Abstract
Key message
Restricted access of light for stems reduced carbon acquisition there and limited the biomass growth of the roots.
Abstract
Light access can affect the microatmosphere within stems, creating favourable conditions for photosynthesis. We tested the hypothesis that stem photosynthesis modifies carbon allocation within plants and also can affect root growth. To verify this hypothesis, parts of Clusia minor L. stems were covered with dark material for 8 months to block light access to stems, and then, we compared morphological traits, biomass increment, photosynthetic activity and carbon isotopic signature (δ13C) in plants with dark- and light-exposed stems. Clusia minor stems were characterized by chlorophyll presence from pith to cortex, active photosystem II and 79% re-assimilation of respired CO2. We also revealed 24-h changes in the δ13C of carbohydrates exported from leaves. Keeping stems in darkness led to a significant lowering in root biomass and shoot-to-root weight index (Iw). Moreover, reductions in stem CO2 efflux and the δ13C in the roots and stems were also observed. Our results indicate that the lack of stem photosynthesis affects photosynthate flux to heterotrophic organs, such as roots, stems and probably expanding leaves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tree stem is thought to have several major functions: support, transport and storage (Givnish 1995). In addition to these well-known functions, photosynthesis seems to be important at least in trees with green (non-lignified) stems. Many living tissues in stems are equipped with chloroplasts, demonstrating that their photochemistry may affect carbon and energy balance (Yiotis and Manetas 2010); and recently, the participation of stem photosynthesis in drought stress tolerance was indicated (Cernusak and Cheesman 2015; Vandegehuchte and Bloemen 2015; Ávila-Lovera et al. 2017; Ávila-Lovera and Tezara 2018).
Stem photosynthesis is often an underestimated process especially in trees where green cells are deeply hidden by the cork layer (Yiotis et al. 2009). When it is transmitted through the cork, light energy in the photosynthetically active radiation (PAR) range can be utilized in stems. Often its intensity is weak and spectrally different in comparison with incident irradiation as a result of the cork reflectance and absorption (Pfanz 1999; Pilarski et al. 2008; Wittmann and Pfanz 2016).
The limited permeability of stem tissue for water vapour and other gases is at least partially responsible for high CO2 concentration and lower O2 abundance when compared with the surrounding atmosphere (Maier and Clinton 2006; Teskey et al. 2008; Kocurek and Pilarski 2012). CO2 refixation measured as percent reduction of CO2 efflux in light is considered a main parameter describing the photosynthetic activity of stems. However, this parameter does not take into account the entire complexity of processes related to photosynthesis and occurring in the veins of midribs, petioles and stems (Yiotis and Manetas 2010; Kuźniak et al. 2016; Miszalski et al. 2017). Photosynthesis in the stems’ cells can be a source of energy (ATP) for these cells or carbohydrate transportation over long distances. Another benefit of this phenomenon is that it provides the amount of oxygen in the stem, necessary for respiration. It is known that the respiration decreases with lowering O2 concentration (Spicer and Holbrook 2005) and stem photosynthesis enables higher respiration rates (Wittmann and Pfanz 2018). In addition, CO2 released during dark respiration can be re-assimilated by stem photosynthesis.
The benefits from stem photosynthesis are not necessarily limited to the anatomical part in which they appear. Saveyn et al. (2010) revealed that light exclusion from stems in three woody species resulted in a reduction in chlorophyll concentration and radial growth. They also revealed that in defoliated plants, darkening of trunks caused a reduction in bud biomass (Saveyn et al. 2010); thus, the photosynthetic activity of stems influences the growth of other organs, likely by affecting carbohydrate allocation.
The mechanism of carbon distribution and carbohydrate transport can be tracked by changes in δ13C in organic matter (OM) and in respiratory CO2 (Moore et al. 2008). The first step of carbon discrimination in plants includes fractionation during CO2 diffusion through the leaf boundary layer and stomata, and discrimination by RubisCO and PEPC. Although carbon isotope discrimination during photosynthetic CO2 fixation is a rather well described and understood phenomenon (Farquhar et al. 1982; Fung et al. 1997; Borland and Dodd 2002), much less is known about the isotopic fractionation associated with the post-photosynthetic processes following carboxylation in leaf tissues (Badeck et al. 2005; Ghashghaie and Badeck 2014; Miszalski et al. 2016). It is proposed that post-photosynthetic carbon fractionation is clearly displayed in whole plant 13C distribution. According to Cernusak et al. (2009), non-photosynthetic/heterotrophic tissues (e.g. stems, fruits and roots) in C3 plants tend to be enriched in 13C compared with the leaves that supply these tissues with photosynthate. Also, young emerging leaves of C3 plants for which growth may be mostly heterotrophic, tend to be 13C enriched (Damesin and Lelarge 2003) and later during the development of the photosynthetic apparatus, δ13C is lowered.
Cernusak et al. (2009) described six hypotheses explaining post-photosynthetic isotope fractionation. One of the hypotheses (Tcherkez et al. 2004) stipulates that post-photosynthetic carbon fractionation is initiated by aldolase, which condenses two trioses to form fructose 1–6 bisphosphate, discriminates carbon isotopes in favour of 13C and enriches hexose molecules (Rossmann et al. 1991; Gleixner and Schmidt 1997; Gilbert et al. 2011, 2012; Ghalagashie and Badeck 2014) and subsequently transitory starch in 13C. The remaining 13C-depleted trioses are transported to the cytosol, forming sucrose that is expected to be 13C depleted compared with sucrose derived from the degradation of transitory starch at night. The proportion between night and day sucrose affects δ13C sucrose levels loaded into the phloem sap. Thus, light period-derived sucrose and other carbohydrates that migrate in the phloem sap are typically 13C depleted compared with those loaded and transported during the night (Gessler et al. 2007, 2008).
