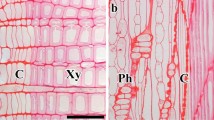
Abstract
Key message
A better understanding of the influence of environmental conditions on wood formation should help to improve the radial growth of trees and to prepare for climate change.
Abstract
The cambial activity of trees is associated with seasonal cycles of activity and dormancy in temperate zones. The timing of cambial reactivation in early spring and dormancy in autumn plays an important role in determination of the cambial growth and the environmental adaptivity of temperate trees. This review focuses on the temperature regulation of the timing of cambial reactivation and xylem differentiation and highlights recent advances of bud growth in relation to cambial activity of temperate trees. In addition, we discuss relationships between the timing of cambial reactivation, start of xylem differentiation and changes in levels of storage materials to identify the source of the energy required for cell division and differentiation. We also present a summary of current understanding of the effects of rapid increases and decreases in temperature on cambial activity, by localized heating and cooling, respectively. Increases in temperature from late winter to early spring influence the physiological processes that are involved in the initiation of cambial reactivation and xylem differentiation both in localized heated stems and under natural conditions. Localized cooling has a direct effect on cell expansion, the thickening of walls of differentiating tracheids, and the rate of division of cambial cells. A rapid decrease in temperature of the stem might be the critical factor in the control of latewood formation and the cessation of cambial activity. Therefore, temperature is the main driver of cambial activity in temperate trees and trees are able to feel changes in temperature through the stem. The climate change might affect wood formation in trees.





Similar content being viewed by others
References
Aloni R (1991) Wood formation in deciduous hardwood trees. In: Raghavendra AS (ed) Physiology of trees. Wiley, New York, pp 175–197
Antonova GF, Stasova VV (1997) Effects of environmental factors on wood formation in larch (Larix sibirica Ldb.) stems. Trees 11:462–468
Arend M, Fromm J (2003) Ultrastructural changes in cambial cell derivatives during xylem differentiation in poplar. Plant Biol 5:255–264
Barnett JR (1992) Reactivation of the cambium in Aesculus hippocastanum L.: a transmission electron microscope study. Ann Bot 70:169–177
Barnett JR, Miller H (1994) The effect of applied heat on graft union formation in dormant Picea sitchensis (Bong.) Carr. J Exp Bot 45:135–143
Beeckman H (2016) Wood anatomy and trait-based ecology. IAWA J 37:127–151
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2007) Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Ann Bot 100:39–447
Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R (2008) Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiol 28:1813–1819
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2010a) Cambial sensitivity to rising temperatures by natural condition and artificial heating from late winter to early spring in the evergreen conifer Cryptomeria japonica. Trees 24:43–52
Begum S, Nakaba S, Oribe Y, Kubo T, Funada R (2010b) Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Ann Bot 106:885–895
Begum S, Shibagaki M, Furusawa O, Nakaba S, Yamagishi Y, Yoshimoto J, Jin HO, Sano Y, Funada R (2012a) Cold stability of microtubules in wood-forming tissues of conifers during seasons of active and dormant cambium. Planta 235:65–179
Begum S, Nakaba S, Yamagishi Y, Yamane K, Islam MdA, Oribe Y, Ko JH, Jin HO, Funada R (2012b) A rapid decrease in temperature induces latewood formation in artificially reactivated cambium of conifer stems. Ann Bot 110:875–885
Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R (2013) Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiol Plant 147:46–54
Begum S, Kudo K, Matsuoka Y, Nakaba S, Yamagishi Y, Nabeshima E, Rahman MH, Nugroho WD, Oribe Y, Jin HO, Funada R (2016) Localized cooling of stems induces latewood formation and cambial dormancy during seasons of active cambium in conifers. Ann Bot 117:465–477
Catesson AM (1994) Cambial ultrastructure and biochemistry: changes in relation to vascular tissue differentiation and the seasonal cycle. Int J Plant Sci 155:251–261
