). Overall, after testing for equal means, we could not find significant differences between medians. Only field maple showed a significant higher recovery value than the other species.
Median resilience values followed the subsequent order: oak (0.86) < wild service tree, European whit elm and European hornbeam (0.90) < European beech (0.94) < field maple (1.02). Between the median of oak and field maple, we could observe a significant difference. For all other species, the hypothesis of equal medians could not be rejected.
Figure 7 shows the relationship between recovery and resistance of the species and the theoretical line of full resilience. European hornbeam, field maple and European beech already showed a very close fit to the line of full resilience for very low values of resistance. Field maple and wild service tree intersected the full-resilience line even for lower resistance values than the other species. The model intercept of European beech was significantly higher (p < 0.05) than the one of wild service tree, European hornbeam, and field maple. In addition, field maple showed a significant higher intercept than oak. For resistance values above 1, field maple, oak and wild service tree showed the highest positive deviation from the line of all species.
In general, we could observe three major curve shapes. European hornbeam and European beech on the one hand showed a good fit to the line of full resilience for all resistance values. On the other hand, European white elm, wild service tree and oak showed a negative deviation from the line for low resistance values but, especially for wild service tree, with a an early intersection and a subsequent clear positive deviation. Field maple, as the only species, shows both a good fit on the line of full resilience for low resistance values and its exceeding for high resistance values.
Influence of site and tree size on drought reaction
Concerning the drought reaction, we could observe significant influences of tree size, drought severity and overall site conditions. However, the expression of the effects strongly differed between species.
The resilience of European hornbeam, European white elm and field maple was significantly affected by the severity of the drought year, displayed by the SPEI values. Field maple and European white elm were more resilient after milder drought years, while European hornbeam showed an opposite reaction. For field maple, this effect was especially visible on sites with a lower DMI, while on sites with more favourable climatic conditions, the resilience was similar over different drought intensities. In addition, for European beech and oak, we could observe an influence of the long-term climatic conditions of the site, displayed by the DMI, and a significant influence of the dbh. For European beech, the resilience values were higher on sites with higher water supply and for thinner trees. For oak, we could observe the higher resilience of thin trees only on sites with a high DMI. On sites with a lower DMI trees of all diameters showed similar resilience values. Notable is, that for wild service tree, we could not find a significant effect of any variable.
Concerning the resistance, we could observe a significant influence of the SPEI values of the drought year for all species beside wild service tree. For oak, European white elm and field maple, a less severe drought led to higher resistance values. European beech showed low resistance values in very dry years on sites with a low DMI, while the resistance of European hornbeams increased with more severe droughts on those sites. Concerning the effect of tree size on the resistance European beech showed a higher resistance in bigger trees on sites with a high DMI and thinner trees on sites with a low DMI. Again, it is notable that for wild service tree, we could not find a significant effect of any variable.
The tree size did not have a significant influence on the recovery of European white elm, field maple, and wild service tree. For oak, we could observe a better recovery for thinner trees. Thinner European hornbeams showed a better recovery on favourable sites than bigger trees, while on sites with a low DMI no such size effect could be observed. For European beech, we could observe the opposite, with a better recovery of thin trees on sites with a low DMI and of bigger trees on sites with a high DMI. This significant influence of the climate conditions on the sites could also be observed for oak and field maple. Field maple and European beech showed generally higher recovery values on sites with low water supply and less severe drought years. On more favourable site, this effect was reversed. The recovery values of oak generally increased with a higher DMI. Wild service tree and European white did not show a significant effect of diameter, site and SPEI on their recovery.
In summary, we could observe a high susceptibility to severe drought for beech on sites with a low DMI, while oak was more stable, especially on good sites and over different tree sizes. Among the rare native species, field maple again stuck out with a high robustness towards drought, even on very dry sites and towards severe drought. European hornbeam reacted similarly as European beech, however, with a higher tolerance towards severe droughts and more constant over different tree sizes. For wild service tree, our data showed a stable drought reaction without any influence of site and tree variables. European white elm showed a particularly high tolerance towards mild droughts.
Discussion
Growth rates and growth variability
Concerning the age-growth relationships, we could observe that the growth of the rare species is within the growth range of European beech and oak on the same sites. When comparing the growth of European hornbeam with existing yield tables for northern Germany (Lockow 2009), sites in Germany reached a site index of II.5 (site WAL) and II (site EBR). Both sites in Poland reached a site index of II.25, despite their lower DMI. For all stands, the mean diameters were higher than the diameters specified by the yield table. This indicates that the validity of the yield table of Lockow (2009) might be regionally limited to stands in northern Germany with their specific climatic and soil conditions. On other sites, the yield table may underestimate the actual growth of European hornbeam. The similar growth patterns of European hornbeam in Poland and Germany underline its high ecological amplitude. Even in eastern Poland, under climate conditions that are limiting the distribution range of European beech, the European hornbeam shows high growth rates.
For wild service tree, the annual increments were similar to the ones observed by Pyttel et al. (2013). They also observed very low annual increments which they explained by the high shade tolerance of wild service tree and its ability to even survive under strong suppression. This indicates that some of the wild service trees used in our study might have been suppressed in the past or growing under a lot of competition. This also explains the high variation in the dbh-age curves showed in Fig. 4, where some trees had much higher diameters for a given age than others.
In general, European beech and oak grew more stable over all sites. The missing significant difference in annual growth between sites with different DMI of oak in the linear mixed model is another indication of the lower climatic sensitivity of oak in comparison to European beech (Scharnweber et al. 2011). The maximum tree-ring widths of the rarer species show, that under good conditions and with proper silvicultural management, they can reach high year ring widths. Combined with the results of the model assessing the sensitivity on site variables, we can see that the high Gini coefficients of rare species are also connected to a high susceptibility of the species to favourable years. Apparently, the species can easily implement good annual growing conditions in annual growth. The high Gini coefficients may also be related to a different silvicultural treatment of the species in the past. European hornbeams are often used as a so called “serving” tree species in oak stands for the shading of stems and the prevention of the development of secondary branches. After a release of suppression by the extraction of the canopy, a higher growth can be provoked. However, this again underlines that with enough growing space high year ring widths of the rare species are possible.