To date, most of the investigations have been focused on the benefits stems gain based on their photosynthetic activity (Pfanz 1999; Eyles et al. 2009; Miszalski et al. 2017; Ávila-Lovera and Tezara 2018; Wittmann and Pfanz 2018). To the best of our knowledge, the only attempt of Saveyn et al. (2010) to show the effect of photoassimilates produced in stems on other organs was observed in buds.
Our hypothesis assumes that limited stem photosynthesis will affect root biomass. For research, we have chosen the tropical tree Clusia minor L. of the Clusiaceae family. The previous results considering Clusia multiflora and C. rosea proved that their stems are characterized by high permeability for water vapour which is an adaptation to low water availability. Also, cross sections of Clusia stems showed well-developed water–storage cortex (Lüttge 2008; Kocurek et al. 2015; Miszalski et al. 2017). This tissue develops a photosynthetically active cell layer, several times thicker than the one in temperate trees (Dima et al. 2006; Berveiller et al. 2007). Here, we report the δ13C values in leaves, stems and roots of C. minor cultivated with full access to light and partially darkened stems. In our experiments, heterogeneity of carbohydrates and its distribution in the diurnal cycle was also tested. Moreover, we discuss other possible explanations concerning δ13C heterogeneity and carbon allocation in woody C3 plants.
Materials and methods
Plant material
The experiments were performed on 8-month-old cuttings from 2-year-old Clusia minor L. plants. Two-leaf pair cuttings were obtained from mother plants. The cuttings were rooted for 4 weeks in tap water. After roots appeared, the cuttings were transferred to individual pots with 500 g soil. This soil was composed of universal substrate (Substral Natural, Substral Evergreen Garden Care Poland, Warsaw, Poland) containing NPK 9:5:10 (pH 6.0–6.5) mixed in a 1:1 ratio with quartz sand. Plants were divided in two groups (5 plants per each). Stems of one group were darkened with black cotton tape; only approximately 10% of whole stem area above the black tape of shoots was not covered with the tape. However, after 8 months of growth (January–September), the portion of the stem above the darkened part accounted for approximately 40% of the whole stem area. Plants were grown under a natural photoperiod in a 12 m2 growth room located in Kraków, Poland (50°08 N, 19°84 E). The experimental plants covered around 20% of the maximal capacity of the well-ventilated growth room, in order to reduce fluctuations in isotope composition of source CO2. However, the exact fluctuation was not measured.
Clusia minor is categorized as a C3-CAM intermediate plant (Borland et al. 1998) that develops CAM when exposed to stress; thus, extensive watering is crucial to sustain C3 metabolism. After an 8-month growth period, plants developed 6–10 new leaves. The plants were thoroughly irrigated every third day using 50 ml of tap water. All measurements except growth analyses were performed after 8 months of experiments, and then phloem sap and fresh material were obtained for δ13C and carbohydrates content determination.
Chlorophyll localization in stems
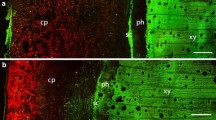
The distribution of chlorophyll in stems was determined based on the autofluorescence of chl a. Hand-cut cross-sections were obtained approximately 2 cm below the upper part of black tape and approximately 1 cm above it (Fig. 1). Cross-sections were observed in distilled water using a Nikon ECLIPSE Ni light and epifluorescence microscope (Nikon, Japan) equipped with a Digital Sight series DS-Fi1c microscope camera and NIS Imaging software (Nikon version 4.11). Red and green colours correspond to autofluorescence of chlorophyll and cell walls, respectively.
Eight-month-old cuttings of C. minor partly darkened with black tape (left) and with access to light (right) (a); partly darkened stem (b); cross-sections of the portion of the stem with access to light (c, e); cross-sections of portion of stem subject to dark for 8 months (d, f) observed by epifluorescence microscopy; pi pith, cp cortex parenchyma, ph phloem, xy xylem, pe peridermis. Red and green colours correspond to autofluorescence of chlorophyll and cell walls, respectively. Black arrows indicate locations where cross-sections were obtained
Fluorescence measurements
Chlorophyll fluorescence imaging was performed using a pulse-amplitude modulated system (Imaging PAM, Walz, Effeltrich, Germany) equipped with a bank of blue (λ = 450 nm) LEDs and a CCD camera capturing fluorescence images. Measurements were performed between 7:00 and 11:00 h on dark-adapted (30 min) plants. The completely dark-adapted parts of C. minor stems were cut and moved to a container filled with distilled water to avoid tissue desiccation. Then, the stems were split with a razor blade and placed into a transparent polyethylene (9 cm × 13 cm) sealable bag completely filled with water. Such packed samples of stems were given a saturating (ca. 3500 μmol m−2 s−1) pulse to assess the maximum PSII photochemical efficiency as Fv/Fm = (Fm–F0)/Fm, where F0 and Fm are the fluorescence yields with open and closed PSII reaction centres, respectively.
