Abstract
Key message
Patterns of non-structural carbohydrate allocation in dry and wet differ between birch and larch in southern boreal forest.
Abstract
An increasing area of boreal forests is currently switching from temperature to drought limitation. It is not sufficiently known how the trees’ non-structural carbohydrate (NSC) pools are affected by alternating dry and wet phases in drought-prone boreal forests and how NSC concentrations are related to growth responses. In the southern boreal forests of Mongolia, NSC concentrations (including soluble sugars and starch) were determined enzymatically in the stemwood of two tree species during a drought year (2017) that was preceded by another drought in 2015 and two subsequent wet years (2018/19). Betula platyphylla as a broadleaved pioneer tree showed reduced radial stem increment in the drought year and rapidly increasing growth in the following wet years. It had low concentrations of NSC and of soluble sugars in particular, suggesting that most assimilate were invested into growth in this early successional tree. Larix sibirica as a late-successional conifer also showed reduced growth in the drought year, but lagged reductions in NSC concentrations and less pronounced growth releases than birch in 2018/19, suggesting a longer aftereffect of the drought than in birch. Larch maintained much higher NSC concentrations throughout the three studied growing seasons, including the drought episode in 2017. The NSC pool in larch was primarily formed by soluble sugars in sapwood and heartwood. In contrast to birch, larch showed a high allocation priority for assimilates in soluble sugars before investment in biomass, which explains the aftereffect of the drought on both growth and NSC. We conclude that the high soluble sugar concentrations in larch are a key determinant of the extreme drought and cold tolerance of L. sibirica and, therefore, growth has lower allocation priority for carbon compared to birch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The productivity of boreal forests has received growing attention because the boreal climate zone is influenced by polar amplification due to its proximity to the Arctic (Stuecker et al. 2018). Thus, the boreal forest experiences more rapid climate warming than most other forested biomes (IPCC 2021). Two lines of evidence suggest that an increasing area of boreal forests is currently switching from temperature to drought limitation of productivity: one is based on remote-sensing analyses of vegetation greenness (Buermann et al. 2014) and the other one on dendrochronological data (Lloyd and Bunn 2007; Babst et al. 2019).
The dendrochronological data from the boreal zone are primarily based on the analysis of wood cores from conifers and mostly from late successional species (Lloyd and Bunn 2007; Tei et al. 2017). Though boreal conifers are not necessarily isohydric tree species (Dulamsuren et al. 2009a, b), they typically follow a water-saving strategy with stringent stomatal regulation (Goulden et al. 1997; Walker et al. 2015). Such a strategy limits C assimilation but lowers the risk of drought mortality. Summer droughts, which are commonplace under continental boreal climates, leave their imprint in the tree-ring data through annual to multi-annual growth depressions (Dulamsuren et al. 2010; Itter et al. 2019). In climate-response analyses, low tree-ring width under drought conditions is usually correlated with high temperature, low precipitation, and high atmospheric drought as expressed by the Standardized Precipitation-Evapotranspiration Index (SPEI). Such analyses also show that the drought’s impact on radial stem increment is sometimes stronger in the year following the drought than during the drought itself (Dulamsuren et al. 2011, 2013; Tumajer and Lehejček 2019).
Birch and aspen, which are the dominant broadleaved pioneer trees in boreal forests (Pfadenhauer and Klötzli 2020), are less in the focus of dendrochronologists because they have shorter life expectancies and the climate signal in the tree-ring series is more likely to be confounded by other factors in disturbed forests where pioneer trees establish than in late-successional forests. Birch and aspen gain the advantage of fast growth by maintaining high transpiration even at high atmospheric vapor pressure deficits (Ponton et al. 2006; Zha et al. 2010), thus accepting a higher drought mortality risk (Kharuk et al. 2013) under declining soil water reserves (Kljun et al. 2006; Welp et al. 2007). In addition, the xylem of these trees is more vulnerable to cavitation than that of late-successional conifers (Dulamsuren et al. 2019). However, the higher stem mortality risk is compensated by the broadleaved pioneer’s capability to resprout (Xu et al. 2018).
While the negative impact of summer droughts on tree-ring width in the current or next year and on vegetation greenness in boreal forests is generally well known, less information is available on the mechanisms that link droughts with immediate or delayed growth depressions. In addition to temperature and plant water status, the size of the non-structural carbohydrates (NSC) pool that is available for cell division and cell elongation exerts an influence on the tree’s ability for biomass production (Oribe et al. 2003). An important function of the NSC pool is to bridge between growing seasons by storing assimilates for tissue formation in the next year (Sauter and Ambrosius 1986; Fierravanti et al. 2019). Especially foliation, flowering and the formation of earlywood with new conduits that reinforce the hydraulic system at the start of the growing season depend on C stored from the previous year (Palacio et al. 2018). Therefore, the NSC pool can be regarded as the material basis of first-order autocorrelation of tree-ring width between consecutive years (von Arx et al. 2017). However, high NSC reserves are no guarantee for wide tree rings, if the climate is unfavorable (Scartazza et al. 2013), because the allocation priority for maintaining a certain NSC level is higher than for stem wood formation (Hartmann et al. 2018, 2020).
Higher C allocation priorities in NSC than in wood growth arise from the circumstance that NSC fulfill multiple functions in addition to assimilate storage for biomass production. NSC form the basis for catabolic and anabolic metabolism (Dietze et al. 2014; Hartmann and Trumbore 2016). The disaccharide sucrose is used for assimilate distribution in the plant through the phloem. NSC and starch in particular are accumulated before the fall of leaves prior to cold and dry seasons to allow for respiration at times without CO2 assimilation (Palacio et al. 2007; Richardson et al. 2013; Furze et al. 2019). In addition, sugars play an important function in osmoregulation (Yoshimura et al. 2016; von Arx et al. 2017; Ozturk et al. 2021) and cold acclimation (Rinne et al. 1998; Charrier et al. 2018; Lintunen et al. 2016; Deslauriers et al. 2021). Despite the importance of sugars for osmotic adjustment, NSC concentration can be depleted under drought conditions as the result of sustained stomatal closure, leading to an imbalance between C assimilation and respiration, which can finally result in death from C starvation (O’Brien et al. 2014; Sevanto et al. 2014; Kono et al. 2019). On the other hand, cell division and cell elongation can be stopped at an earlier stage of a beginning drought than the Calvin cycle (Muller et al. 2011; O’Brien et al. 2015), resulting in NSC accumulation. However, Thompson et al. (2023) recently found simultaneous downregulation of photosynthesis and growth preventing NSC accumulation in a semiarid southern North American conifer woodland. Therefore, neither NSC depletion nor accumulation can be interpreted as a reliable drought stress indicator in plants (Dickman et al. 2019; Thompson et al. 2023).
Drought responses of NSC concentrations in trees are variable dependent on drought intensity, duration and the tree species (Regier et al. 2009; Dietze et al. 2014). Most trees show an increase in soluble sugar concentrations in the stemwood during moderate drought, which matches with their osmoregulatory function, but a decrease after long-lasting intense drought pointing to the consumption of C reserves (Maguire and Kobe 2015; He et al. 2020). Correspondingly, large NSC reservoirs reduce the mortality risk during drought due to their function in osmoregulation and the prevention of C starvation after stomatal closure (Canham et al. 1999). In addition to their osmoregulatory function, NSC (e.g. raffinose and fructans) are involved in protecting membranes from drought-induced oxidative stress (Jiménez et al. 2013; Van den Ende 2013; ElSayed et al. 2014). Furthermore, NSC have a function in refilling embolized xylem conduits, though the extent to which this occurs as a regular process after drought in mature trees is controversial and apparently different between species (Nardini et al. 2011; Klein et al. 2018).