The lower sensitivity to drier years of trees with a smaller dbh that we observed could be related to their smaller height and thus the lower gravimetric potential they have to overcome to transport water in the tree trunk and the higher stomatal conductance and sensitivity that make smaller trees less prone to transpirational stress (Grote et al. 2016; Ryan et al. 2006; Ryan and Yoder 1997). The decline in growth with an increasing tree size on dry sites can be traced back to a typical age effect and the culmination of growth already at lower diameters on poorer sites. The decrease of annual growth in field maple and wild service tree even on better sites indicates that both species culminate earlier in their growth than European beech and oak. Again, it also indicates a different silvicultural treatment of the species in the past. The increase of growth with higher diameters on better sites for European beech, oak and European hornbeam points to a release from suppression or competition in higher ages. Outstanding is the high potential of annual growth of European white elm. Especially on good sites and in young stands, this species can easily obtain year ring widths of more than 1 cm.
Drought reaction
When comparing the Lloret indices of all species, not considering site and tree variables, we could not find many significant differences between species. Nevertheless, it was noticeable that field maple showed especially high values of recovery and resilience, however, with low resistance values. The resistance values of European beech being higher than the ones of field maple is contradicting the results of Kunz et al. (2016) who had reverse findings. This could be due to a different selection of drought years and stands in both studies and also the missing consideration between resistance and recovery values (Schwarz et al. 2020). However, when combining resistance and recovery values and comparing them with the line of full resilience, field maple and wild service tree stick out as drought tolerant species. Wild service tree due to its capability to surpass the line of full resilience for milder droughts and field maple for both its high recovery values in years with low resistance and its good performance after milder drought. This again matches the findings of Kunz et al. (2016). It once more shows the importance of rather combining both resistance and recovery than looking at the single indices when evaluating the drought tolerance of different species. Thereby, it can be considered that species with high resistance values were often showing low recovery values and vice versa, as after a strong decline, there is naturally a stronger potential for recovery. Species with higher values of recovery for lower resistance values, as wild service tree and field maple, can recover more easily even in years with a strong growth decline. Oak showed better overall resistance to drought than the rare species, however, in severe drought years field maple and wild service tree seem to be better adapted.
The good recovery of oak and European white elm in years with milder drought can be attributed to their wood anatomy. In contrast to the other species considered in this study, oak and elm are ring porous species. They are less sensitive to climate and drought than diffuse-porous species (Elliott et al. 2015) as they produce larger early wood vessels in which then the majority of the hydraulic transport takes place (Hacke and Sauter 1996; Zimmermann 1983). After a drought event, they can, therefore, recover more quickly as larger vessels are already produced at the start of the next growing season. Diffuse porous species must use their smaller vessels, created throughout the previous growing period. Embolised conduits can be repaired by refilling them (Hacke and Sauter 1996). Furthermore, our results correspond to the findings of Leonova et al. (2022), who found an increased fine root growth of European white elm during drought. This also leads to an enhanced growth during the next growing season.
Influence of size and site variables
Regarding the drought reaction, we could observe a higher drought tolerance of European hornbeam towards severe drought and on dry sites, as already observed by Leuzinger et al. (2005). This could explained by differences in their water consumption and stomatal regulation strategies, where European hornbeam follows a more isohydric behaviour (Köcher et al. 2009; Leuschner et al. 2019). In contrast to our results, Scharnweber et al. (2020) observed a strong growth decline for European hornbeam after two consecutive drought years. The drought tolerance of European beech and European hornbeam was similar in their study, with even a higher growth decline for European hornbeam. However, they suppose that a part of this growth decline can be attributed to an increased growth of roots in drought years as an adaptation strategy. This again matches our results where the higher resistance of European hornbeam towards severe drought could be observed for dry sites, where the European hornbeam might already be adapted to drought events. For field maple on very dry sites, we could observe a high resilience and very high recovery towards milder droughts. On more favourable sites field maple also recovered very well after severe drought events. This one more time points out the high drought tolerance of the species and its high potential on dry sites. As already studied by Kunz et al. (2018), field maple is a highly drought tolerant species with a high potential under climate change. Beloiu et al. (2022) found a low recovery and high mortality after drought for field maple saplings, while Kunz et al. (2016) found much younger seedlings to be highly drought resistant. This indicates that the initial drought resistance of the species may decrease with age. However, the high mortality after drought events may lead to a natural selection of trees with a higher drought resistance and, therefore, an adaptation to drought. At the same time, it may result in a higher drought tolerance of surviving trees due to an acclimation effect after the drought event (Kozlowski and Pallardy 2002). This again results in a higher drought tolerance of mature trees. For European white elm, we could not differentiate between different climatic site conditions, as the two sites had very similar DMI values. However, we could observe a high resistance against milder drought. Although more detailed research is needed, our study confirms the assumptions of Walentowski et al. (2014), that European white elm could be a suitable species under drier climate conditions. Site variables did not have any influence on drought resilience, recovery, and resistance of wild service tree. This is also a sign of the high stability of wild service tree under different site conditions. On both sites, we could find a good recovery after mild droughts, which was already observed by Kunz et al. (2016) for seedlings.
Oak could cope better with drought on wetter sites, however, showing higher resistance values for large trees and a faster recovery of small trees on dry sites. This can be attributed to the weaker stomatal control on photosynthesis of small oaks trees (Zang et al. 2012). In our study, we could observe the dependence of drought tolerance of European beech on climatic site conditions. On wetter sights, the tolerance of severe droughts was higher than on dry sites, while the tolerance towards mild droughts was lower. This higher susceptibility to mild drought on sites with a good water supply was already observed in other studies dealing with the growth of European beech towards its distribution margins (Cavin and Jump 2017; Muffler et al. 2020; Weber et al. 2013). It might be the result of a phenotypical reaction (Cavin and Jump 2017; Leuschner 2020) to site conditions and an acclimation after multiple drought years (Petrik et al. 2022). This could point to a more isohydric behaviour of trees on drier sites (Nguyen et al. 2017). The good recovery of beech in milder droughts on dry sites can be connected to the rapid recovery of leaf metabolism (Leuschner 2020). High recovery values on wet sites after droughts might be related to the stimulation of fine root growth, as observed for saplings of European beech (Zang et al. 2014).