Phloem sap samples
To obtain phloem sap, leaves were detached at the base of the petiole. Then, each leaf was inserted with the petiole into a vial containing 1.5 ml of 15 mM sodium hexametaphosphate (Sigma-Aldrich, St Louis, MO, USA). Exudates from the first hour were discarded, and the vials replaced by new vials containing the same amount of solution. Sap collections were stopped after 5 h, and samples were boiled for 1 min to stop enzyme activities (Wild et al. 2010). In the obtained exudates, δ13C and carbohydrate content were determined.
Carbon isotope analysis in organic samples
Frozen samples of leaves, barks and roots were oven-dried for 24 h at 105 °C before being ground to a fine powder for isotope analysis. The samples of phloem sap were lyophilized for 24 h before δ13C determination. Isotope ratio measurements of 13C were performed on a DELTAplus Mass Spectrometer (Finnigan, Germany) coupled with a Flash1112 NC Series Elemental Analyzer (ThermoFinnigan, Italy) in a continuous flow mode. Laboratory isotope standards (sorghum flour δ13C = − 13.68‰ and protein − 26.98‰, IVA Analysentechnik, Germany) were used in measurements to calculate the final δ13C results as follows (Malec-Czechowska and Wierzchnicki 2013):
where R is the isotope ratio 13C/12C in the sample, and Rstnd is the isotope ratio 13C/12C in international standard PDB (Pee Dee Belemnite).
Gas exchange measurements
The measurements of gas exchange were conducted with a Portable Photosynthesis System LI-6400XT (LI-COR Inc., Lincoln, NE, USA) using leaves and stems under greenhouse conditions. Gas (CO2 and H2O) efflux rates were measured using a standard chamber for leaves and a conifer chamber 6400-05 for stems. Measurements of gas exchange of the leaves were performed continuously (every 15 min) under greenhouse conditions under sun exposure (30–37 °C, 28–48% relative humidity (RH), 400–900 µmol mol−1 CO2, and 0–1250 µmol PAR m−2 s−1). Refixation of stems was estimated from CO2 efflux under laboratory conditions (30 °C, 30% RH, CO2 concentration 400 µmol mol−1, in darkness or 1000 µmol PAR m−2 s−1). Refixation by stems was estimated according to Cernusak and Marshall (2000) and obtained from CO2 efflux in the dark (Rd) and under illumination (Rlt) as follows:
Carbohydrate contents
The contents of soluble sugars were determined according to Black et al. (1996) with some modifications according to Janeczko et al. (2010). Approximately 5 mg of the lyophilized samples (LABCONCO, USA) were homogenized with 80% (v:v) ethanol at 80 °C for 40 min and then centrifuged at 13 000 × g for 10 min. After dissolving in 100 ml of ultra-pure water, samples were filtered through a 0.22-µm filter (Costar Spin-x, Coring, NY, USA). Samples (1–2 ml) were injected onto a Hamilton RCX-10 (250 mm × 4.1 mm) column (Hamilton, Reno, NV, USA) and separated by high-performance liquid chromatography (HPLC, Beckman, Fullerton, CA, USA) using the Beckman System Gold 125 NM Solvent Module equipped with an ESA Coulochem II Analytical Cell 5040 and gold electrode (ESA Chelmsford, MA, USA).
Statistics
Statistical analyses of the data were performed using Statistica 12.0 (Statsoft, Tulsa, OK, USA). Morphological parameters, δ13C values, gas exchange and carbohydrate levels were evaluated by statistical analysis of variance (ANOVA). Images from chlorophyll a fluorescence and epifluorescence microscopy represent typical examples of at least five repetitions. Detailed information about statistic tests and the number of replicates is indicated in the description of tables and figures.
Results
Stems were strongly affected by the 8-month period of darkening treatment (Fig. 1). The darkened parts of the stem became brown because of the cork layer, whereas the light exposed parts exhibited a visibly green colour. The changes also included the interior of stems and concerned chlorophyll amount, which was clearly visible on the cross-sections as a red signal. Initially, chlorophyll presence was observed in all tissues of the stem and subsequently disappeared from the pith, pith rays and phloem. Ultimately, only small amounts of chlorophyll remained in the upper cortex parenchyma.
The maximum PSII photochemical efficiency (Fv/Fm) measured at the cross-section of stems exposed to light (Fig. 2) was observed in all tissues based on the presence of chlorophyll as noted above on the epifluorescence pictures (Fig. 1). Fv/Fm values varied from 0.84 in cortex parenchyma to 0.83 in the piths, whereas areas containing no chlorophyll, such as wood, appeared black, which is similar to the background signal.
The gas exchange values of plants with lightened and darkened stems are shown in Table 1. The net photosynthesis of illuminated stems was slightly negative at 1000 µmol m–2 s–1 PAR, whereas dark respiration of those stems reached 0.9 µmol m–2 s–1. Plants with darkened stems were characterized by reduced dark respiration. Refixation calculated from respiration and net photosynthesis was possible only in the case of illuminated stems. Conductance of water vapour was significantly increased in lightened stems when compared with darkened ones.
The δ13C values are presented in Fig. 3. The lowest δ13C was noted in leaves of plants with light-exposed stems. These values were significantly higher in the barks of stems and roots. In plants with darkened stems, δ13C values for leaves, barks and roots were reduced by approximately 1.0, 2.5 and 2.7‰, respectively. Moreover, in plants with darkened stems, clear differences in isotopic ratio between organs were not observed.
