Abstract
Subalpine conifers are highly sensitive to climatic changes. In these trees, the intra-annual dynamics of cambial activity and phenological process in xylem development are closely associated with climatic conditions. However, these scientific findings have not been verified for subalpine conifers in the Republic of Korea. Therefore, we initiated such a study with four subalpine conifers, viz. Abies koreana, Pinus koraiensis, Taxus cuspidata, and Picea jezoensis, growing between 1573 and 1594 m a.s.l. at Deogyusan National Park. Microcores (Ø 2 mm) of these trees were obtained using a mini borer, called as Trephor, every week between April 7 and September 25 in 2017 to monitor their growing seasons, the intra-annual dynamics of the cambial activity, and the number of cells during phenological phases of cell enlargement and cell-wall thickening. For the study, five trees were selected for each conifer species. Results showed that the cambial activity of A. koreana, P. koraiensis, and P. jezoensis required at least 73.8 heat-sum values, whereas the T. cuspidata needed 109.6 heat-sum. The durations of cambial activity of A. koreana, P. koraiensis, T. cuspidata, and P. jezoensis were 134 (127–144), 113 (92–128), 113 (106–120), and 100 (76–128) days, respectively. The intra-annual variations of the number of cells in the cambium, during the cell enlargement phase and cell-wall thickening phase showed predominantly a bell-shaped curve, with a delay of approximately 2–4 weeks between each other. On the other hand, the number of cumulated mature cells showed an S-shaped curve. Through this study, the first fundamental data on phenological process in xylem development of subalpine conifers in the Republic of Korea have been successfully presented.
Similar content being viewed by others
Introduction
The total forest area in the Republic of Korea is 6383000 ha which corresponds to 64% of the total land of Korea (Korea Forest Service 2018). Furthermore, the net absorption of greenhouse gases due to land use, land use change and by the forestry (LULUCF) sector is 41.6 million metric tons CO2 eq. or 5.9% of the total national emissions in 2017, with forests accounting for 99.9% of the total absorption [1]. Therefore, it can be seen that trees, the main component of forests, play the most vital role in reducing the vast amount of carbon emissions from the atmosphere [2, 3]. However, the trees growing at high elevations and/or latitudes face mortality risk and extinction threat due to the ever-increasing global warming and drought risks due to climatic changes [4,5,6,7,8].
Subalpine forest, with harsh conditions for tree growth [9, 10], serves as an ecotone between mixed deciduous forest and alpine vegetation communities [11]. In the Republic of Korea, the subalpine forests start from 800 to 1000 m a.s.l. [12], and trees such as Abies koreana, Abies nephrolepis, Picea jezoensis, Juniperus chinensis, Pinus pumila and Taxus cuspidata dominantly occupy the forests [13,14,15,16]. Although the conifer species in the subalpine zone can grow under extreme growth conditions, these species are vulnerable to the effect of rapid climate change [7, 17,18,19]. In a recent publication [16], it has been reported that the area of conifers in the subalpine zones of the Republic of Korea had reduced by 25%, from 93.27 to 69.90 km2, over the last 21 years (1994–2015).
Deogyusan, the fourth highest mountain peak (1614 m a.s.l.) of the Republic of Korea, is one of the important habitats of plants, containing 811 different plant species [20]. The Deogyusan National park serves as a habitat for subalpine conifers; however, their spread is only restricted within 3 km2 [12]. Although the subalpine conifers are under the threat of extinction due to climatic change, surprisingly, only a handful of studies have been done so far on the climatic effect on their growth or growth strategy. Moreover, most of the research focused on the subalpine ecosystem [21,22,23].
Tree growth mainly occurs due to the activities of meristem tissues, viz. apical meristem and vascular cambium [24, 25]. The growth of shoot is induced by the former and is an externally visible phenology, whereas radial growth is due to the latter and remains hidden [26, 27]. The radial growth integrates exogenous influences as well as endogenous factors, which are archived in each annual ring [28, 29]. Unlike the annual rings, the cambial activity and xylem formation can be monitored in a short time rather than a growing season [30,31,32,33]. Based on these advantages, researchers explored the intra-annual dynamics of cambium, the xylem phenology and their growth rates to achieve a deeper insight into the tree-growth strategy depending upon climate [32, 34,35,36,37].
Trees at cold temperatures, such as subalpines, are particularly vulnerable to frost damage during winter season at the beginning and cessation of meristem tissue growth [10, 38,39,40]. Therefore, temperature plays an important role in activating their growth. A large volume of research [41,42,43] confirmed that trees growing under cold conditions can initiate cambial activity with lower heat-sum value than those in warmer environment. The heat-sum value is the amount of heat accumulated by a plant during its growth or up to its the maturity [44]. Furthermore, the trees in cold environment have a shorter growing season than those at warmer locations [45,46,47,48,49,50,51]. Some research groups presented that even if the growing conditions remain the same, the growing seasons do not remain the same and vary according to tree species [32, 46, 50,51,52,53]. However, these general findings, have not been verified to-date for the conifer trees in the subalpine zones of the Republic of Korea. Therefore, the present study aimed to monitor the intra-annual variations of the cambial activity and xylem phenology for some dominant subalpine conifer species (Abies koreana, Pinus koraiensis, Taxus cuspidata, and Picea jezoensis) at the Deogyusan National Park in the Republic of Korea, which are facing the threat of disappearance due to continuous climate change. To evaluate the influence of temperature on the initiation of their cambial activities, heat-sum values, the so-called degree-days, were applied. The results are expected to serve as fundamental data to evaluate the growth conditions of subalpine conifer tree species under climate change as well as to understand their growth phenology at the cellular level.