Study limitations
Our study results show some limitation as tree cores were partially taken of different stem heights. The expression of climate sensitivity decreases on lower parts of the stem (Hoffmann et al. 2018) which may lead to some inaccuracy when comparing values of stands with different retrieval height. Furthermore, we did not sample dead trees as proposed by Schwarz et al. (2020). Therefore, we were not able to assess climatic factors that might have a very negative, even lethal, effect on tree growth. The low mean-Gleichläufigkeit values of European white elm indicate that the growth of trees on the plots was not primarily limited by precipitation and evapotranspiration, but rather by other site factors. As both sampled sites of European white elm were in riparian forests, the flooding regime and groundwater access could be factors determining tree growth. This follows the critique of Schwarz et al. (2020) and Zang et al. (2020), who suggest including more soil parameters in studies. For our study this was not possible, as, besides the SMI, no data on past soil moisture were available for those stands. The SMI, however, only has a resolution of 4 × 4 km, which again leads to imprecise results for our forest stands.
By sampling two sites per species, we tried to cover a variety of different site conditions. However, this was not always possible, as the occurrence of our species was often limited to sites where European beech or coniferous species were not dominating. These were, in case of field maple and wild service tree, very dry sites, in case of European white elm riparian forests. However, as seen in Fig. 2, our selected stands were located mostly in the core distribution range of the species and thereby represent typical climatic conditions. Nevertheless, an expansion of the study area with more plots on the edges of the distribution range could be interesting for future studies.
In our study, we did not account for the fact that repeated drought events may influence the drought reaction of the species. Especially for younger stands, the selected drought years might be the first droughts that the trees were experiencing. However, as all species covered younger and older trees with a different number of past drought events, we decided to neglect that effect in our analysis.
Conclusion: implications for silviculture
Even if European beech shows a certain drought resistance on sites with a good water supply and during mild droughts, it is generally displaying a high sensitivity towards drought. This points out a possible limitation on its occurrence and cultivation. Even on more favourable sites, the risk of drought events will increase in the future. As other rare species were more resistant towards drought on these sites, they could increase their shares in the species composition while the share of European beech might decrease.
Oak turned out to be a stable species, even under dry conditions. Comparably high increments combined with a high drought resistance make it a solid basis for future forest stands. For sites were in the future many very dry years are expected, wild service tree and field maple are good options to enrich these stands. These two species are very well adapted to drought and can keep up with oak with their ability in transforming good weather conditions into annual growth. The proportions of mixture should increase with increasing drought potential.
Concerning the drought sensitivity and growth, European hornbeam showed overall a similar behaviour as European beech, however, with a slightly smaller climate sensitivity and a stable growth even over different climate zones in Poland and Germany. Furthermore, our results show a good resistance against severe drought years. We, therefore, recommend European hornbeam as a complementary species for the enrichment of European beech stands. As for both species drought sensitivity increased with diameter, an adjustment of target diameters might be considered. In addition, oak stands with slightly better water supply could be suitable for an admixture of European hornbeam.
The higher drought tolerance of younger trees of wild service tree, field maple and European white elm is especially important concerning the relative uncertainty of weather development in the next years. Our results suggest that young stands with those species, that were recently established or will be established in the next years, can cope with a variety of different weather conditions and are, therefore, a low-risk option to sustain unstable stands on dry sites.
European white elm can be highly recommended as a species for riparian forest systems. Even if we could only get a limited assertion of its behaviour under drought conditions, our results generally indicate a high drought tolerance which implies a suitability also on dry sites. European white elm is prone to the invasive Dutch elm disease (DED), but much less than other native elm species like wych elm (Ulmus glabra Huds.) (Jürisoo et al. 2019, 2021). As common ash (Fraxinus excelsior L.) is also suffering from ash dieback, European white elm remains as a relatively stable species on floodplain sites, fulfilling both ecological and economical functions. The high growth rates also make it an interesting species concerning the carbon sequestration in forests.
In general, rare native species are a promising option to enrich the species composition on dry sites and to contribute to the establishment of climate tolerant forest stands. However, with ongoing climate change, even those species will eventually reach the limits of their drought tolerance. Therefore, the fight against climate change stays the most important factor to guarantee the stability of future forests.
Author contribution statement
JS collected and analysed data and wrote the manuscript; GS supervised the tree-ring analysis and manuscript writing process; EU supervised data collection, analysis and writing process; HP initiated the study and supervised the whole data collection, analysis and writing process.
Data availability
The materials described in the manuscript including all relevant raw data will be freely available upon request from the corresponding author.