The δ13C values were also measured in the phloem sap obtained from petioles of the plants with lightened stems (Fig. 4). The values were rather stable during the light period of the day and were significantly higher between 8.00 and 13.00 h compared with evening and night (18.00–23.00 h). Plants were maintained in the mode of C3 photosynthesis by regular watering, so the daily net photosynthesis plot in leaves showed positive values only during the day with two depressions around midday and 15.30 h. Leaves performed dark respiration at levels not exceeding 0.85 µmol m–2 s–1 and mostly at the level of 0.2–0.5 µmol m–2 s–1. The daily trend graph also reveals sugar levels in phloem sap (Fig. 5). The highest sugar concentration was recorded at the beginning of the day between 5.00 and 10.00 h and gradually decreased thereafter. Fluctuations in sugar content were low (2.4–3.9 mM) and significantly different only between morning (5.00–10.00 h) and late evening hours (18.00–23.00 h).
Diurnal changes in δ13C carbohydrate levels in phloem exudates (shaded bars) and net photosynthesis (black curve) in leaves of plants with lightened stems. Exudation lasted for 5 h. The solid bar on the x-axis indicates the dark period. Data on δ13C are the means ± SD of five replicates. The mean values marked with the same letters are not statistically significant according to the Duncan's test (P ≤ 0.05, n = 5). Net photosynthesis curve is representative of three replicates (n = 3)
Diurnal changes in carbohydrate concentrations in phloem exudates (shaded bars) in C. minor with lightened stems. Exudation lasted for 5 h. The solid bar on the x-axis indicates the dark period. Data are the means ± SD of five replicates. The mean values marked with the same letters are not statistically significant, according to the Duncan's test (P ≤ 0.05, n = 5)
Plant biomass of cuttings (shoots + roots) with darkened stems was characterized by approximately 23% reduced weight compared with illuminated stems. In the case of roots, this value was more pronounced and reached 34% (Table 2). This result indicates that darkening affected shoots and roots differently. This divergence between organs was also evident in the shoot/root weight index (lw), which was increased in darkened (1.39) compared with lightened plants (1.02). Consequently, the shoot length of plants with lightened stems (15 cm) was approximately twofold higher when compared with darkened stems. However, darkening did not significantly affect leaf development. During the experiment, the leaf area increased by approximately 200% in plants with darkened stems and 230% in light-exposed stems (however not statistically significant).
Discussion
In cross-sections of C. minor stems, using the epifluorescence microscopy technique, we can observe particular layers of tissues emitting a red signal originating from chlorophyll molecules. Using this technique, Dima et al. (2006) also detected chloroplasts in the cortex of 20 examined woody species, and these chloroplasts were also observed in perimedullar rays and piths of 19 and 16 species, respectively. In our study, chloroplasts were present in the cortex and pith. Moreover, fluorescence images indicate that chlorophyll-rich tissues showed a highly efficient PSII apparatus (Fv/Fm above 0.82). Similar observations have been made by Yotis et al. (2009) who revealed a continuous decrease in the Fv/Fm value (from 0.80 to 0.65) along the depth of stems of Eleaganus angustifolia L. A CO2-rich environment in stems impedes photochemical activity possibly through acidification of the cytoplasm (Pfanz 1999; Manetas 2004), and an active PSII will produce O2 that can be used for respiration and can support photorespiration. Thus, it seems that the stem photochemical activity will provide energy (ATP) and photosynthate and counteracts O2 deficiency (Kuźniak et al. 2016; Wittmann and Pfanz 2018).
Stems responsible for transport of water and photoassimilates often are very tight, impeding gas exchange with the surrounding atmosphere. As shown in our experiments on Clusia species (Kocurek et al. 2015), stems are well protected from water evaporation. In young stems of C. minor, H2O conductance was approximately 0.8 mmol m−2 s−1, while this value reached 30 mmol m−2 s−1 in leaves (data not shown). Other examined trees also showed similar conductance, e.g. ca. 1.0 mmol m−2 s−1 in 4-year old stems of Pinus monticola Dougl. ex D. Don (Cernusak and Marshall 2000) or 1.1 mmol m−2 s−1 in young stems of Betula pendula Roth (Wittmann et al. 2006). The presence of cork increases diffusion resistance values that typically cause an even more drastic reduction in the stems' conductance, e.g. 0.15–0.20 mmol m−2 s−1 in 8- to 10-year-old stems of Clusia multiflora Kunth and Clusia rosea Jacq. (Kocurek et al. 2015). The limited conductance of the cork favours accumulation of CO2 produced via respiration or/and transported from roots in xylem sap (Teskey et al. 2008). Finally, stem CO2 efflux is modulated by actual efficiency of photosynthesis. Based on the CO2 efflux calculations (in light and dark conditions), efficient refixation of respiratory CO2 is considered a photosynthetic activity of plant stems (Pfanz and Aschan 2001; Pfanz et al. 2002; Cerasoli et al. 2009).
In our experiments, the relatively high conductance of 2-year-old stems was manifested by a high CO2 efflux (0.9 μmol m−2 s−1), and approximately 79% of CO2 was refixed by stem tissues. Our calculations of CO2 refixation in strong light (1000 µmol m−2 s−1 PAR) in C. minor yield high levels compared with those (7–123%) reported for other species (Cernusak and Marshall 2000; Teskey et al. 2008; Cerasoli et al. 2009; Ávila et al. 2014).