Being interested in potential interrelations between NSC concentrations and radial stemwood increment and their dependence on climate, we compared the seasonal and interannual variability of NSC concentrations in the stemwood between an early successional broadleaved pioneer (Betula platyphylla Sukaczev, Siberian silver birch) and a late successional conifer (Larix sibirica Ledeb., Siberian larch) in a drought-limited southern boreal forest of Mongolia. Our study covered a sequence of three consecutive growing seasons, where the first one in 2017 represented a drought year (preceded by a drought in 2015), whereas the subsequent years 2018 and 2019 represented wet years. Since birch as a pioneer tree is specialized in rapidly occupying as much canopy space as possible, we hypothesized (1) that birch has lower NSC concentrations than larch, as birch can be assumed to invest more of the assimilated C directly into growth. Therefore, we also tested hypothesis (2) that the drought episode in 2017 (that itself might have been still affected by the preceding drought of 2015) exerted no long-lasting effect on NSC concentrations in birch that extended to the subsequent wet years. As L. sibirica often shows stronger growth depressions in the years following the drought than in the year of the drought (Dulamsuren et al. 2013; Khansaritoreh et al. 2017a, b), we tested the hypothesis (3) that the drought caused a reduction in the NSC concentration in larch that persisted in the year(s) following the drought year 2017.
Materials and methods
Study area
The study was carried out in the mountain taiga of northern Mongolia, which occurs at the southern distribution limit of the Eurosiberian boreal forest in Inner Asia. Fieldwork was conducted in the western Khentei Mountains at 48°40ʹ N, 106°55ʹ E near the town of Tunkhel, ca. 85 km north of the Mongolian capital Ulan Bator and 230 km south of Lake Baikal. The Khentei Mountains range from Ulan Bator northeastward into Siberia (where they are called Yablonoviy Mountains) to Transbaikalia. Geologically, the mountain range consists of Proterozoic and Paleozoic rock, especially granite. Forests below 1500 m a.s.l. are mostly composed of Larix sibirica, but are frequently replaced by Betula platyphylla after natural and anthropogenic disturbance, which is mainly caused by fire, windthrow and logging (Dulamsuren 2004). A local timber industry contributing to anthropogenic disturbance was developed in Tunkhel, which is located 12 km from our study site, in the 1960s.
Climate in the study region is coined by the Siberian High, which has its center over Lake Baikal from September to April and generates extremely cold and dry winters. Mean January temperatures are typically around − 25 °C and daily temperature minima regularly fall to values between − 45 and − 50 °C in the montane forest belt (Dulamsuren and Hauck 2008). In summer, when the Siberian High has collapsed, warm and moist air masses flow in resulting in a strong precipitation peak in summer and in July in particular. Meteorological data for our study area were obtained from the CRU TS 4.05 dataset edited by the Climate Research Unit of the University of East Anglia, Norwich and the Met Office in Exeter, UK. Data were downloaded for the period from 1901 to 2020 from the website of the World Meteorological Organization (WMO, https://climexp.knmi.nl) selecting a resolution of 0.5° × 0.5° for the grid field of 48.5–49.0°N and 106.5–107.0°E. Mean annual (July/January) temperatures in the region amounts to − 1.7 ± 0.1 °C (17.6 ± 0.1 °C/− 24.0 ± 0.3 °C). Mean annual precipitation is 330 ± 5 mm with 90% falling between May and September (298 ± 5 mm); 68% (226 ± 4 mm) of the annual precipitation is received from June to August. The Standardized Precipitation-Evapotranspiration Index (SPEI) was calculated as one-monthly means (SPEI1) using the R package SPEI 1.7 (Vicente-Serrano et al. 2010). Potential evapotranspiration was calculated after Thornthwaite (1948) for a latitude of 48.666667°N.
Sampling design
Fieldwork was conducted on a mountain range ca. 12 km ENE of the town of Tunkhel (Selenge Province, Mongolia) during the growing seasons of 2017, 2018, and 2019 (Dulamsuren 2021). The field site is part of a larger project on forest functioning in late-successional conifers and broadleaved pioneers at the drought-prone southern fringe of the boreal forest and is located near a field station of the German Corporation for International Cooperation (Deutsche Gesellschaft für Internationale Zusammenarbeit, GIZ). The mountain range is covered with the light taiga forest of Larix sibirica and Betula platyphylla on its north-facing slope at the studied elevational range of 1300–1500 m a.s.l. and steppe grassland on south-facing slopes. Three sample plots (48°40ʹ N, 106°55ʹ E) were studied representing mixed larch-birch forest, pure birch forest and pure larch forest, respectively. These plots were similar in terms of elevation (1290–1395 m a.s.l.), aspect (north) and were 210–325 m apart from one another.
Samples were collected in the three subsequent growing seasons of 2017, 2018 (both weekly sampling), and 2019 (biweekly sampling) from May to late September/early October. Six trees per plot and tree species were sampled in 2017 and 2018. In 2019, sampling was limited to three replicate trees. Wood cores of 5 mm diameter and a length of ca. 5 cm were collected with an increment borer (Haglöf, Långsele, Sweden) along the entire stem circumference at a height of 1.3 m above the ground keeping a distance of 2 cm from the last sampling point. Sample trees were selected to represent dominant to subdominant trees with representative stem diameter and height for the stands. Diameters at breast height were 25.9 ± 8.6 cm (birch in pure stand), 27.3 ± 0.8 cm (birch in mixed forest), 38.4 ± 0.6 cm (larch in pure stand), and 39.2 ± 0.7 cm (larch in mixed forest). At the start of each growing season, new tree individuals were selected to avoid bias by previous sampling activities. Wood cores were treated in a microwave at 600 W for 90 s min at the day of sampling to inactivate enzymes and prevent biochemical conversions like starch hydrolysis and subsequently air-dried. Wood cores were stored dry until analysis. Analyses were done for the complete stemwood core for the individual samples (separately for each tree and sampling date). A subset of samples from the growing seasons of 2017–2019 was analyzed separately differentiating for sapwood and heartwood. This analysis was restricted to larch, as birch usually has no distinct sapwood/heartwood separation. Another subset of weekly samples from 2017/18 and biweekly samples from 2019 was used for the analysis of the NSC content in the phloem.
NSC analysis
NSC analysis included the measurement of free sugar (glucose, fructose, sucrose) and starch concentrations following ethanol extraction (80% at 90 °C for 10 min) of the homogenized wood (Landhäusser et al. 2018). Extracts were centrifuged at 13,000g for 1 min at room temperature. Supernatants were used for the analysis of free sugars, whereas pellets were subjected to two further iterations of ethanol extraction and centrifugation to remove residual sugar extract from the pellet and then used for starch analysis. After the ethanol had been evaporated from the supernatant, the remnant was dissolved in deionized water at 90 °C, cooled down to room temperature and then centrifuged at 13,000g for 1 min. Free sugar content was determined enzymatically through the reduction of NAD+ to NADH by glucose-6-phosphate. This reaction is catalyzed by glucose-6-phosphate dehydrogenase and the change in NADH concentration was recorded as an increase in absorbance at λ = 340 nm using a microplate reader (BioTek, Agilent, Santa Clara, California, USA). Sucrose was broken down into glucose and fructose with invertase. Hexokinase was used to phosphorylate glucose and fructose to glucose-6-phosphate and fructose-6-phosphate, respectively; the latter was converted to glucose-6-phosphate with isomerase. Starch was broken down into glucose in two steps: first, α-amylase was added to the starch pellet and incubated at 85 °C, cooled to room temperate and afterwards centrifuged at 13,000g for 1 min. Amyloglucosidase was added to the supernatant, incubated at 55 °C and then also cooled to room and centrifuged at 13,000g for 1 min. The resulting glucose solution was phosphorylated and then treated like the free sugar samples for further analysis. Three replicate measurements were made per extract and the results averaged. All concentrations presented in the paper refer to glucose equivalents. Glucose and fructose were used as standards. All biochemicals were obtained from Sigma-Adrich (St. Louis, Missouri, USA).