References
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim J-H, Allard G, Running SW, Semerci A, Cobb N (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684. https://doi.org/10.1016/j.foreco.2009.09.001
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:art129. https://doi.org/10.1890/ES15-00203.1
Bachmair S, Tanguy M, Hannaford J, Stahl K (2018) How well do meteorological indicators represent agricultural and forest drought across Europe? Environ Res Lett 13:34042. https://doi.org/10.1088/1748-9326/aaafda
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft. https://doi.org/10.18637/jss.v067.i01
Beguería S, Vicente-Serrano SM (2017) SPEI: Calculation of the Standardised Precipitation-Evapotranspiration Index.: R package version 1.7
Beguería S, Vicente-Serrano SM, Reig F, Latorre B (2014) Standardized precipitation evapotranspiration index (SPEI) revisited: parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int J Climatol 34:3001–3023. https://doi.org/10.1002/joc.3887
Beloiu M, Stahlmann R, Beierkuhnlein C (2020) High recovery of saplings after severe drought in temperate deciduous forests. Forests 11:546. https://doi.org/10.3390/f11050546
Beloiu M, Stahlmann R, Beierkuhnlein C (2022) Drought impacts in forest canopy and deciduous tree saplings in Central European forests. For Ecol Manag 509:120075. https://doi.org/10.1016/j.foreco.2022.120075
Bhuyan U, Zang C, Menzel A (2017) Different responses of multispecies tree ring growth to various drought indices across Europe. Dendrochronologia 44:1–8. https://doi.org/10.1016/j.dendro.2017.02.002
Biondi F, Qeadan F (2008) Inequality in paleorecords. Ecology 89:1056–1067. https://doi.org/10.1890/07-0783.1
Black-Samuelsson S, Whiteley RE, Junzhan G (2003) Growth and leaf morphology response to drought stress in the riparian broadleaved tree, Ulmus laevis (Pall.). Silvae Genetica 52:292–298
Bolte A, Ammer C, Löf M, Madsen P, Nabuurs G-J, Schall P, Spathelf P, Rock J (2009) Adaptive forest management in central Europe: climate change impacts, strategies and integrative concept. Scand J for Res 24:473–482. https://doi.org/10.1080/02827580903418224
Bottero A, Forrester DI, Cailleret M, Kohnle U, Gessler A, Michel D, Bose AK, Bauhus J, Bugmann H, Cuntz M, Gillerot L, Hanewinkel M, Lévesque M, Ryder J, Sainte-Marie J, Schwarz J, Yousefpour R, Zamora-Pereira JC, Rigling A (2021) Growth resistance and resilience of mixed silver fir and Norway spruce forests in central Europe: contrasting responses to mild and severe droughts. Glob Change Biol 27:4403–4419. https://doi.org/10.1111/gcb.15737
Brzeziecki B, Bielak K, Bolibok L, Drozdowski S, Zajączkowski J, Żybura H (2018) Structural and compositional dynamics of strictly protected woodland communities with silvicultural implications, using Białowieża Forest as an example. Ann for Sci. https://doi.org/10.1007/s13595-018-0767-x
Brzeziecki B, Woods K, Bolibok L, Zajączkowski J, Drozdowski S, Bielak K, Żybura H (2020) Over 80 years without major disturbance, late-successional Białowieża woodlands exhibit complex dynamism, with coherent compositional shifts towards true old-growth conditions. J Ecol 108:1138–1154. https://doi.org/10.1111/1365-2745.13367
Bunn AG (2010) Statistical and visual crossdating in R using the dplR library. Dendrochronologia 28:251–258. https://doi.org/10.1016/j.dendro.2009.12.001
Bunn AG, Korpela M, Biondi F, Campelo F, Mérian P, Qeadan F, Zang C (2021) dplR: Dendrochronology Program Library in R. https://CRAN.R-project.org/package=dplR
Buras A (2017) A comment on the expressed population signal. Dendrochronologia 44:130–132. https://doi.org/10.1016/j.dendro.2017.03.005
Buras A, Rammig A, Zang CS (2020) Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences 17:1655–1672. https://doi.org/10.5194/bg-17-1655-2020
Castro-Díez P, Vaz AS, Silva JS, van Loo M, Alonso Á, Aponte C, Bayón Á, Bellingham PJ, Chiuffo MC, DiManno N, Julian K, Kandert S, La Porta N, Marchante H, Maule HG, Mayfield MM, Metcalfe D, Monteverdi MC, Núñez MA, Ostertag R, Parker IM, Peltzer DA, Potgieter LJ, Raymundo M, Rayome D, Reisman-Berman O, Richardson DM, Roos RE, Saldaña A, Shackleton RT, Torres A, Trudgen M, Urban J, Vicente JR, Vilà M, Ylioja T, Zenni RD, Godoy O (2019) Global effects of non-native tree species on multiple ecosystem services. Biol Rev 94:1477–1501. https://doi.org/10.1111/brv.12511
Cavin L, Jump AS (2017) Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob Chang Biol 23:362–379. https://doi.org/10.1111/gcb.13366
Collin E, Bozzano M (2015) Implementing the dynamic conservation of elm genetic resources in Europe: case studies and perspectives. Iforest Biogeosci for 8:143–148. https://doi.org/10.3832/ifor1206-008
Cook ER (ed) (1992) Methods of dendrochronology: Applications in the environmental sciences. Kluwer, Dordrecht
Cook ER, Peters K (1997) Calculating unbiased tree-ring indices for the study of climatic and environmental change. The Holocene 7:361–370. https://doi.org/10.1177/095968369700700314
Cook BI, Mankin JS, Marvel K, Williams AP, Smerdon JE, Anchukaitis KJ (2020) Twenty-first century drought projections in the CMIP6 forcing scenarios. Earth’s Future. https://doi.org/10.1029/2019EF001461
Coudun C, Gégout J-C, Piedallu C, Rameau J-C (2006) Soil nutritional factors improve models of plant species distribution: an illustration with Acer campestre (L.) in France. J Biogeogr 33:1750–1763. https://doi.org/10.1111/j.1365-2699.2005.01443.x