De Micco V, Campelo F, De Luis M, Bräuning A, Grabner M, Battipaglia G, Cherubini P (2016) Intra-annual density fluctuations in tree rings: how, when, where and why? IAWA J 37:232–259
Denne MP, Dodd RS (1981) The environmental control of xylem differentiation. In: Barnett JR (ed) Xylem cell development. Castle House, Tunbridge Wells, pp 236–255
Deslauriers A, Rossi S, Anfodillo T, Saracino A (2008) Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol 28:863–871
Deslauriers A, Beaulieu M, Balducci L, Giovannelli A, Gagnon MJ, Rossi S (2014) Impact of warming and drought on carbon balance related to wood formation in black spruce. Ann Bot 114:335–345
Deslauriers A, Huang JG, Balducci L, Beaulieu M, Rossi S (2016) The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiol 170:2072–2084
Dié A, Kitin P, Kouamé FN, Van den Bulcke J, Van Acker J, Beeckman H (2012) Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann Bot 110:861–873
Dünisch O (2010) Low night temperatures cause reduced tracheid expansion in Podocarpus latifolius. IAWA J 31:245–255
Farrar JJ, Evert RF (1997a) Seasonal changes in the ultrastructure of the vascular cambium of Robinia pseudoacacia. Trees 11:191–202
Farrar JJ, Evert RF (1997b) Ultrastructure of cell division in fusiform cells of the vascular cambium of Robinia pseudoacacia. Trees 11:203–215
Frankenstein C, Eckstein D, Schmitt U (2005) The onset of cambium activity—a matter of agreement? Dendrochronologia 23:57–62
Fritts HC (1976) Tree rings and climate. Academic Press, New York
Funada R (2008) Microtubules and the control of wood formation. In: Nick P (ed) Plant microtubules: microtubules and the control of wood formation. Springer, Heidelberg, pp 83–119
Funada R, Catesson AM (1991) Partial cell wall lysis and the resumption of meristematic activity in Fraxinus excelsior cambium. IAWA Bull 12:439–444
Funada R, Kubo T, Fushitani M (1987a) Relationship between patterns of latewood formation and levels of endogenous auxin in the trunk of sugi (Cryptomeria japonica). Mokuzai Gakkaishi 33:253–260
Funada R, Sugiyama T, Kubo T, Fushitani M (1987b) Determination of indole-3-acetic acid levels in Pinus densiflora using the isotope dilution method. Mokuzai Gakkaishi 33:83–87
Funada R, Kubo T, Fushitani M (1990) Early and latewood formation in Pinus densiflora trees with different amounts of crown. IAWA Bull 11:281–288
Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M (2001) Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora stems in relation to earlywood-latewood transition and cessation of tracheid production. Holzforschung 55:128–134
Funada R, Kubo T, Sugiyama T, Fushitani M (2002) Changes in levels of endogenous plant hormones in cambial regions of stems of Larix kaempferi at the onset of cambial activity in springtime. J Wood Sci 48:75–80
Funada R, Yamagishi Y, Begum S, Kudo K, Nabeshima E, Nugroho WD, Rahman MH, Oribe Y, Nakaba S (2016) Xylogenesis in trees: from cambial cell division to cell death. In: Kim YS, Funada R, Singh AP (eds) Secondary xylem biology: origins, functions and applications. Elsevier, Academic Press, New York, pp 25–43
Gindl W, Grabner M (2000) Characteristics of spruce (Picea abies L. Karst) latewood formed under abnormal low temperatures. Holzforschung 54:9–11
Gričar J, Zupančič M, Čufar K, Koch G, Schmitt U, Oven P (2006) Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Ann Bot 97:943–951
Gričar J, Zupančič M, Čufar K, Oven P (2007) Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci Technol 41:463–475
Heide OM, Prestrud AK (2005) Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol 25:109–114
Hölttä T, Mäkinen H, Nöjd P, Mäkelä A, Nikinmaa E (2010) A physiological model of softwood cambial growth. Tree Physiol 30:1235–1252
Jenkins PA (1975) Influence of temperature change on wood formation in Pinus radiata grown in controlled environments. N Z J Bot 13:579–592
Jenkins PA, Shepherd KR (1972) Influence of temperature on cambial activity and cell diameter in Pinus radiata D. Don. J Ins Wood Sci 6:36–39
Kalcsits LA, Silim S, Tanino K (2009) Warm temperature accelerates short photoperiod-induced growth cessation and dormancy induction in hybrid poplar (Populus × spp.). Trees 23:971–979
Kitin P, Funada R (2016) Earlywood vessels in ring-porous trees become functional for water transport after bud burst and before the maturation of the current-year leaves. IAWA J 37:315–331