The physiological role of stem photosynthesis is clearly observed when stems are kept for a long time in darkness. Restricted access of light led to the disappearance of chlorophyll as observed in cross-sections of C. minor stems. Such treatment also limited the biomass of the whole plant by approximately 20%. This result is attributed to reduced carbon acquisition in the darkened stem area that was estimated at approximately 60% of the whole stem and limited transport of carbohydrates due to ATP deficiency, as suggested above. A significant photosynthesis rate in the entire plant area (stem + leaves) allows estimation of the stem’s contribution to total plant photosynthetic production (Aschan and Pfanz 2003). Cernusak and Hutley (2011) estimated that 11% of wood in the branches of Eucalyptus miniata A. Cunn. ex Schauer originates from corticular photosynthate. Kharouk et al. (1995) also reported 30–50% re-assimilation of respiratory CO2 by the bark of Populus tremuloides Michx., which constitutes a 10–15% contribution to the CO2 acquisition during the summer months.
Interestingly, stem darkening affected root biomass even more than shoots. Such treatment lowers shoot weight by approximately 10% and root weight by 34%. To our knowledge, this is the first time that the effect of stem photosynthesis on root development has been quantified. Earlier, Saveyn et al. (2010) observed reduced bud development on darkened stems and revealed that buds are supplied photosynthate derived from stem photosynthesis.
Darkened stems become a more heterotrophic organ likely because photosynthates are transported from other organs via the phloem. We expect that changes in carbon distribution between auto- and heterotrophic organs can be predicted based on δ13C.
Daily carbohydrate concentrations in phloem depend on the species. In general, a higher concentration is noted during the day in conditions favourable for photosynthesis (Sharkey and Pate 1976; Wild et al. 2010; Kallarackal et al. 2012). Transport of carbohydrates in phloem is regulated by source and sink organs and the circadian rhythm of growth (Paul and Foyer 2001; Borland and Dodd 2002; Ceusters et al. 2009a, b). Leaves grow during the day and use “in situ” produced photosynthate (Walter and Schurr 2005). In contrast, woody stem growth occurs mostly at night (Steppe et al. 2005; Saveyn et al. 2007), whereas roots do not exhibit a diurnal cycle (Walter and Schurr 2005). In our experiments in C. minor, phloem sap showed diurnal variations in δ13C of up to 2.2‰, and we also observed higher carbohydrates concentration during morning (significant) and midday (not significant) compared to late evening hours. We expect that carbohydrate distribution can change the 13C discrimination pattern between organs. The lack of photosynthates produced by the darkened stem likely led to a change in the distribution of sugars reaching the new leaves, stems and roots. The δ13C heterogeneity in C. minor organs revealed that leaves of plants with light-exposed stems export 13C-enriched sugars mainly derived from transitory starch to stems and roots (Fig. 6). Darkening of stems disturbed this process because heterotrophic stems need more photosynthate. Thus, more sugars with reduced 13C levels are directed to the stems and roots and subsequently results in no differences in δ13C between organs. The changes in δ13C distribution after darkening of the stems correspond with biomass limitations: lower in shoots and higher in roots. It seems that stems kept in the dark and unable to drive photosynthesis consume a portion of sugars transferred to roots. Thus, root growth suffers more than other organs.
Carbohydrates produced in photosynthetically active tissues and exported to heterotrophic organs are used in respiration (Borland and Dodd 2002). Thus, another explanation of the δ13C distribution in C. minor organs after darkening is the possibility of 13C fractionation during respiration. The respiratory CO2 efflux from stem of trees and shrubs was widely observed in the range of 0.2–4 µmol m–2 s–1 (Wittmann et al. 2006; Saveyn et al. 2007; Cerasoli et al. 2009, Yiotis and Manetas 2010). In C. minor, respiration evaluated as CO2 efflux in dark- and in light-exposed stems reached 0.9 µmol m–2 s–1, whereas the maximum respiration of leaves is 0.85 µmol m–2 s–1. These results indicate intensive stem metabolism.
Trunk and branch respiration in some species may represent 26% of the total carbon assimilated by leaves in a beech forest (Damesin et al. 2002). Thus, it seems that disturbances in respiration levels may substantially modify δ13C in stem tissues. The CO2 respired in the dark by C3 leaves is generally 13C enriched compared with leaf organic matter (or leaf sugar pools), and the latter is subsequently depleted in 13C (Ghashghaie et al. 2003; Badeck et al. 2005; Werner et al. 2012). On the other hand, respiring roots generally release 13C-depleted CO2 compared with root organic matter. However, roots of lignified plants show 13C enrichment in respired CO2. Conversely, in stems and leaves of woody plants (herbaceous plants were not investigated), effluxing CO2 is 13C enriched (Damesin et al. 2002; Saveyn et al. 2010), and the remaining organic matter of leaves is 13C depleted. Moreover, the intensity of stem photosynthesis is reflected in the δ13C value of CO2 released from stems. Cernusak et al. (2001) revealed strong stem photosynthesis modulation in δ13C of CO2 released from stems of two woody plants. As a result of darkening, an increased δ13C in organic matter of stems was observed. Similar results were also published by Saveyn et al. (2010) and Cernusak and Hutley (2011). These results (Cernusak et al. 2001; Saveyn et al. 2010; Cernusak and Hutley 2011) are clearly in contrast to the δ13C levels observed in organic matter in stems of C. minor kept in the dark. However, without detailed analyses of the δ13C level of CO2 released from C. minor stems, we can only hypothesize that respiration in darkened stems fractionates carbon isotopes in an opposite manner as reported for other investigated plants (release of increased 13C levels of CO2 than organic matter). Hypothetical changes in respiratory metabolism consistent with the observed δ13C levels in stems and roots of C. minor should involve a high contribution of the pyruvate dehydrogenase reaction (PDH) pathway (Ghashghaie and Badeck 2014).