Del Martinez CE, Zang CS, Buras A, Hacket-Pain A, Esper J, Serrano-Notivoli R, Hartl C, Weigel R, Klesse S, Resco de Dios V, Scharnweber T, Dorado-Liñán I, van der Maaten-Theunissen M, van der Maaten E, Jump A, Mikac S, Banzragch B-E, Beck W, Cavin L, Claessens H, Čada V, Čufar K, Dulamsuren C, Gričar J, Gil-Pelegrín E, Janda P, Kazimirovic M, Kreyling J, Latte N, Leuschner C, Longares LA, Menzel A, Merela M, Motta R, Muffler L, Nola P, Petritan AM, Petritan IC, Prislan P, Rubio-Cuadrado Á, Rydval M, Stajić B, Svoboda M, Toromani E, Trotsiuk V, Wilmking M, Zlatanov T, de Luis M (2022) Climate-change-driven growth decline of European beech forests. Commun Biol 5:163. https://doi.org/10.1038/s42003-022-03107-3
Dell’Oro M, Mataruga M, Sass-Klaassen U, Fonti P (2020) Climate change threatens on endangered relict Serbian spruce. Dendrochronologia 59:125651. https://doi.org/10.1016/j.dendro.2019.125651
Dixon PM, Weiner J, Mitchell-Olds T, Woodley R (1987) Bootstrapping the Gini coefficient of inequality. Ecology 68:1548–1551. https://doi.org/10.2307/1939238
Droogers P, Allen RG (2002) Estimating reference evapotranspiration under inaccurate data conditions. Irrig Drain Syst 16:33–45. https://doi.org/10.1023/A:1015508322413
DWD Climate Data Center (2022a) Grids of monthly averaged daily air temperature (2m) over Germany
DWD Climate Data Center (2022b) Grids of monthly total precipitation over Germany
Elliott KJ, Miniat CF, Pederson N, Laseter SH (2015) Forest tree growth response to hydroclimate variability in the southern Appalachians. Glob Chang Biol 21:4627–4641. https://doi.org/10.1111/gcb.13045
Eyring V, Bony S, Meehl GA, Senior CA, Stevens B, Stouffer RJ, Taylor KE (2016) Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci Model Dev 9:1937–1958. https://doi.org/10.5194/gmd-9-1937-2016
Grote R, Gessler A, Hommel R, Poschenrieder W, Priesack E (2016) Importance of tree height and social position for drought-related stress on tree growth and mortality. Trees 30:1467–1482. https://doi.org/10.1007/s00468-016-1446-x
Hacke U, Sauter JJ (1996) Xylem dysfunction during winter and recovery of hydraulic conductivity in diffuse-porous and ring-porous trees. Oecologia 105:435–439. https://doi.org/10.1007/BF00330005
Hargreaves GH (1994) Defining and using reference evapotranspiration. J Irrig Drain Eng 120:1132–1139. https://doi.org/10.1061/(ASCE)0733-9437(1994)120:6(1132)
Harris I, Osborn TJ, Jones P, Lister D (2020) Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci Data 7:109. https://doi.org/10.1038/s41597-020-0453-3
Helmholtz Centre for Environmental Research (2021) SMI-Daten 1951–2020, Gesamtboden. https://www.ufz.de/index.php?de=37937
Hoffmann N, Schall P, Ammer C, Leder B, Vor T (2018) Drought sensitivity and stem growth variation of nine alien and native tree species on a productive forest site in Germany. Agric for Meteorol 256–257:431–444. https://doi.org/10.1016/j.agrformet.2018.03.008
Hothorn T, Bretz F, Westfall P (2015) Simultaneous inference in general parametric models. Biomet J 50(3):346–363. https://doi.org/10.1002/bimj.200810425
Ionita M, Nagavciuc V (2021) Changes in drought features at the European level over the last 120 years. Nat Hazards Earth Syst Sci 21:1685–1701. https://doi.org/10.5194/nhess-21-1685-2021
Ionita M, Nagavciuc V, Kumar R, Rakovec O (2020) On the curious case of the recent decade, mid-spring precipitation deficit in central Europe. Npj Clim Atmos Sci. https://doi.org/10.1038/s41612-020-00153-8
Jacobs M, Rais A, Pretzsch H (2021) How drought stress becomes visible upon detecting tree shape using terrestrial laser scanning (TLS). For Ecol Manag 489:118975. https://doi.org/10.1016/j.foreco.2021.118975
Jürisoo L, Adamson K, Padari A, Drenkhan R (2019) Health of elms and Dutch elm disease in Estonia. Eur J Plant Pathol 154:823–841. https://doi.org/10.1007/s10658-019-01707-0
Jürisoo L, Selikhovkin AV, Padari A, Shevchenko SV, Shcherbakova LN, Popovichev BG, Drenkhan R (2021) The extensive damage to elms by Dutch elm disease agents and their hybrids in northwestern Russia. Urban for Urban Green 63:127214. https://doi.org/10.1016/j.ufug.2021.127214
Kasper J, Leuschner C, Walentowski H, Petritan AM, Weigel R (2022) Winners and losers of climate warming: declining growth in Fagus and Tilia vs. stable growth in three Quercus species in the natural beech–oak forest ecotone (western Romania). For Ecol Manag 506:119892. https://doi.org/10.1016/j.foreco.2021.119892
Klesse S (2021) Critical note on the application of the “two-third” spline. Dendrochronologia 65:125786. https://doi.org/10.1016/j.dendro.2020.125786
Koch O, de Avila AL, Heinen H, Albrecht AT (2022) Retreat of major European tree species distribution under climate change—minor natives to the rescue? Sustainability 14:5213. https://doi.org/10.3390/su14095213
Köcher P, Gebauer T, Horna V, Leuschner C (2009) Leaf water status and stem xylem flux in relation to soil drought in five temperate broad-leaved tree species with contrasting water use strategies. Ann for Sci 66:101. https://doi.org/10.1051/forest/2008076
Kozlowski TT, Pallardy SG (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334. https://doi.org/10.1663/0006-8101(2002)068[0270:AAAROW]2.0.CO;2
Kreyling J, Schmid S, Aas G (2015) Cold tolerance of tree species is related to the climate of their native ranges. J Biogeogr 42:156–166. https://doi.org/10.1111/jbi.12411
Kumar R, Samaniego L, Attinger S (2013) Implications of distributed hydrologic model parameterization on water fluxes at multiple scales and locations. Water Resour Res 49:360–379. https://doi.org/10.1029/2012WR012195
Kunz J, Räder A, Bauhus J (2016) Effects of drought and rewetting on growth and gas exchange of minor European broadleaved tree species. Forests 7:239. https://doi.org/10.3390/f7100239
Kunz J, Löffler G, Bauhus J (2018) Minor European broadleaved tree species are more drought-tolerant than Fagus sylvatica but not more tolerant than Quercus petraea. For Ecol Manage 414:15–27. https://doi.org/10.1016/j.foreco.2018.02.016