Radial stem increment
At the end of the last field season in 2019, wood cores for dendrochronological analysis were collected from all NSC sample trees with the increment borer at 1.3 m height parallel to the contour lines of the slope. Annual ring width was measured with a precision of 10 µm on a movable object table (Lintab 6, Rinntech, Heidelberg, Germany), the movements of which were electronically transmitted to a computer system equipped with TSAP (Time Series Analysis and Presentation)-Win software (Rinntech). TSAP-Win and visual control were used for cross-dating. Tree-ring data were used to extract the annual radial stem increment of the years 2015–2019. Data were not standardized, because they were only used for the calculation of mean tree-ring width and all sample trees of each species were of similar size and age.
Light microscopy
In addition to the wood cores of 5 mm diameter used for NSC and tree-ring analysis, wood microcores of 2 mm diameter and 15 mm length were collected with a Trephor cutting tube manufactured at the University of Padova, Italy (Rossi et al. 2006). The microcores were preserved in 50% ethanol, embedded in polyethylene glycol (PEG 2000) at 70 °C and cut with the microtome (Reichert, Leica Microsystems, Wetzlar, Germany) to thin sections of 5–10 µm. Thin sections were washed with ethanol, placed on an object holder and treated with Lugol’s iodine (1%), before the solution was removed with deionized water. Micrographs were taken using an Eclipse Ni-U research microscope (Nikon, Tokyo, Japan).
Statistical analysis
Arithmetic means ± standard errors (SE) are presented throughout the paper. All statistical calculations were done with R 4.0.3 software. Data were tested for normal distribution with the Shapiro–Wilk test. Linear mixed effect analysis was calculated with the R package ‘lme4’ 1.1–31 (Bates et al. 2015) to analyze the effect of tree species, tree mixture, and time on NSC concentrations. We entered tree species, tree mixture, year (for interannual variation) and month (for seasonal variability) as fixed effects into the model and the sample plot as random effect. Duncan’s multiple range test was used as a posthoc test (following analysis of variance) for multiple comparisons of means; it was calculated with the R package ‘agricolae’ 1.3–5. Phloem NSC concentrations and dendrochronological data (tree-ring width, basal area increment) were log10-transformed before Duncan’s multiple range test in order to remove skewness by mainly positive outliers.
Results
Climatic variability
The growing season of 2017 was characterized by a strong drought from late June to July, which is normally the most precipitation-rich month in Mongolia. July precipitation amounted to 46 mm in 2017 compared to 119 mm in 2018 and 97 mm in 2019 (Fig. 1a) and 86 ± 2 mm on the long-term average (1901–2020). The annual precipitation did not show such a sharp contrast between the years (Fig. 1d), as the low July precipitation in 2017 was partly compensated by high rainfall in August (115 mm in 2017 vs. 90 mm in 2018 and 70 mm in 2019). The long-term average of August precipitation for the area is 79 ± 2 mm. An SPEI of − 2.1 in July 2017 indicated strong atmospheric drought (Fig. 1b), whereas SPEI values were positive, indicating moist conditions, in July 2018 (1.3) and 2019 (0.1). July air temperature was 20 °C in 2017, 18 °C in 2018, and 19 °C in 2019 (Fig. 1c).
Variation of a, d precipitation sums, b, e one-monthly values of the Standardized Precipitation-Evapotranspitration Index (SPEI), and c, f mean air temperature in a–c the study period from 2017 to 2019 (monthly variation) and d–f from 2005 to 2019, including annual, monthly (June to August) and growing season (May to September, MJJAS) values. Data are modeled from the CRU TS 4.05 dataset edited by the Climate Research Unit of the University of East Anglia, Norwich and the Met Office in Exeter, UK (https://climexp.knmi.nl) at a resolution of 0.5° × 0.5° for the grid field of 48.5–49.0°N and 106.5–107.0°E. July values a–c of the drought year 2017 are highlighted in red color, those of the wet years 2018 and 2019 in green
The year 2017 was preceded by a moderately dry year where 90% (296 mm) of the mean annual precipitation and 88% (75 mm) of the mean July precipitation were received (Fig. 1d). June precipitation in 2016 (64 mm) was close the long-term average (104%), while August precipitation (53 mm) was less than normal (67%). The summer of 2015 was characterized by a very dry June (18 mm or 29% of the long-term mean), followed by a moderately dry July (73 mm, 85%) and August (33 mm, 42%). Since both years had warm summers with July temperatures of 20 °C (Fig. 1f), this resulted in moderate meteorological drought in July 2015 and 2016 (SPEI = − 1.1 in both years), but strong drought in June (SPEI = − 2.1) and August 2015 (SPEI = − 2.5; Fig. 1e).
Radial stem increment
Annual radial stem increment showed a growth depression in 2017 in both tree species (Fig. 2). Growth was rapidly resumed after the drought year 2017 in both species with increased tree-ring width and basal area increment in the first wet year 2018 and further accelerated growth in the second wet year 2019. Growth releases in the second wet year were much higher in birch than in larch. The reduction of tree-ring width and basal area increment in 2017 compared to the preceding and the following years were consistently significant for larch, but not in birch, which showed high growth variability between individuals.
a Radial stem increment and b basal area increment of Betula platyphylla and Larix sibirica from 2015 to 2019 including the drought year 2017 (red color) and the two wet years 2018 and 2019 (green color). Means sharing a common letter within a tree species, do not differ significantly (P ≤ 0.05, Duncan’s multiple range test, dfmodel, error = 9, 196)
NSC concentrations in stemwood
Linear mixed-effects modeling yielded a strong effect of the tree species on the concentrations of total NSC, soluble sugars, and starch, as evidenced by P ≤ 0.001 in the maximum likelihood estimates, high t-values and modest confidence intervals (Table 1). By contrast, tree mixture (i.e. the occurrence of larch and birch in pure or mixed forest) had with high certainty no effect on NSC concentrations (P = 0.98 in all cases). Random effects, including influences by the sample plots, were also negligible. Therefore, we merged the data from pure and mixed forests for each species and present the separate data only in the Supplementary Information (Figs. S2 to S4). A significant effect of the interaction of species × tree mixture was minor and was (particularly for total NSC and soluble sugars) connected with high uncertainty as indicated by very high SE and confidence intervals. Temporal variation in NSC concentrations was also minor, as shown by only small and often statistically insignificant effects of year and month.
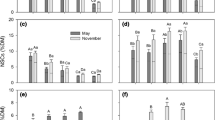
Total NSC concentrations were much higher in larch than in birch stemwood (Fig. 3a) with mean values of 2.50 ± 0.04% in larch and 0.79 ± 0.02% in birch across all data. Birch and larch differed fundamentally in how much of the total NSC was allocated to soluble sugars or starch (Fig. 4). Soluble sugars represented one-third of total stemwood NSC (32.5 ± 2.0%) in birch, while the remaining two-thirds were deposited as starch (67.1 ± 6.6%). By contrast, larch kept the vast majority of NSC as soluble sugars (93.7 ± 0.9%) and only 6.6 ± 0.8% as starch. These differences are consistent with significantly higher sugar concentrations in larch than in birch (Fig. 3b) and (on a lower absolute level) higher starch concentrations in birch (Fig. 3c).