Furthermore, the observed patterns could at least partially result from the anaplerotic fixation of HCO3− by PEPC into 13C-enriched malate. PEPC activity in stems and roots of C3 plants is significantly higher than that in the leaves (Gao et al. 1996; Berveiler and Damesin 2008; Kocurek and Pilarski 2011). According to the hypothesis formulated by Hibberd and Quick (2002) and Brown et al. (2010), the roots are to some extent the source of malate transported in xylem sap and then decarboxylated in the vascular bundles of stems, petioles and leaves where CO2 is re-assimilated. We hypothesize that an enhanced anaplerotic pathway in heterotrophic roots and stems after darkening increased malate production. In addition, 13C-enriched malate is transported to leaves, and the carbon remaining in organic matter is 13C depleted, which may explain the more negative δ13C signal due to stem darkening.
Conclusion
A portion of energy needed for transport, storage and development of stems is provided from photochemical activity itself. The darkening of stems reduces this important energy source and significantly reduces root weight. Additionally, darkening of stems reduces the level of CO2 efflux, which indicates a reduction in the level of metabolism. Changes in δ13C levels in Clusia organs with darkened stems reveal the impact on the flow of carbohydrates produced by the leaf.
In lightened stems, carbohydrates with high δ13C levels derived from leaves flow to the roots and stems. Darkening disturbs this allocation, and carbohydrates with lower δ13C levels are transferred to stems and roots. We do not know which particular carbohydrates are involved in this mechanism, and whether this phenomenon equally occurs in trees or plants from other families.
We conclude that the role of stem photosynthesis in shaping root growth should require further, more extensive research.
Author contribution statement
ZM, MK: conceived and designed experiments; MK UL and ZM: wrote the manuscript; MK: performed most experimental analyses; RW: performed isotopic analysis; AK: made microscopic plant anatomy observations.
References
Aschan G, Pfanz H (2003) Non-foliar photosynthesis—a strategy of additional carbon acquisition. Flora 198(2):81–97
Ávila E, Herrera A, Tezara W (2014) Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species. Photosynthetica 52(1):3–15
Ávila-Lovera E, Tezara W (2018) Water-use efficiency is higher in green stems than in leaves of a tropical tree species. Trees Struct Funct 32:1547–1558
Ávila-Lovera E, Zerpa AJ, Santiago LS (2017) Stem photosynthesis and hydraulics are coordinated in desert plant species. New Phytol 216(4):1119–1129
Badeck FW, Tcherkez G, Nogues S, Piel C, Ghashghaie J (2005) Postphotosynthetic fractionation of stable carbon isotopes between plant organs—a widespread phenomenon. Rapid Commun Mass Spectrom 19:1381–1391
Berveiller D, Damesin C (2008) Carbon assimilation by tree stems: potential involvement of phosphoenolpyruvate carboxylase. Trees Struct Funct 22:149–157
Berveiller D, Kierzkowski D, Damesin C (2007) Interspecific variability of stem photosynthesis among tree species. Tree Physiol 27:53–61
Black M, Corbineau F, Grzesik M, Guy P, Côme D (1996) Carbohydrate metabolism in the developing and maturing wheat embryo in relation to its desiccation tolerance. J Exp Bot 47:161–169
Borland AM, Dodd AN (2002) Carbohydrate partitioning in crassulacean acid metabolism plants: reconciling potential conflicts of interest. Funct Plant Biol 29(6):707–716
Borland AM, Tecsi LI, Leegood RC, Walker RP (1998) Inducibility of crassulacean acid metabolism (CAM) in Clusia species: physiological/biochemical characterization and intercellular localization of carboxylation and decarboxylation processes in three species which exhibit different degrees of CAM. Planta 205:342–351
Brown NJ, Palmer BG, Stanley S, Hajaji H, Janacek SH, Astley HM, Parsley K, Kajala K, Quick WP, Trenkamp S, Fernie AR, Maurino VG, Hibberd JM (2010) C4 acid decarboxylases required for C4 photosynthesis are active in the mid-vein of the C3 species Arabidopsis thaliana, and are important in sugar and amino acid metabolism. Plant J 61(1):122–133
Cerasoli S, McGuire MA, Faria J, Mourato M, Schmidt M, Pereira JS, Chaves MM, Teskey RO (2009) CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). J Exp Bot 60:99–105
Cernusak LA, Cheesman AW (2015) The benefits of recycling: how photosynthetic bark can increase drought tolerance. New Phytol 208(4):995–997
Cernusak LA, Hutley LB (2011) Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiol 155(1):515–523
Cernusak LA, Marshall JD (2000) Photosynthetic refixation in branches of Western White Pine. Funct Ecol 14:300–311
Cernusak LA, Marshall JD, Comstock JP, Balster NJ (2001) Carbon isotope discrimination in photosynthetic bark. Oecologia 128(1):24–35
Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A, Barbour MM, Williams DG, Reich PB, Ellsworth DS (2009) Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36:199–213