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Soft. https://doi.org/10.18637/jss.v082.i13
Leonova A, Heger A, Vásconez Navas LK, Jensen K, Reisdorff C (2022) Fine root mortality under severe drought reflects different root distribution of Quercus robur and Ulmus laevis trees in hardwood floodplain forests. Trees 36:1105–1115. https://doi.org/10.1007/s00468-022-02275-3
Leuschner C (2020) Drought response of European beech (Fagus sylvatica L.)—a review perspectives in plant ecology. Evol Syst 47:125576. https://doi.org/10.1016/j.ppees.2020.125576
Leuschner C, Wedde P, Lübbe T (2019) The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann for Sci. https://doi.org/10.1007/s13595-019-0838-7
Leuzinger S, Zotz G, Asshoff R, Körner C (2005) Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol 25:641–650. https://doi.org/10.1093/treephys/25.6.641
Lloret F, Keeling EG, Sala A (2011) Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120:1909–1920. https://doi.org/10.1111/j.1600-0706.2011.19372.x
Lockow K-W (2009) Die Hainbuche im nordostdeutschen Tiefland: Wuchsverhalten und Bewirtschaftungshinweise;[Waldwirtschaft-aber natürlich]. MLUV, Referat Presse-und Öffentlichkeitsarbeit
Martonne ME (1926) Areisme et indice d’aritile. Compt Rend Acad Sci 182:1395–1398
Matevski D, Schuldt A (2021) Tree species richness, tree identity and non-native tree proportion affect arboreal spider diversity, abundance and biomass. For Ecol Manag 483:118775. https://doi.org/10.1016/j.foreco.2020.118775
Mauri A, Strona G, San-Miguel-Ayanz J (2017) EU-Forest, a high-resolution tree occurrence dataset for Europe. Sci Data 4:160123. https://doi.org/10.1038/sdata.2016.123
Mette T, Dolos K, Meinardus C, Bräuning A, Reineking B, Blaschke M, Pretzsch H, Beierkuhnlein C, Gohlke A, Wellstein C (2013) Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 4:art145. https://doi.org/10.1890/ES13-00115.1
Meyer BF, Buras A, Rammig A, Zang CS (2020) Higher susceptibility of beech to drought in comparison to oak. Dendrochronologia 64:125780. https://doi.org/10.1016/j.dendro.2020.125780
Muffler L, Weigel R, Hacket-Pain AJ, Klisz M, Maaten E, Wilmking M, Kreyling J, Maaten-Theunissen M (2020) Lowest drought sensitivity and decreasing growth synchrony towards the dry distribution margin of European beech. J Biogeogr 47:1910–1921. https://doi.org/10.1111/jbi.13884
Nguyen QN, Polle A, Pena R (2017) Intraspecific variations in drought response and fitness traits of beech (Fagus sylvatica L.) seedlings from three provenances differing in annual precipitation. Trees 31:1215–1225. https://doi.org/10.1007/s00468-017-1539-1
Obladen N, Dechering P, Skiadaresis G, Tegel W, Keßler J, Höllerl S, Kaps S, Hertel M, Dulamsuren C, Seifert T, Hirsch M, Seim A (2021) Tree mortality of European beech and Norway spruce induced by 2018–2019 hot droughts in central Germany. Agric for Meteorol 307:108482. https://doi.org/10.1016/j.agrformet.2021.108482
Petrik P, Petek-Petrik A, Kurjak D, Mukarram M, Klein T, Gömöry D, Střelcová K, Frýdl J, Konôpková A (2022) Interannual adjustments in stomatal and leaf morphological traits of European beech (Fagus sylvatica L.) demonstrate its climate change acclimation potential. Plant Biol (stuttg). https://doi.org/10.1111/plb.13401
Pfenninger M, Reuss F, Kiebler A, Schönnenbeck P, Caliendo C, Gerber S, Cocchiararo B, Reuter S, Blüthgen N, Mody K, Mishra B, Bálint M, Thines M, Feldmeyer B (2021) Genomic basis for drought resistance in European beech forests threatened by climate change. Elife. https://doi.org/10.7554/eLife.65532
Philipp M, Wegmann M, Kübert-Flock C (2021) Quantifying the response of German forests to drought events via satellite imagery. Remote Sensing 13:1845. https://doi.org/10.3390/rs13091845
Pilli R, Alkama R, Cescatti A, Kurz WA, Grassi G (2022) The European forest carbon budget under future climate conditions and current management practices. Biogeosciences 19:3263–3284. https://doi.org/10.5194/bg-19-3263-2022
Pinheiro JC, Bates DM (2000) Extending the basic linear mixed-effects model. In: Mixed-effects models in S and S-PLUS. Springer, New York, NY. pp 201–270. https://doi.org/10.1007/0-387-22747-4_5
Pötzelsberger E, Spiecker H, Neophytou C, Mohren F, Gazda A, Hasenauer H (2020) Growing non-native trees in european forests brings benefits and opportunities but also has its risks and limits. Curr Forestry Rep 6:339–353. https://doi.org/10.1007/s40725-020-00129-0
Pretzsch H, Schütze G, Uhl E (2013) Resistance of European tree species to drought stress in mixed versus pure forests: evidence of stress release by inter-specific facilitation. Plant Biol (stuttg) 15:483–495. https://doi.org/10.1111/j.1438-8677.2012.00670.x
Pyttel P, Kunz J, Bauhus J (2013) Growth, regeneration and shade tolerance of the Wild Service Tree (Sorbus torminalis (L.) Crantz) in aged oak coppice forests. Trees 27:1609–1619. https://doi.org/10.1007/s00468-013-0908-7
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rasmussen KK, Kollmann J (2004) Defining the habitat niche of Sorbus torminalis from phytosociological releves along a latitudinal gradient. Phyto 34:639–662. https://doi.org/10.1127/0340-269X/2004/0034-0639
Rita A, Camarero JJ, Nolè A, Borghetti M, Brunetti M, Pergola N, Serio C, Vicente-Serrano SM, Tramutoli V, Ripullone F (2020) The impact of drought spells on forests depends on site conditions: The case of 2017 summer heat wave in southern Europe. Glob Change Biol 26:851–863. https://doi.org/10.1111/gcb.14825
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242. https://doi.org/10.2307/1313077