NSC concentrations (given as glucose equivalents) in stemwood of Betula platyphylla and Larix Syllabification: si-bi-ri-ca in the growing seasons (May to September/October) of 2017–2019: a total NSC, b soluble sugars, and c starch. Sample numbers indicate a number of sampling dates from weekly sampling in 2017/18 and biweekly sampling in 2019. Means of samples sharing a common letter, do not differ significantly (P ≤ 0.05, Duncan’s multiple range test, dfmodel, error = 5, 202)
In larch, total NSC and soluble sugar concentrations were significantly reduced in the two years (2018 and 2019) following the drought year 2017 (Fig. 3a, b). The low starch concentrations in larch remained unchanged over the years (Fig. 3c). Birch did not show any depression in total NSC and starch concentrations in 2018, but concentrations increased significantly in 2019 (Fig. 3a, c). Soluble sugar concentrations in birch were very low (especially when compared to larch), but nevertheless showed a reduction in 2018, with a significantly lower concentration than in 2019 (Fig. 3b).
As already pointed out in connection with the linear mixed effect analysis (Table 1), seasonal variation of NSC concentrations was remarkably low (Fig. 5). Some of the small seasonal variations was due to the conversion of starch into soluble sugar before winter (especially in birch, Fig. 5d, e, g, h) or caused by decreasing sugar and total NSC concentrations in spring in both species. Soluble sugar (and thus also total NSC) concentrations in larch remained stable from the start of the growing season in 2017 throughout the drought in late June and July and showed some drift towards lower values in autumn (Fig. 5d). In the two following years, sugar (and total NSC) concentrations in larch decreased rapidly at the start of the growing season (Fig. 5e, f) to a level significantly below that in 2017 (Fig. 3). Despite this decline, soluble sugar concentrations were always much higher than in birch. Birch showed a buildup of the starch pool during the growing season in the wet years 2018 and 2019 (Fig. 5h, i). In 2017, however, a very weak depression occurred in the starch concentration in birch during the drought in July (Fig. 5g), simultaneously with a slight increase in soluble sugar concentrations (Fig. 5d), but these alterations in birch concerned low relative changes at low concentration values.
Sapwood vs. heartwood NSC concentrations
The separate analysis of NSC concentrations in sapwood and heartwood of larch yielded strong differences between these wood types (Fig. 6). Most soluble sugars were found in the heartwood and not in the sapwood. Starch was not detectable in the heartwood. Growing season mean concentrations of soluble sugars varied between 2.6 and 3.7% in heartwood, but only 0.3 and 0.8% in sapwood.
Phloem NSC concentrations
Total NSC concentrations in the phloem exceeded that in stemwood by a factor of 5.6 in birch and by 4.0 in larch. Linear mixed-effects analysis revealed a dominant effect of the tree species on NSC concentrations in the phloem (Table 2). Like in the stemwood, phloem NSC concentrations were significantly higher in larch than in birch (Fig. 7; Fig. S5 for species occurrence in the pure or mixed forest). Tree mixture and year had no significant effect on NSC concentrations, while seasonal variation (month) showed a very weak effect in the analysis (Table 2). Unlike in stemwood, starch was the dominant fraction in the phloem NSC in both tree species. Soluble sugar concentrations increased toward the end of the growing season, markedly in larch and to a lesser extent (and not consistently) in birch (Fig. 8d-f; Fig. S6). Sugar concentrations also partly showed declines in spring. Starch concentrations showed trends for an increase during the growing season (Fig. 8g–i). Before winter, the starch content decreased in birch (especially in 2017 and 2018) and in the drought year 2017 also in larch. Starch (Fig. 8g) and total NSC concentrations (Fig. 8a) showed depressions during the drought in late June and July 2017, but recovered in August in both tree species.
NSC concentrations (given as glucose equivalents) in the phloem of Betula platyphylla and Larix sibirica in the growing seasons (May to September/October) of 2017–2019: a total NSC, b soluble sugars, and c starch. Means of samples sharing a common letter, do not differ significantly (P ≤ 0.05, Duncan’s multiple range test calculated from log-transformed data, dfmodel, error = 5, 200)
Localization of starch granules
Micrographs of stem thin sections stained with Lugol’s iodine visualized higher densities of starch deposits in the phloem than the xylem in both tree species (Fig. 9). Starch granules were more abundant in the phloem and outer xylem of B. platyphylla than L. sibirica (Fig. 9a, d). In the inner sapwood (Fig. 9b), but not the heartwood (Fig. 9c) of larch, starch was detectable in the ray parenchyma. In birch, the abundance of starch deposits in the xylem parenchyma also decreased strongly with increasing distance from the xylem, until the starch was no longer detectable by staining (Fig. 9e, f).
Localization of starch granules stained bluish black with Lugol’s iodine in a–c Larix sibirica and d–f Betula platyphylla. Larch: a relatively high starch density in phloem and starch in radial ray parenchyma of outer sapwood, b starch in ray parenchyma of inner sapwood, c no starch in heartwood. Birch: d high starch density in phloem and peripheral xylem parenchyma, e low starch density and f no starch in xylem parenchyma with increasing distance from the phloem
Interrelation between stemwood NSC concentrations and tree-ring width
In both tree species, the drought year 2017 was characterized by low stemwood increment, but high NSC concentrations (Fig. 10). By contrast, higher growth rates in the moist years 2018 and 2019 were associated with low NSC concentrations. The increase in soluble sugars in the wood of trees growing at very low levels in 2017 was about 10 times higher in larch than in birch. In birch, the increment in the following year was independent of the NSC concentration in the previous year, and regardless of whether the year of NSC sampling was a wet or dry year (Fig. 10b, f, j). In larch, however, trees with high NSC concentrations (found in 2017) also showed low growth in the following year (Fig. 10d, h, l).
Discussion
Despite their different life strategies and systematic positions as a broadleaved pioneer and late-successional conifer, birch and larch did not differ fundamentally in their growth response during the drought of 2017. Both tree species showed marked growth depressions. That these depressions were only partly significant compared to 2016 and 2018 in birch is attributable to the higher growth variability between tree individuals compared to larch. 2015 also was a drought year, though with a timing that differed from the drought in 2017, which apparently caused a lagged growth reduction in the moderately dry year 2016, but could even have an impact still in 2017. The main difference between 2015 and 2017 was that the 2017 drought spell lasted from late June to July, which is usually the most precipitation-rich month in Mongolia, whereas the drought in 2015 occurred in June and August, but was interrupted by a relatively moist July. Drought recovery after 2017 contrasted between the two tree species, as birch translated the additional water supply during the two subsequent wet years 2018/19 into much stronger growth releases than larch, at least in the second year. It is likely that the rapid drought recovery in 2017 was enabled by the wet August of that year.
The approximately threefold higher NSC content in the stemwood of Larix sibirica than in that of Betula platyphylla is consistent with our first hypothesis that birch as a fast-growing pioneer invests a higher share of the total C gain in growth, whereas C allocation in growth and NSC stocks in larch as the late-successional pioneer L. sibirica is more a tradeoff between survival probability, on the one hand, and biomass production, on the other hand (Sala et al. 2012). The resource allocation priorities in birch are clearly driven by the target to outcompete other trees through rapid growth and occupation of canopy space. Fast growth is achieved by accepting low wood density (Koizumi et al. 2003; Dulamsuren et al. 2016; Erdene-Ochir et al. 2020) and low hydraulic safety (Dulamsuren et al. 2019) compared to L. sibirica and, thus, at the expense of longevity (Büntgen et al. 2019; Duchesne et al. 2019). Slower growth in larch is connected with larger mechanical stability, while the thicker bark produces greater fire tolerance.