Ceusters J, Borland AM, Ceusters N, Verdoodt V, Godts C, De Proft MP (2009a) Seasonal influences on carbohydrate metabolism in the CAM bromeliad Aechmea 'Maya': consequences for carbohydrate partitioning and growth. Ann Bot 105(2):301–309
Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP (2009b) Differential usage of storage carbohydrates in the CAM bromeliad Aechmea 'Maya' during acclimation to drought and recovery from dehydration. Physiol Plant 135(2):174–184
Damesin C, Lelarge C (2003) Carbon isotope composition of current-year shoots from Fagus sylvatica in relation to growth, respiration and use of reserves. Plant Cell Environ 26:207–219
Damesin C, Ceschia E, Le Goff N, Ottorini J-M, Dufrêne E (2002) Stem and branch respiration of beech: from tree measurements to estimations at the stand level. New Phytol 153:159–172
Dima E, Manetas Y, Psaras GK (2006) Chlorophyll distribution pattern in inner stem tissues: evidence from epifluorescence microscopy and reflectance measurements in 20 woody species. Trees Struct Funct 20:515–521
Eyles A, Pinkard EA, O'Grady AP, Worledge D, Warren CR (2009) Role of corticular photosynthesis following defoliation in Eucalyptus globulus. Plant Cell Environ 32(20):1004–1014
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Fung I, Field CB, Berry JA, Thompson MV, Randerson JT, Malmström CM, Vitousek PM, Collatz GJ, Sellers PJ, Randall DA, Denning AS, Badeck F, John J (1997) Carbon-13 exchanges between the atmosphere and biosphere. Glob Biogeochem Cycles 11:507–533
Gao Z, Sagi M, Lips M (1996) Assimilate allocation priority as affected by nitrogen compounds in the xylem sap of tomato. Plant Physiol Biochem 34:807–815
Gessler A, Keitel C, Kodama N, Weston C, Winters AJ, Keith H, Grice K, Leuning R, Farquhar GD (2007) δ13C of organic matter transported from the leaves to the roots in Eucalyptus delegatensis: short term variations and relation to respired CO2. Funct Plant Biol 34:692–706
Gessler A, Tcherkez G, Peuke A, Ghashghaie J, Farquhar GD (2008) Diel variations of the carbon isotope composition in leaf, stem and phloem sap organic matter in Ricinus communis. Plant Cell Environ 3:941–953
Ghashghaie J, Badeck FW (2014) Opposite carbon isotope discrimination during dark respiration in leaves versus roots—a review. New Phytol 201:751–769
Ghashghaie J, Badeck FW, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochem Rev 2:145–161
Gilbert A, Silvestre V, Robins RJ, Tcherkez G, Remaud GS (2011) A 13C NMR spectrometric method for the determination of intramolecular δ13C values in fructose from plant sucrose samples. New Phytol 191:579–588
Gilbert A, Robins R, Remaud G, Tcherkez G (2012) Intramolecular 13C-pattern in hexoses from autotrophic and heterotrophic C3 plant tissues: causes and consequences. Proc Natl Acad Sci USA 109:18204–18209
Givnish TJ (1995) Plants stems: biomechanical adaptation for energy capture and influence on species distribution. In: Gartner BL (ed) Plant stems: physiology and functional morphology. Academic Press, San Diego, pp 3–49
Gleixner G, Schmidt HL (1997) Carbon isotope effects on the fructose-1,6-bisphosphate aldolase reaction, origin for non-statistical 13C distribution in carbohydrates. J Biol Chem 272:5382–5387
Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415:451–454
Janeczko A, Biesaga-Kościelniak J, Oklešt′ková J, Filek M, Dziurka M, Szarek-Łukaszewska G, Kościelniak J (2010) Role of 24-epibrasenoide in wheat production: physiological effects and uptake. J Agron Crop Sci 196:311–321
Kallarackal J, Bauer SN, Nowak H, Hajirezaei M-R, Komor E (2012) Diurnal changes in assimilate concentrations and fluxes in the phloem of castor bean (Ricinus communis L.) and tansy (Tanacetum vulgare L.). Planta 236:209–223
Kharouk VI, Middleton EM, Spencer SL, Rock BN, Williams DL (1995) Aspen bark photosynthesis and its significance to remote-sensing and carbon budget estimates in the boreal ecosystem. Water Air Soil Pollut 82:483–497
Kocurek M, Pilarski J (2011) Activity of C4 enzymes in C3-type herbaceous plants. Photosynthetica 49:473–477
Kocurek M, Pilarski J (2012) Implication of stem structures for photosynthetic functions in select herbaceous plants. Pol J Environ Stud 21:1687–1696
Kocurek M, Kornas A, Pilarski J, Tokarz K, Lüttge U, Miszalski Z (2015) Photosynthetic activity of stems in two Clusia species. Trees Struct Funct 29:1029–1040
Kuźniak E, Kornas A, Kaźmierczak A, Rozpądek P, Nosek M, Kocurek M, Zellnig G, Müller M, Miszalski Z (2016) Photosynthesis-related characteristics of the midrib and the interveinal lamina in leaves of the C3-CAM intermediate plant Mesembryanthemum crystallinum. Ann Bot 117:1141–1151
Lüttge U (2008) Clusia: Holy Grail and enigma. J Exp Bot 59:1503–1514
Maier CA, Clinton BD (2006) Relationship between stem CO2 efflux, stem sap velocity and xylem CO2 concentration in young loblolly pine trees. Plant Cell Environ 29(8):1471–1483
Malec-Czechowska K, Wierzchnicki R (2013) A study of stable isotope composition of chosen foodstuffs from the Polish market. Nukleonika 58:323–327