Ryan MG, Phillips N, Bond BJ (2006) The hydraulic limitation hypothesis revisited. Plant Cell Environ 29:367–381. https://doi.org/10.1111/j.1365-3040.2005.01478.x
Samaniego L, Kumar R, Attinger S (2010) Multiscale parameter regionalization of a grid-based hydrologic model at the mesoscale. Water Resour Res. https://doi.org/10.1029/2008WR007327
Samaniego L, Kumar R, Zink M (2013) Implications of parameter uncertainty on soil moisture drought analysis in Germany. J Hydrometeorol 14:47–68. https://doi.org/10.1175/JHM-D-12-075.1
Sapsford SJ, Brandt AJ, Davis KT, Peralta G, Dickie IA, Gibson RD, Green JL, Hulme PE, Nuñez MA, Orwin KH, Pauchard A, Wardle DA, Peltzer DA (2020) Towards a framework for understanding the context dependence of impacts of non-native tree species. Funct Ecol 34:944–955. https://doi.org/10.1111/1365-2435.13544
Scharnweber T, Manthey M, Criegee C, Bauwe A, Schröder C, Wilmking M (2011) Drought matters—declining precipitation influences growth of Fagus sylvatica L. and Quercus robur L. in north-eastern Germany. For Ecol Manag 262:947–961. https://doi.org/10.1016/j.foreco.2011.05.026
Scharnweber T, Smiljanic M, Cruz-García R, Manthey M, Wilmking M (2020) Tree growth at the end of the 21st century—the extreme years 2018/19 as template for future growth conditions. Environ Res Lett 15:74022. https://doi.org/10.1088/1748-9326/ab865d
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Schmied G, Hilmers T, Mellert K-H, Uhl E, Buness V, Ambs D, Steckel M, Biber P, Šeho M, Hoffmann Y-D, Pretzsch H (2023) Nutrient regime modulates drought response patterns of three temperate tree species. Sci Total Environ 868:161601. https://doi.org/10.1016/j.scitotenv.2023.161601
Schuldt B, Buras A, Arend M, Vitasse Y, Beierkuhnlein C, Damm A, Gharun M, Grams TE, Hauck M, Hajek P, Hartmann H, Hiltbrunner E, Hoch G, Holloway-Phillips M, Körner C, Larysch E, Lübbe T, Nelson DB, Rammig A, Rigling A, Rose L, Ruehr NK, Schumann K, Weiser F, Werner C, Wohlgemuth T, Zang CS, Kahmen A (2020) A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl Ecol 45:86–103. https://doi.org/10.1016/j.baae.2020.04.003
Schwarz J, Skiadaresis G, Kohler M, Kunz J, Schnabel F, Vitali V, Bauhus J (2020) Quantifying growth responses of trees to drought—a critique of commonly used resilience indices and recommendations for future studies. Curr for Rep 6:185–200. https://doi.org/10.1007/s40725-020-00119-2
Senf C, Buras A, Zang CS, Rammig A, Seidl R (2020) Excess forest mortality is consistently linked to drought across Europe. Nat Commun 11:6200. https://doi.org/10.1038/s41467-020-19924-1
Signorell A, et al. (2022) DescTools: Tools for Descriptive Statistics, R package version 0.99.45
Silver NC, Dunlap WP (1987) Averaging correlation coefficients: should Fisher’s z transformation be used? J Appl Psychol 72:146–148. https://doi.org/10.1037/0021-9010.72.1.146
Skiadaresis G, Schwarz JA, Bauhus J (2019) Groundwater extraction in floodplain forests reduces radial growth and increases summer drought sensitivity of Pedunculate Oak Trees (Quercus robur L.). Front for Glob Change. https://doi.org/10.3389/ffgc.2019.00005
Slette IJ, Post AK, Awad M, Even T, Punzalan A, Williams S, Smith MD, Knapp AK (2019) How ecologists define drought, and why we should do better. Glob Change Biol 25:3193–3200. https://doi.org/10.1111/gcb.14747
Speer JH (2010) Fundamentals of tree-ring research. Univ. of Arizona Press, Tucson
Spinoni J, Naumann G, Vogt JV (2017) Pan-European seasonal trends and recent changes of drought frequency and severity. Global Planet Change 148:113–130. https://doi.org/10.1016/j.gloplacha.2016.11.013
Spinoni J, Barbosa P, de Jager A, McCormick N, Naumann G, Vogt JV, Magni D, Masante D, Mazzeschi M (2019) A new global database of meteorological drought events from 1951 to 2016. J Hydrol Reg Stud 22:100593. https://doi.org/10.1016/j.ejrh.2019.100593
Steckel M, Del Río M, Heym M, Aldea J, Bielak K, Brazaitis G, Černý J, Coll L, Collet C, Ehbrecht M, Jansons A, Nothdurft A, Pach M, Pardos M, Ponette Q, Reventlow D, Sitko R, Svoboda M, Vallet P, Wolff B, Pretzsch H (2020) Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt). Liebl.)—site water supply and fertility modify the mixing effect. For Ecol Manag 461:117908. https://doi.org/10.1016/j.foreco.2020.117908
Stimm K, Heym M, Uhl E, Tretter S, Pretzsch H (2021) Height growth-related competitiveness of oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) under climate change in Central Europe. Is silvicultural assistance still required in mixed-species stands? For Ecol Manag 482:118780. https://doi.org/10.1016/j.foreco.2020.118780
Sturm J, Santos MJ, Schmid B, Damm A (2022) Satellite data reveal differential responses of Swiss forests to unprecedented 2018 drought. Glob Change Biol 28:2956–2978. https://doi.org/10.1111/gcb.16136
Süßel F, Brüggemann W (2021) Tree water relations of mature oaks in southwest Germany under extreme drought stress in summer 2018. Plant Stress 1:100010. https://doi.org/10.1016/j.stress.2021.100010
Thonfeld F, Gessner U, Holzwarth S, Kriese J, da Ponte E, Huth J, Kuenzer C (2022) A first assessment of canopy cover loss in Germany’s forests after the 2018–2020 drought years. Remote Sensing 14:562. https://doi.org/10.3390/rs14030562
Thurm EA, Hernandez L, Baltensweiler A, Ayan S, Rasztovits E, Bielak K, Zlatanov TM, Hladnik D, Balic B, Freudenschuss A, Büchsenmeister R, Falk W (2018) Alternative tree species under climate warming in managed European forests. For Ecol Manag 430:485–497. https://doi.org/10.1016/j.foreco.2018.08.028
van der Maaten-Theunissen M, van der Maaten E, Bouriaud O (2015) pointRes: an R package to analyze pointer years and components of resilience. Dendrochronologia 35:34–38. https://doi.org/10.1016/j.dendro.2015.05.006