In birch, low growth in 2017 (combined with a slight but insignificant trend for temporarily reduced starch concentrations in stemwood and phloem during the drought spell), suggest that the trees were seriously affected during the drought. Reduced growth (and the slight depression of starch concentrations) could partly be the result of leaf shedding during the drought, which concerned approximately 30% of the foliage in birch, as was observed during our fieldwork. The overall low NSC pool in birch suggests that the species has not much capacity to buffer drought-induced reductions in CO2 assimilation by falling back to its sugar and starch reserves. After the drought, B. platyphylla recovered rapidly. After the drought, the original leaf area was restored despite the late point in time within the growing season (early August), which is consistent with increasing starch concentrations at that time (Figs. 5, 8). It is plausible to assume that hydraulic failure of the xylem was associated with the period of reduced growth and leaf shedding during the drought, because xylem vulnerability in B. platyphylla is known to be considerably greater than in L. sibirica and other southern boreal conifers of southern Siberia and Inner Asia (Dulamsuren et al. 2019). However, the drought did not leave a long-lasting imprint neither on NSC concentrations, nor growth that exceeded the drought. It is known that B. platyphylla is capable of xylem refilling (Ogasa et al. 2010, 2013), which is consistent with quick drought recovery. Birch used the two consecutive wet years following the drought year for strong growth releases, but only for minor NSC accumulation, which was found in the second wet year. In agreement with our second hypothesis, the growth release after the drought was not achieved at the expense of falling NSC concentrations. Yet, it remains unclear whether the NSC content would have risen earlier or more strongly during the two consecutive wet years without the preceding drought in 2017 (and 2015). However, the strong growth releases in birch during the wet years at initially constant and later only moderately increasing NSC concentrations suggest a strong C allocation priority of birch for growth.
Larch responded to the 2017 drought with a growth depression (which occurred in 2017 but could have been influenced by aftereffects of the 2015 drought), but a lagged reduction in NSC concentrations lasting over the two years following the drought, confirming our third hypothesis. In addition, it cannot be ruled out that growth reduction in 2017 were partly influenced by aftereffects of the 2015 drought. Whether L. sibirica responds to drought with immediate or lagged or multiannual reductions in tree-ring width depends on the timing of the drought spell and its intensity (Dulamsuren et al. 2011, 2013; De Grandpré et al. 2011; Khansaritoreh et al. 2017a). The NSC data show that the 2017 drought episode (perhaps partly influenced by the preceding drought of 2015) left an imprint despite growth recovery in 2018 and even more 2019, as NSC concentrations in larch were significantly and even increasingly reduced compared to 2017. This aftereffect of the drought on the NSC pool explains how drought exposure weakens the first-order autocorrelation in tree-ring chronologies of L. sibirica (Khansaritoreh et al. 2017a) and reduces the resistance of growth to repeated consecutive drought spells, which may lead to persistent growth and vitality reductions (Dulamsuren et al. 2010) or increased tree mortality (Liu et al. 2013; Khansaritoreh et al. 2017a). Climate change-induced drought mortality has become widespread in L. sibirica in Mongolia’s boreal forest, although the xylem of L. sibirica is highly cavitation-resistant (Dulamsuren et al. 2019) and can acclimate to low water supply (Chenlemuge et al. 2015a, b).
That NSC concentrations show this lagged decline (contrasting with simultaneously increasing NSC concentrations in birch) suggests that reduced assimilate availability is a key determinant of delayed drought responses in L. sibirica. If cambium damage due to insufficient hydration occurred, the NSC pool should have increased, rather than decreased, as assimilates not consumed for growth and respiration would accumulate (Blackman et al. 2019; Dickman et al. 2019). We can only speculate about the mechanism leading to the drought’s aftereffect on the NSC pool. Persistent hydraulic failure of the xylem, where conduits would have to be replaced by the slow formation of new wood, would be a possible explanation (Creek et al. 2018), which would thwart transpiration, impair leaf hydration, and reduce photosynthesis. Such dependence of the post-drought restoration of hydraulic conductivity on the formation of new conduits would activate a downward spiral of reduced NSC pools and reduced growth, as the underlying CO2 assimilation is a function of stomatal conductance and transpiration rate. It has not been investigated whether L. sibirica can significantly refill its xylem after drought; therefore, this explanation for the lagged and persistent drought response on NSC concentrations in our study and sometimes delayed growth responses found in other studies (De Grandpré et al. 2011; Khansaritoreh et al. 2017a) remains a hypothesis.
It is evident that L. sibirica has a high C allocation priority in soluble sugars, unlike B. platyphylla, which apparently spends a high share of assimilates in growth. Rather, larch splits its C resources between allocation in sugars and biomass. High allocation priority for NSC was also observed in Larix decidua from Europe (Peters et al. 2020). As L. sibirica maintained high soluble sugar concentrations during the drought in stemwood and phloem and increased sugar concentrations in phloem before winter, it is plausible to assume a key role in the exceptional drought and cold tolerance of this species. While several boreal tree species occur under the relatively moist climates of upper mountain forests in Mongolia or of the western Siberian dark taiga, the highly continental southernmost boreal forests of Inner Asia are monospecific larch forests (Gunin et al. 1999; Dulamsuren 2004). Soluble sugars are needed for osmotic adjustment to generate low plant water potentials (Dulamsuren et al. 2009a). Maintenance of high soluble sugar concentrations (Mitchell et al. 2013) or conversion of starch into sugars (Maguire and Kobe 2015; Cui et al. 2021) during drought and positive relationships between drought survival and NSC pools (Galiano et al. 2011; Adams et al. 2013) have been demonstrated repeatedly. The difference in the NSC pools suggests lower cold hardiness of B. platyphylla compared to L. sibirica, which is probably the cause of distribution gaps in particularly cold parts of Mongolia’s boreal forest area, including the Mongolian Altai (Dulamsuren et al. 2014) or the northwestern Khangai Mountains (Khansaritoreh et al. 2017a, b).
Remarkably, larch allocated soluble sugars at much higher concentrations to heartwood than to sapwood. Though we did not separate between different tissue types within heartwood, sapwood and phloem in our analyses, it is plausible to assume that the soluble sugars in the heartwood were located in the ray parenchyma that extends from the sapwood into the stem interior (Hartmann and Trumbore 2016). While high sugar concentrations are known to occur in the transition zone between sapwood and heartwood, because the sugars are utilized for secondary metabolite synthesis (Niamke et al. 2018; Cui et al. 2021), NSC concentrations usually decrease from the sapwood toward the stem interior heartwood (Hoch et al. 2003). Yet, comparing NSC concentrations in the sapwood and heartwood of several angiosperm and gymnosperm trees, Piper et al. (2019) found lower concentrations in heartwood in some species, but similar concentrations in other species. Replicate analyses from other L. sibirica forests should be performed to clarify whether high heartwood concentrations are consistently found in this species.