Manetas Y (2004) Probing corticular photosynthesis through in vivo chlorophyll fluorescence measurements: evidence that high internal CO2 levels suppress electron flow and increase the risk of photoinhibition. Physiol Plant 120:509–517
Miszalski Z, Skoczowski A, Silina E, Dymova O, Golovko T, Kornas A, Strzalka K (2016) Photosynthetic activity of vascular bundles in Plantago media leaves. J Plant Physiol 204:36–43
Miszalski Z, Kornas A, Kuźniak E (2017) Photosynthesis-related functions of vasculature-associated chlorenchymatous cells. In: Cánovas F, Lüttge U, Matyssek R (eds) Progress in botany, vol 79. Springer, Cham, pp 173–196
Moore DM, Gonzalez-Meler MA, Taneva L, Pippen JS, Kim HS, Delucia EH (2008) The effect of carbon dioxide enrichment on apparent stem respiration from Pinus taeda L. is confounded by high levels of soil carbon dioxide. Oecologia 158(1):1–10
Paul M, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400
Pfanz H (1999) Photosynthetic performance of twigs and stems of trees with and without stress. Phyton 39:29–33
Pfanz H, Aschan G (2001) The existence of bark and stem photosynthesis in woody plants and its significance for the overall carbon gain. An eco-physiological and ecological approach. Prog Bot 62:477–510
Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M (2002) Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften 89:147–162
Pilarski J, Tokarz K, Kocurek M (2008) Optical properties of the cork stems and trunks of beech (Fagus sylvatica L.). Pol J Environ Stud 17:773–779
Rossmann A, Butzenlechner M, Schmidt H-L (1991) Evidence for a nonstatistical carbon isotope distribution in natural glucose. Plant Physiol 96:609–614
Saveyn A, Steppe K, Lemeur R (2007) Drought and the diurnal patterns of stem CO2 efflux and xylem CO2 concentration in young oak (Quercus robur). Tree Physiol 27:365–375
Saveyn A, Steppe K, Ubierna N, Dawson TE (2010) Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant Cell Environ 33:1949–1958
Sharkey PJ, Pate JS (1976) Translocation from leaves to fruits of a legume, studied by a phloem bleeding technique: diurnal changes and effects of continuous darkness. Planta 128(1):6
Spicer R, Holbrook NM (2005) Within-stem oxygen concentration and sapflow in four temperate tree species: does long-lived xylem parenchyma experience hypoxia? Plant Cell Environ 28:192–201
Steppe K, De Pauw DJW, Lemeur R, Vanrolleghem PA (2005) A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol 26:257–273
Tcherkez G, Farquhar G, Badeck F, Ghashghaie J (2004) Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Funct Plant Biol 31:857–877
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Vandegehuchte MW, Bloemen J (2015) Woody tissue photosynthesis in trees: salve on the wounds of drought? New Phytol 208(4):998–1002
Walter A, Schurr U (2005) Dynamics of leaf and root growth—endogenous control versus environmental impact. Ann Bot 95:891–900
Werner C, Badeck FW, Brugnoli E, Cohn B, Cuntz M, Dawson T, Gessler A, Ghashghaie J, Grams TEE, Kayler Z, Keitel C, Lakatos M, Lee X, Máguas C, Ogée J, Rascher KG, Schnyder H, Siegwolf R, Unger S, Welker J, Wingate L, Zeeman MJ (2012) Linking carbon and water cycles using stable isotopes across scales: progress and challenges. Biogeosciences 9:3083–3111
Wild B, Wanek W, Postl W, Richter A (2010) Contribution of carbon fixed by Rubisco and PEPC to phloem export in the Crassulacean acid metabolism plant Kalanchoë daigremontiana. J Exp Bot 61(5):1375–1383
Wittmann C, Pfanz H (2016) The optical, absorptive and chlorophyll fluorescence properties of young stems of five woody species. Environ Exp Bot 121:83–93
Wittmann C, Pfanz H (2018) More than just CO2-recycling: corticular photosynthesis as a mechanism to reduce the risk of an energy crisis induced by low oxygen. New Phytol 219:551–564
Wittmann C, Pfanz H, Loreto F, Centritto M, Pietrini F, Alessio G (2006) Stem CO2 release under illumination: corticular photosynthesis, photorespiration or inhibition of mitochondrial respiration? Plant Cell Environ 29:1149–1158
Yiotis C, Manetas Y (2010) Sinks for photosynthetic electron flow in green petioles and pedicels of Zantedeschia aethiopica: evidence for innately high photorespiration and cyclic electron flow rates. Planta 232:523–531
Yiotis C, Petropoulou Y, Manetas Y (2009) Evidence for light-independent and steeply decreasing PSII efficiency along twig depth in four tree species. Photosynthetica 47:223–231
Acknowledgements
This research was financially supported by the Ministry of Science and Higher Education of the Republic of Poland. Additional funding is also greatly appreciated: the support to Z. Miszalski by the Alexander von Humboldt Foundation (AvH Stiftung).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Guy.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kocurek, M., Kornas, A., Wierzchnicki, R. et al. Importance of stem photosynthesis in plant carbon allocation of Clusia minor. Trees 34, 1009–1020 (2020). https://doi.org/10.1007/s00468-020-01977-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-020-01977-w