van der Maaten-Theunissen M, Trouillier M, Schwarz J, Skiadaresis G, Thurm EA, van der Maaten E (2021) pointRes 2.0: new functions to describe tree resilience. Dendrochronologia 70:125899. https://doi.org/10.1016/j.dendro.2021.125899
van der Werf GW, Sass-Klaassen UG, Mohren G (2007) The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 25:103–112. https://doi.org/10.1016/j.dendro.2007.03.004
Varol T, Cetin M, Ozel HB, Sevik H, Zeren Cetin I (2022) The effects of climate change scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil Pollut. https://doi.org/10.1007/s11270-022-05516-w
Venables WN, Ripley BD (2002) time series analysis. In: Chambers J, Eddy W, Härdle W, Sheather S, Tierney L, Venables WN, Ripley BD (eds) Modern applied statistics with S. Springer New York, New York, pp 387–418
Venturas M, Fuentes-Utrilla P, López R, Perea R, Fernández V, Gascó A, Guzmán P, Li M, Rodríguez-Calcerrada J, Miranda E, Domínguez J, González-Gordaliza G, Zafra E, Fajardo-Alcántara M, Martín JA, Ennos R, Nanos N, Lucena JJ, Iglesias S, Collada C, Gil L (2015) Ulmus laevis in the Iberian Peninsula: a review of its ecology and conservation. Iforest Biogeosci for 8:135–142. https://doi.org/10.3832/ifor1201-008
Vicente-Serrano SM, Beguería S, López-Moreno JI (2010) A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J Clim 23:1696–1718. https://doi.org/10.1175/2009JCLI2909.1
Vicente-Serrano SM, Beguería S, Lorenzo-Lacruz J, Camarero JJ, López-Moreno JI, Azorin-Molina C, Revuelto J, Morán-Tejeda E, Sanchez-Lorenzo A (2012) Performance of drought indices for ecological, agricultural, and hydrological applications. Earth Interact 16:1–27. https://doi.org/10.1175/2012EI000434.1
Vitasse Y, Bottero A, Cailleret M, Bigler C, Fonti P, Gessler A, Lévesque M, Rohner B, Weber P, Rigling A, Wohlgemuth T (2019) Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob Chang Biol 25:3781–3792. https://doi.org/10.1111/gcb.14803
Walentowski H, Falk W, Mette T, Kunz J, Bräuning A, Meinardus C, Zang C, Sutcliffe LM, Leuschner C (2014) Assessing future suitability of tree species under climate change by multiple methods: a case study in southern Germany. Ann for Res. https://doi.org/10.15287/afr.2016.789
Weber P, Bugmann H, Pluess AR, Walthert L, Rigling A (2013) Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees 27:171–181. https://doi.org/10.1007/s00468-012-0786-4
Wigley TML, Briffa KR, Jones PD (1984) On the Average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J Clim Appl Meteor 23:201–213. https://doi.org/10.1175/1520-0450(1984)023%3c0201:OTAVOC%3e2.0.CO;2
Zang C, Biondi F (2015) treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography 38:431–436. https://doi.org/10.1111/ecog.01335
Zang C, Pretzsch H, Rothe A (2012) Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 26:557–569. https://doi.org/10.1007/s00468-011-0617-z
Zang U, Goisser M, Häberle K-H, Matyssek R, Matzner E, Borken W (2014) Effects of drought stress on photosynthesis, rhizosphere respiration, and fine-root characteristics of beech saplings: a rhizotron field study. Z Pflanzenernähr Bodenk 177:168–177. https://doi.org/10.1002/jpln.201300196
Zang CS, Buras A, Esquivel-Muelbert A, Jump AS, Rigling A, Rammig A (2020) Standardized drought indices in ecological research: why one size does not fit all. Glob Change Biol 26:322–324. https://doi.org/10.1111/gcb.14809
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer Series in Wood Science. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-22627-8
Zink M, Samaniego L, Kumar R, Thober S, Mai J, Schäfer D, Marx A (2016) The German drought monitor. Environ Res Lett 11:74002. https://doi.org/10.1088/1748-9326/11/7/074002
Zohner CM, Mo L, Renner SS, Svenning J-C, Vitasse Y, Benito BM, Ordonez A, Baumgarten F, Bastin J-F, Sebald V, Reich PB, Liang J, Nabuurs G-J, Miguel-de S, Alberti G, Antón-Fernández C, Balazy R, Brändli U-B, Chen HYH, Chisholm C, Cienciala E, Dayanandan S, Fayle TM, Frizzera L, Gianelle D, Jagodzinski AM, Jaroszewicz B, Jucker T, Kepfer-Rojas S, Khan ML, Kim HS, Korjus H, Johannsen VK, Laarmann D, Lang M, Zawila-Niedzwiecki T, Niklaus PA, Paquette A, Pretzsch H, Saikia P, Schall P, Šebeň V, Svoboda M, Tikhonova E, Viana H, Zhang C, Zhao X, Crowther TW (2020) Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc Natl Acad Sci U S A 117:12192–12200. https://doi.org/10.1073/pnas.1920816117
Zscheischler J, Seneviratne SI (2017) Dependence of drivers affects risks associated with compound events. Sci Adv 3:e1700263. https://doi.org/10.1126/sciadv.1700263
Acknowledgements
We want to thank Monika Bradatsch and Gerhard Schütze for all the hard work and patience with very poorly visible tree rings and their measurements. In addition, we want to thank the department of silviculture of the SGGW in Warsaw for their support in the field work in Poland. Furthermore, we want to thank Peter Biber for support in statistical problems and questions.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was financed by the Bavarian State Ministry for Food, Agriculture and Forestry within the framework of the project W048 ‘Growth dynamics and climate sensitivity of rare domestic tree species’. In addition, this research has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 778322 (CARE4C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by van der Maaten.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmucker, J., Uhl, E., Schmied, G. et al. Growth and drought reaction of European hornbeam, European white elm, field maple and wild service tree. Trees 37, 1515–1536 (2023). https://doi.org/10.1007/s00468-023-02441-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02441-1