Our results for soluble sugar and starch concentrations agree well with the magnitudes found in other analyses of stemwood or phloem (Palacio et al. 2007; Galiano et al. 2011; Richardson et al. 2013, 2015; Scartazza et al. 2013; Zhang et al. 2014; Landhäusser et al. 2018; Smith et al. 2018). However, our values are not comparable with the results of Liu et al. (2019) from stemwood of B. platyphylla and Pinus sylvestris samples from the Mongolian boreal forest because of differences in the analytical method. While our measurements were based on enzymatic analyses, which can reliably separate between different sugar compounds, Liu et al. (2019) employed the phenol–sulfuric acid method, which results in much higher sugar concentrations. These higher concentrations are measured, because the phenol–sulfuric acid method also subsumes oligosaccharides other than glucose, fructose, and sucrose as well as other compounds with glycosidic bonds in the result (Landhäusser et al. 2018).
Conclusion
Birch and larch contrasted in their stemwood NSC concentrations reflecting the different life strategies as early- and late-successional tree species. Birch invested most assimilated C in growth and not in NSC during the growth recovery period. It recovered quickly from an intense summer drought and used the two wet years after the drought for strong growth releases, whereas larch always kept a high C allocation priority for soluble sugars over dry and wet periods. Larch showed also growth releases during the wet years, but they were more modest than in birch, which is consistent with the birches’ strategy to rapidly occupy canopy space and the larches’ strategy to find a tradeoff between canopy space occupation and longevity. The high soluble sugar pool in L. sibirica can be seen as a key strategy causing the species’ extreme drought and cold tolerance in addition to its fairly cavitation-resistant xylem (Dulamsuren et al. 2019). The intense summer drought of 2017 resulted in lagged reductions of NSC concentrations in larch, while growth was reduced in the drought year. We cannot rule out that these responses were also partly influenced by the preceding drought in 2015.
Author contribution statement
CD designed the study, analyzed data and wrote the paper. CD, GBY and SO conducted field and laboratory work. CD, RN and TG established the analytical method for NSC analysis in the laboratory. All authors read and commented on the paper.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adams HD, Germino MJ, Breshears DD, Barron-Gafford GA, Guardiola-Claramonte M, Zou CB, Huxman TE (2013) Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol 197:1142–1151
Babst F, Bouriaud O, Poulter B, Trouet V, Girardin MP, Frank DC (2019) Twentieth century redistribution in climatic drivers of global tree growth. Sci Adv 5:eaat4313
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Software 67:1–48
Blackman CJ, Creek D, Maier C, Aspinwall MJ, Drake JE, Pfautsch S, O’Grady A, Delzon S, Medlyn BE, Tissue DT, Choat B (2019) Drought response strategies and hydraulic traits contribute to mechanistic understanding of plant dry-down to hydraulic failure. Tree Physiol 39:910–924
Buermann W, Parida BR, Jung M, MacDonald GM, Tucker CJ, Reichstein M (2014) Recent shift in Eurasian boreal forest greening response may be associated with warmer and drier summers. Geophys Res Lett 41:1995–2002
Büntgen U, Krusic PJ, Piermattei A, Coomes DA, Esper J, Myglan VS, Kirdyanov AV, Camarero JJ, Crivellaro A, Körner C (2019) Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat Commun 10:2171
Canham CD, Kobe RK, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121:1–11
Charrier G, Lacointe A, Améglio T (2018) Dynamic modelling of carbon metabolism during the dormant period accurately predicts the changes in frost hardiness in walnut trees Juglans regia L. Front Plant Sci 9:1746
Chenlemuge T, Dulamsuren C, Hertel D, Schuldt B, Leuschner C, Hauck M (2015a) Hydraulic properties and fine root mass of Larix sibirica along forest edge-interior gradients. Acta Oecol 63:28–35
Chenlemuge T, Schuldt B, Dulamsuren C, Hertel D, Leuschner C, Hauck M (2015b) Stem increment and hydraulic architecture of a boreal conifer (Larix sibirica) under contrasting macroclimates. Trees 29:623–636
Creek D, Blackman CJ, Brodribb TJ, Choat B, Tissue DT (2018) Coordination between leaf, stem, and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell Environ 41:2869–2881
Cui Z, Yang Z, Xu D, Li X (2021) Drought could promote the heartwood formation in Dalbergia odorifera by enhancing the transformation of starch to soluble sugars. Scand J for Res. https://doi.org/10.1080/02827581.2021.2009022
De Grandpré L, Tardif JC, Hessl A, Pederson N, Conciatori F, Green TR, Oyunsanaa B, Baatarbileg N (2011) Seasonal shift in the climate responses of Pinus sibirica, Pinus sylvestris, and Larix sibirica trees from semi-arid, north-central Mongolia. Can J for Res 41:1242–1255
Deslauriers A, Garcia L, Charrier G, Buttò V, Pichette A, Paré M (2021) Cold acclimation and deacclimation in wild blueberry: direct and indirect influence of environmental factors and non-structural carbohydrates. Agric for Meteorol 301–302:108349
Dickman LT, McDowell NG, Grossiord C, Collins AD, Wolfe BT, Detto M, Wright SJ, Medina-Vega JA, Goodsman D, Rogers A, Serbin SP, Wu J, Ely KS, Michaletz ST, Xu C, Kueppers L, Chambers JQ (2019) Homoeostatic maintenance of nonstructural carbohydrates during the 2015–2016 El Niño drought across a tropical forest precipitation gradient. Plant Cell Environ 42:1705–1714
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Ann Rev Plant Biol 65:667–687
Duchesne L, Houle D, Ouimet R, Caldwell L, Gloor M, Brienen R (2019) Large apparent growth increases in boreal forests inferred from tree-rings are an artefact of sampling biases. Sci Rep 9:6832
Dulamsuren C (2004) Floristische Diversität, Vegetation und Standortbedingungen in der Gebirgstaiga des Westkhentey, Nordmongolei. Ber Forschungszentr Waldökosyst A 191:1–290
Dulamsuren C (2021) Organic carbon stock losses by disturbance: comparing broadleaved and late-successional conifer forests in Mongolia’s boreal forest. For Ecol Manag 499:119636
Dulamsuren C, Hauck M (2008) Spatial and seasonal variation of climate on steppe slopes of the northern Mongolian mountain taiga. Grassl Sci 54:217–230
Dulamsuren C, Hauck M, Bader M, Osokhjargal D, Oyungerel S, Nyambayar S, Runge M, Leuschner C (2009a) Water relations and photosynthetic performance in Larix sibirica growing in the forest-steppe ecotone of northern Mongolia. Tree Physiol 29:99–110
Dulamsuren C, Hauck M, Bader M, Osokhjargal D, Oyungerel S, Nyambayar S, Leuschner C (2009b) The different strategies of Pinus sylvestris and Larix sibirica to deal with summer drought in a northern Mongolian forest-steppe ecotone suggest a future superiority of pine in a warming climate. Can J for Res 39:2520–2528
Dulamsuren C, Hauck M, Leuschner C (2010) Recent drought stress leads to growth reductions in Larix sibirica in the western Khentey, Mongolia. Glob Change Biol 16:3024–3035
Dulamsuren C, Hauck M, Leuschner HH, Leuschner C (2011) Climate response of tree-ring width in Larix sibirica growing in the drought-stressed forest-steppe ecotone of northern Mongolia. Ann for Sci 68:275–282