Krause C, Rossi S, Thibeault-Martel M, Plourde PY (2010) Relationship of climates and cell features in stems and roots of black spruce and balsam fir. Ann For Sci 67:402–408
Kudo K, Nabeshima E, Begum S, Yamagishi Y, Nakaba S, Oribe Y, Yasue K, Funada R (2014) The effects of localized heating and disbudding on cambial reactivation and earlywood vessels in seedlings of the deciduous ring-porous hardwood, Quercus serrata. Ann Bot 113:1021–1027
Kudo K, Yasue K, Hosoo Y, Funada R (2015) Relationship between formation of earlywood vessels and leaf phenology in two ring-porous hardwoods, Quercus serrata and Robinia pseudoacacia, in early spring. J Wood Sci 61:455–464
Kurczynska EU, Dmuchowski W, Wloch W, Bytnerowicz A (1997) The influence of air pollutants on needles and stems of Scots pine (Pinus sylvestris L.) trees. Environ Pollut 98:325–334
Lachaud S (1989) Participation of auxin and abscisic acid in the regulation of seasonal variations in cambial activity and xylogenesis. Trees 3:125–137
Lachaud S, Catesson AM, Bonnemain JL (1999) Structure and functions of the vascular cambium. CR Acad Sci Paris 322:633–650
Larson PR (1964) Some indirect effects of environment on wood formation. In: Zimmermann MH (ed) The formation of wood in forest tress. Academic Press, New York, pp 345–365
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin, Heidelberg, New York
Li X, Liang E, Gricar J, Prislan P, Rossi S, Cufar K (2013) Age-dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol 33:48–56
Li X, Rossi S, Liang E, Camarero JJ (2016) Temperature thresholds for the onset of xylogenesis in alpine shrubs on the Tibetan Plateau. Trees 30:2091–2099
Little CHA, Bonga JM (1974) Rest in the cambium of Abies balsamea. Can J Bot 59:342–348
Little CHA, Wareing PF (1981) Control of cambial activity and dormancy in Picea sitchensis by indole-3-acetic acid and abscisic acid. Can J Bot 59:1480–1493
Lugo JB, Deslauriers A, Rossi S (2012) Duration of xylogenesis in black spruce lengthened between 1950 and 2010. Ann Bot 110:1099–1108
Lupi C, Morin H, Deslauriers A, Rossi S (2010) Xylem phenology and wood formation: resolving the chicken-or-egg dilemma. Plant Cell Environ 33:1721–1730
Mellerowicz EJ, Coleman WK, Riding RT, Little CHA (1992) Periodicity of cambial activity in Abies balsamea. I. Effects of temperature and photoperiod on cambial dormancy and frost hardiness. Physiol Plant 85:515–525
Nabeshima E, Kubo T, Yasue K, Hiura T, Funada R (2015) Changes in radial growth of earlywood in Quercus crispula between 1970 and 2004 reflect climate change. Trees 29:1273–1281
Oribe Y, Kubo T (1997) Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiol 17:81–87
Oribe Y, Funada R, Shibagaki M, Kubo T (2001) Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta 212:684–691
Oribe Y, Funada R, Kubo T (2003) Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees 17:185–192
Rahman MH, Begum S, Nakaba S, Yamagishi Y, Kudo K, Nabeshima E, Nugroho WD, Oribe Y, Funada R (2016) Relationship between the earlywood-to-latewood transition and changes in levels of stored starch around the cambium in locally heated stems of the evergreen conifer Chamaecyparis pisifera. Trees 30:1619–1631
Rensing KH, Samuels AL (2004) Cellular changes associated with rest and quiescence in winter-dormant vascular cambium of Pinus contorta. Trees 18:373–380
Riding RT, Little CHA (1984) Anatomy and histochemistry of Abies balsamea cambial zone cells during the onset and breaking of dormancy. Can J Bot 62:2570–2579
Riding RT, Little CHA (1986) Histochemistry of the dormant vascular cambium of Abies balsamea: changes associated with tree age and crown position. Can J Bot 64:2082–2087
Rohde A, Bastien C, Boerjan W (2011) Temperature signals contribute to the timing of photoperiodic growth cessation and bud set in poplar. Tree Physiol 31:472–482
Rossi S, Deslauriers A, Anfodillo T (2007) Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152:1–12
Rossi S, Deslauriers A, Anfodillo T, Carrer M (2008a) Age-dependent xylogenesis in timberline conifers. New Phytol 177:199–208
Rossi S, Deslauriers A, Griçar J, Seo JW, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R (2008b) Critical temperatures for xylogenesis in conifers of cold climates. Global Eco Biol 17:696–707