Dulamsuren C, Wommelsdorf T, Zhao F, Xue Y, Zhumadilov BZ, Leuschner C, Hauck M (2013) Increased summer temperatures reduce the growth and regeneration of Larix sibirica in southern boreal forests of eastern Kazakhstan. Ecosystems 16:1536–1549
Dulamsuren C, Khishigjargal M, Leuschner C, Hauck M (2014) Response of tree-ring width to climate warming and selective logging in larch forests of the Mongolian Altai. J Plant Ecol 7:24–38
Dulamsuren C, Klinge M, Degener J, Khishigjargal M, Chenlemuge T, Bat-Enerel B, Yeruult Y, Saindovdon D, Ganbaatar K, Tsogtbaatar J, Leuschner C, Hauck M (2016) Carbon pool densities and a first estimate of the total carbon pool in the Mongolian forest-steppe. Glob Change Biol 22:830–844
Dulamsuren C, Abilova S, Bektayeva M, Eldarov M, Schuldt B, Leuschner C, Hauck M (2019) Hydraulic architecture and vulnerability to drought-induced embolism in southern boreal tree species of Inner Asia. Tree Physiol 39:463–473
ElSayed AI, Rafudeen MS, Golldack D (2014) Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biol 16:1–8
Erdene-Ochir T, Ishiguri F, Nezu I, Tumenjargal B, Baasan B, Chultem G, Ohshima J, Yokota S (2020) Utilization potential of naturally regenerated Mongolian Betula platyphylla wood based on growth characteristics and wood properties. Silva Fennica 54:10284
Fierravanti A, Rossi S, Kneeshaw D, De Grandpré L, Deslauriers A (2019) Low non-structural carbon accumulation in spring reduces growth and increases mortality in conifers defoliated by spruce budworm. Front for Glob Change 2:15
Furze ME, Huggett BA, Aubrecht DM, Stolz CD, Carbone MS, Richardson AD (2019) Whole-tree nonstructural carbohydrate storage and seasonal dynamics in five temperate species. New Phytol 221:1466–1477
Galiano L, Martínez-Vilalta J, Lloret F (2011) Carbon reserves and canopy defoliation determine the recovery of Scots pine 4 yr after a drought episode. New Phytol 190:750–759
Goulden ML, Daube BC, Fan S-M, Sutton DJ, Bazzaz A, Munger JW, Wofsy SC (1997) Physiological responses of a black spruce forest to weather. J Geophys Res 102:28987–28996
Gunin PD, Vostokova EA, Dorofeyuk NI, Tarasov PE, Black CC (1999) Vegetation dynamics of Mongolia. Kluwer, Dordrecht
Hartmann H, Trumbore S (2016) Understanding the roles of non-structural carbohydrates in forest trees—from what we can measure to what we want to know. New Phytol 211:386–403
Hartmann H, Adams HD, Hammond WM, Hoch G, Landhäusser SM, Wiley E, Zaehle S (2018) Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ Exp Bot 152:7–18
Hartmann H, Bahn M, Carbone M, Richardson AD (2020) Plant carbon allocation in a changing world—challenges and progress: introduction to a Virtual Issue on carbon allocation. New Phytol 227:981–988
He W, Liu H, Qi Y, Liu F, Zhu X (2020) Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob Change Biol 26:3627–3638
Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081
IPCC (2021) Climate change 2021: the physical science basis. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Itter MS, D’Orangeville LD, Dawson A, Kneeshaw D, Duchesne L, Finley AO (2019) Boreal tree growth exhibits decadal-scale ecological memory to drought and insect defoliation, but no negative response to their interaction. J Ecol 107:1288–1301
Jiménez S, Dridi J, Gutiérrez D, Moret D, Irigoyen JJ, Moreno MA, Gogorcena Y (2013) Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiol 33:1061–1075
Khansaritoreh E, Dulamsuren C, Klinge M, Ariunbaatar T, Bat-Enerel B, Batsaikhan G, Ganbaatar K, Saindovdon D, Yeruult Y, Tsogtbaatar J, Tuya D, Leuschner C, Hauck M (2017a) Higher climate warming sensitivity of Siberian larch in small than large forest islands in the fragmented Mongolian forest steppe. Glob Change Biol 23:3675–3689
Khansaritoreh E, Eldarov M, Ganbaatar K, Saindovdon D, Leuschner C, Hauck M, Dulamsuren C (2017b) Age structure and trends in annual stem increment of Larix sibirica in two neighboring Mongolian forest-steppe regions differing in land use history. Trees 31:1973–1986
Kharuk VI, Ranson KJ, Oskorbin PA, Im ST, Dvinskaya ML (2013) Climate induced birch mortality in Trans-Baikal lake region, Siberia. For Ecol Manag 289:385–439
Klein T, Zeppel MJB, Anderegg WRL, Bloemen J, De Kauwe MG, Hudson P, Ruehr NK, Powell TL, von Arx G, Nardini A (2018) Xylem embolism refilling and resilience against drought-induced mortality in woody plants: processes and trade-offs. Ecol Res 33:839–855
Kljun N, Black TA, Griffis TJ, Barr AG, Gaumont-Guay D, Morgenstern K, McCaughey JH, Nesic Z (2006) Response of net ecosystem productivity of three boreal forest stands to drought. Ecosystems 9:1128–1144
Koizuma A, Takata K, Yamashita K, Nakada R (2003) Anatomical characteristics and mechanical properties of Larix sibirica grown in south-central Siberia. IAWA J 24:355–370
Kono Y, Ishida A, Saiki S-T, Yoshimura K, Dannoura M, Yazaki K, Kimura F, Yoshimura J, Aikawa S-I (2019) Initial hydraulic failure followed by late-stage carbon starvation leads to drought-induced death in the tree Trema orientalis. Commun Biol 2:8
Landhäusser SM, Chow PS, Dickman LT, Furze ME, Kuhlman I, Schmid S, Wiesenbauer J, Wild B, Gleixner G, Hartmann H, Hoch G, McDowell NG, Richardson AD, Richter A, Adams HD (2018) Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol 38:1764–1778
Lintunen A, Paljakka T, Jyske T et al (2016) Osmolality and non-structural carbohydrate composition in the secondary phloem of trees across a latitudinal gradient in Europe. Front Plant Sci 7:726
Liu H, Williams AP, Allen CD, Guo D, Wu X, Anenkhonov OA, Liang EY, Sandanov DV, Yin Y, Qi Z, Badmaeva NK (2013) Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob Change Biol 19:2500–2510
Liu H, Shangguan H, Zhou M, Airebule P, Zhao P, He W, Xiang C, Wu X (2019) Differentiated responses of nonstructural carbohydrate allocation to climatic dryness and drought events in the Inner Asian arid timberlime. Agric for Meteorol 271:355–361
Lloyd AH, Bunn AG (2007) Responses of the circumpolar boreal forest to 20th century climate variability. Environ Res Lett 2(045013):1–13
Maguire AJ, Kobe RK (2015) Drought and shade deplete nonstructural carbohydrate reserves in seedlings of five temperate tree species. Ecol Evol 5:5711–5721
Mitchell PJ, O’Grady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA (2013) Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol 197:862–872
Muller B, Pantin F, Génard M, Turc O, Freixes S, Piques M, Gibon Y (2011) Water deficits uncouple growth from photosynthesis, increases C content, and modify the relationships between C and growth in sink organs. J Exp Bot 62:1715–1729
Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Sci 180:604–611
Niamke BF, Adima AA, Seraphin K-C, Amusant N, Jay-Allemand C (2018) Heartwood formation process in teak (Tectonia grandis L. f): fate of non-structural carbohydrates and characterization of forsythoside B. Int J Biol Chem Sci 12:1102–1112
O’Brien MJ, Leuzinger S, Philipson CD, Tay J, Hector A (2014) Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat Clim Change 4:710–714
O’Brien MJ, Burslem DFRP, Caduff A, Tay J, Hector A (2015) Contrasting nonstructural carbohydrate dynamics of tropical tree seedlings under water deficit and variability. New Phytol 205:1083–1094
Ogasa M, Miki NH, Yoshikawa K (2010) Changes of hydraulic conductivity during dehydration and rehydration in Quercus serrata Thunb. and Betula platyphylla var. japonica Hara: the effect of xylem structures. Tree Physiol 33:335–344
Ogasa M, Miki NH, Murakami Y, Yoshikawa K (2013) Recovery performance in xylem hydraulic conductivity is correlated with cavitation resistance for temperate deciduous tree species. Tree Physiol 33:335–344
Oribe Y, Funada R, Kubo T (2003) Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sacchalinensis (Schmidt) Masters. Trees 17:185–192
Ozturk M, Unal BT, García-Caparrós P, Khursheed A, Gul A, Hasanuzzaman M (2021) Osmoregulation and its actions during the drought stress in plants. Physiol Plant 172:1321–1335
Palacio S, Maestro M, Montserrat-Martí G (2007) Seasonal dynamics of non-structural carbohydrates in two species of mediterranean sub-shrubs with different leaf phenology. Environ Exp Bot 59:34–42
Palacio S, Camarero JJ, Maestro M, Alla AQ, Lahoz E, Montserrat-Martí G (2018) Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees 32:777–790
Peters RL, Miranda JC, Schönbeck L, Nievergelt D, Fonti MV, Saurer M, Stritih A, Fonti P, Wermelinger B, von Arx G, Lehmann GG (2020) Tree physiological monitoring of the 2018 larch budmoth outbreak: preference for leaf recovery and carbon storage over stem wood formation in Larix decidua. Tree Physiol 40:1697–1711
Pfadenhauer JS, Klötzli FA (2020) Global vegetation: fundaments, ecology and distribution. Springer, Cham
Piper FI, Hoch G, Fajardo A (2019) Revisiting the relative growth rate hypothesis for gymnosperm and angiosperm species co-occurrence. Am J Bot 106:101–112
Ponton S, Flanagan LB, Alstad KP, Johnson BG, Morgenstern K, Kljun N, Black TA, Barr AG (2006) Comparison of ecosystem water-use efficiency among Douglas-fir forest, aspen forest and grassland using eddy covariance and carbon isotope techniques. Glob Change Biol 12:294–310
Regier N, Streb S, Cocozza C, Schaub M, Cherubini P, Zeeman SC, Frey B (2009) Drought tolerance of two black poplar (Populus nigra L.) clones: contribution of carbohydrates and oxidative stress defence. Plant Cell Environ 32:1724–1736
Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, Schaberg PG, Xu X (2013) Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol 197:850–861
Richardson AD, Carbone MS, Huggett BA, Furze ME, Czimczik CI, Walker JC, Xu X, Schaberg PG, Murakami P (2015) Distribution of old and new nonstructural carbon in two temperate trees. New Phytol 206:590–597
Rinne P, Welling A, Kaikuranta P (1998) Onset of freezing tolerance in birch (Betula pubescens Ehrh.) involves LEA proteins and osmoregulation and is impaired in an ABA-deficient genotype. Plant Cell Environ 21:601–611
Rossi S, Anfodillo T, Menardi R (2006) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27:89–97
Sala A, Woodruff DR, Meinzer FC (2012) Carbon dynamics in trees: feast or famine? Tree Physiol 32:764–775
Sauter JJ, Ambrosius T (1986) Changes in the partitioning of carbohydrates in the wood during bud break in Betula pendula Roth. J Plant Physiol 124:31–43
Scartazza A, Moscatella S, Matteucci G, Battistelli A, Brugnoli E (2013) Seasonal and inter-annual dynamics of growth, non-structural carbohydrates and C stable isotopes in a Mediterranean beech forest. Tree Physiol 33:730–742
Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ 37:153–161
Smith MG, Miller RE, Arndt SK, Kasel S, Bennett LT (2018) Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol 38:570–581
Stuecker MF, Bitz CM, Armour KC, Proistosescu C, Kang SM, Xie S-P, Kim D, McGregor S, Zhang W, Zhao S, Cai W, Dong Y, Jin F-F (2018) Polar amplification dominated by local forcing and feedbacks. Nat Clim Change 8:1076–1081
Tei S, Sugimoto A, Yonenobu H, Matsuura Y, Osawa A, Sato H, Fujinuma J, Maximov TC (2017) Tree-ring analysis and modelling approaches yield contrary response of circumboreal forest productivity to climate change. Glob Change Biol 23:5179–5188
Thompson RA, Adams HD, Breshears DD, Collins AD, Dickman LT, Grossiord C, Manrique-Alba À, Peltier DM, Ryan MG, Trowbridge AM, McDowell NG (2023) No carbon storage in growth-limited trees in a semi-arid woodland. Nat Commun 14:1959
Tumajer J, Lehejček J (2019) Boreal tree-rings are influenced by temperature up to two years prior to their formation: a trade-off between growth and reproduction? Environ Res Lett 14:124024
Van den Ende W (2013) Multifunctional fructans and raffinose family oligosaccharides. Front Plant Sci 4:247
Vicente-Serrano SM, Beguería S, López-Moreno JI (2010) A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J Clim 23:1696–1718
von Arx G, Arzac A, Fonti P, Frank D, Zweifel R, Rigling A, Galiano L, Gessler A, Olano JM (2017) Responses of sapwood ray parenchyma and non-structural carbohydrates of Pinus sylvestris to drought and long-term irrigation. Funct Ecol 31:1371–1382
Walker XJ, Mack MC, Johnstone JF (2015) Stable carbon isotope analysis reveals widespread drought stress in boreal black spruce forests. Glob Change Biol 21:3102–3113
Welp LR, Randerson JT, Liu HP (2007) The sensitivity of carbon fluxes to spring warming and summer drought depends on plant functional type in boreal forest ecosystems. Agric for Meteorol 147:172–185
Xu C, Liu H, Zhou M, Xue J, Zhao P, Shi L, Shangguan H (2018) Enhanced sprout-regeneration offsets warming-induced forest mortality through shortening the generation time in semiarid birch forest. For Ecol Manag 409:298–306
Yoshimura K, Saiki S-T, Yazaki K, Ogasa MY, Shirai M, Nakano T, Yoshimura J, Ishida A (2016) The dynamics of carbon stored in xylem sapwood to drought-induced hydraulic stress in mature trees. Sci Rep 6:24513
Zha T, Barr AG, van der Kamp G, Black TA, McCaughey JH, Flanagan LB (2010) Interannual variation of evapotranspiration from forest and grassland ecosystems in western Canada in relation to drought. Agric for Meteorol 150:1476–1484
Zhang H, Wang C, Wang X (2014) Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees 28:77–89
Acknowledgements
The German Corporation for International Cooperation (GIZ, K. Schmidt-Corsitto, M. Trockenbrodt) is thanked for permitting the use of their Tunkhel field station and related support for the study. T. Ariunbaatar and D. Mukhjargal (Ulan Bator) are thanked for their work during wood core sampling for NSC analysis in the field. M. Scherer-Lorenzen kindly provided the facilities of his laboratory at the Chair of Geobotany, University of Freiburg.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was funded by the German Science Foundation (Deutsche Forschungsgemeinschaft, DFG) with the grant “Climate control of wood formation measured at high precision in two southern boreal tree species (Larix sibirica and Betula platyphylla)” to Ch. Dulamsuren (Du 1145/4-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by A. Nardini.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dulamsuren, C., Byamba-Yondon, G., Oyungerel, S. et al. Non-structural carbohydrate concentrations in contrasting dry and wet years in early- and late-successional boreal forest trees. Trees 37, 1315–1332 (2023). https://doi.org/10.1007/s00468-023-02420-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02420-6