Rossi S, Morin H, Deslauriers A, Plourde PY (2011) Predicting xylem phenology in black spruce under climate warming. Global Change Biol 17:614–625
Rossi S, Morin H, Deslauriers A (2012) Causes and correlations in cambium phenology: towards an integrated framework of xylogenesis. J Exp Bot 63:2117–2126
Rossi S, Girard MJEE, Morin H (2014) Lengthening of the duration of xylogenesis engenders disproportionate increases in xylem production. Global Change Biol 20:2261–2271
Samuels AL, Kaneda M, Rensing KH (2006) The cell biology of wood formation: from cambial divisions to mature secondary xylem. Can J Bot 84:631–639
Savidge RA, Wareing PF (1981) Plant growth regulators and the differentiation of vascular elements. In: Barnett JR (ed) Xylem cell development. Castle House, London, pp 192–235
Savidge RA, Wareing PF (1982) Apparent auxin production and transport during winter in the non-growing pine tree. Can J Bot 60:681–691
Schmitt U, Jalkanen R, Eckstein D (2004) Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn 38:167–178
Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Nat Acad Sci USA 100:10096–10101
Schweingruber FH (1988) Tree rings: basics and applications of dendrochronology. Reidel Publishing Company, Dordrecht
Seo JW, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U (2008) Estimating the onset of cambial activity in scots pine in northern Finland by means of the heat-sum approach. Tree Physiol 28:105–112
Shepherd KR (1964) Some observations on the effect of drought on the growth of Pinus radiata D. Don. Aust Forestry 28:7–22
Shestakovaa TA, Gutiérreza E, Kirdyanovb AV, Camarerod JJ, Génovae M, Knorre AA, Linaresf JC, Dios VRD, Salguero RS, Voltas J (2016) Forests synchronize their growth in contrasting Eurasian regions in response to climate warming. Proc Nat Acad Sci USA 113:662–667
Simard S, Giovannelli A, Treydte K, Traversi ML, King GM, Frank D, Fonti P (2013) Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiol 33:913–923
Sundberg B, Little CHA, Riding RT, Sandberg G (1987) Levels of endogenous indole-3-acetic acid in the vascular cambium region of Abies balsamea trees during the activity-rest-quiescence transition. Physiol Plant 71:163–170
Sundberg B, Little CHA, Cui K, Sandberg G (1991) Level of endogenous indole-3-acetic acid in the stem of Pinus sylvestris in relation to the seasonal variation of cambial activity. Plant Cell Environ 14:241–246
Sundberg B, Uggla C, Tuominen H (2000) Cambial growth and auxin gradients. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific Publishers, Oxford, pp 169–188
Svendsen E, Wilen R, Stevenson R, Liu R, Tanino K (2007) A molecular marker associated with low-temperature induction of dormancy in red osier dogwood (Cornus sericea L.). Tree Physiol 27:385–397
Timell TE (1986) Formation of compression wood. In: Compression wood in gymnosperms 1. Springer, Berlin, Heidelberg, New York, Tokyo
Utsumi Y, Sano Y, Funada R, Ohtani J, Fujikawa S (2003) Seasonal and perennial changes in the distribution of water in the sapwood of conifers in a sub-frigid zone. Plant Physiol 131:1826–1833
Yasue K, Funada R, Fukazawa K, Ohtani J (1997) Tree-ring width and maximum density of Picea glehnii as indicators of climatic changes in northern Hokkaido, Japan. Can J For Res 27:1962–1970
Yasue K, Funada R, Kobayashi O, Ohtani J (2000) The effects of tracheid dimensions on variations in maximum density of Picea glehnii and relationships to climatic factors. Trees 14:223–229
Young PJ, Megonigal JP, Sharitz RR, Day FP (1993) False ring formation in baldcypress (Taxodium distichum) saplings under two flooding regimes. Wetlands 13:293–298
Ziaco E, Biondi FB, Heinrich I (2016) Wood cellular dendroclimatology: testing new proxies in great basin bristlecone pine. Front Plant Sci 7:1602
Acknowledgements
This work was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Nos. 19580183, 20120009, 205659, 21380107, 2200104, 23380105, 24380090, 15K07508, 15H04527 and 16K14954).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
Additional information
Communicated by M. Buckeridge.
Rights and permissions
About this article
Cite this article
Begum, S., Kudo, K., Rahman, M.H. et al. Climate change and the regulation of wood formation in trees by temperature. Trees 32, 3–15 (2018). https://doi.org/10.1007/s00468-017-1587-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1587-6