Materials and methods
Study sites and tree species
The study site was chosen between the peaks of the Hyangjeokbong (1614 m a.s.l.) and Jungbong (1594 m a.s.l.) in Deogyusan National Park (35°51ʹN, 127°44ʹE) (Fig. 1). The natural vegetation between the Hyangjeokbong and Jungbong peaks consists of Quercus mongolica, Pinus koraiensis, Taxus cuspidata, Abies koreana, and Rhododendron schlippenbachii. Among these, the tree species older than 100 years are Taxus cuspidata and Abies koreana at Hyangjeokbong [54, 55]. As recorded by a meteorological station close to the study site, the mean annual temperature and total precipitation during 2011–2017 were 5.2 °C and 1677 mm, respectively (Fig. 2). The coldest and warmest months were January (− 9.6 °C) and July (17.8 °C), whereas the driest and wettest months were January (54 mm) and August (348 mm), respectively.
In the present study, four conifer tree species, viz. Abies koreana, Taxus cuspidata, Picea jezoensis and Pinus koraiensis were selected for experiments. Among these, A. koreana, T. cuspidata and P. jezoensis were the representative subalpine species whose habitats are higher than 1300 m a.s.l. at the Deogyusan National Park. By contrast, the primary habitat of P. koraiensis is between low and high altitudes (550–1500 m a.s.l.), and only a few P. koraiensis are distributed at the study site [13, 56]. Considering the effect of size and age of the trees on the cambial activity [31, 57], the trees were selected according to their vitality and diameter at breast height. Due to limited population of each species at the study site, only five trees were monitored and no trees of the same age were selected (Table 1). The tree ages were verified using increment cores extracted from portions of the test trees at 1.2 m from the ground after finishing micro sampling at the end of October, 2017.
Microcore sampling and preparation
Microcores were extracted from the trees weekly using a Trephor [58] between April and September in 2017. To minimize any difference between the timings of cambial activity or xylem phenology due to sampling height [59], sampling was done from the same height, 1 m from the ground. Furthermore, to avoid any wounding effect from prior sampling action, the sampling points were kept separated by at least 2.5 cm horizontally direction in a zigzag pattern (Fig. 3a). Two microcores were weekly extracted from each tree, and a total of 1000 microcores were collected. In general, the microcores comprised the developing tree ring and 2 more previous tree rings.
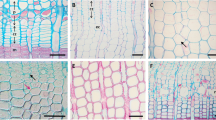
Sample preparation process. a collection of micro-samples using a Trephor, b cutting a cross-section of the embedded micro-samples in PEG2000 (polyethylene glycol 2000) using a sliding microtome of thickness 6–12 μm, and c observation of thin sections under light (left) and polarized microscopes (right) to identify the cambial zone and xylem cells during enlarging, cell-wall thickening, and mature phases (scale bars, 100 μm)
Immediately after extraction, the microcores were soaked in distilled water and after coming back to the laboratory, they were stored in refrigerator at 8 ˚C to prevent tissue deterioration before embedding [50]. Within 1 month, all the microcores were embedded within PEG 2000 (Polyethylene glycol) [30], followed by cutting transverse planes from them using a sliding microtome (H/I Sliding Microtome, Hacker, USA) of thickness 6–12 μm (Fig. 3b). The cut sections were next stained with a mixture of safranine (1%) and astra blue (0.5%) [49, 59,60,61] to visibly observe the cambium and the developing xylem cells under light microscope (Fig. 3c).
Microscopic observation
The cambial cells and the developing xylem cells were examined under a light and/or a polarized light microscope to observe the criteria reported in literature [30, 36, 37, 52, 62]. A cambial cell has a primary wall and a small radial diameter. Therefore, these cells were blue stained by astra blue due to absence of lignin in the primary wall (Fig. 4). On the other hand, the xylem cells have much larger radial diameter than the cambial cells during their enlargement stage, and possess only primary wall; so, they can be easily distorted and blue stained with astra blue. However, during the cell-wall thickening phase, the xylem cells thickens slightly and the walls become stiffer due to development of secondary walls. These characteristics can be easily observed as purple under light microscope and slightly glistened under polarized light microscope due to crystalline cellulose microfibrils with biaxial anisotropic structure in the secondary wall [63]. The mature cells are distinctly glistened under a polarized light microscope and normally appear red when stained by safranin, which stains the lignin content [64] formed on complete lignification in the secondary walls. During each developmental phase, the cells were counted every week along five radial files [36, 45, 65]. In the current study, the day of a year (DOY) for the initiation of cambial activity was set when an enlarging cell was first observed in the cross-section under light microscope. The cessation of cambial activity was marked when no more cell division in the cambial zone was observed and the youngest xylem cells start cell-wall thickening phase.
Heat sum
Heat sum, the so-called degree-days (hereafter d.d.), was calculated using the Sarvas model [66], as shown in Eq. 1:
where d.d. is the sum of \({T}_{\mathrm{Diff}}\), i.e., the sum of the differences between the daily mean temperature (\({T}_{i}\)) and the threshold of + 5 °C; \(j\) is the day of year (DOY) when the mean daily temperature is greater than or equal to the threshold for at least five consecutive days [41].
Results and discussion
Effects of degree-day (d.d.) on triggering the cambial activity
Among the experimental tree species, A. koreana and P. koraiensis initiated the cambial activity between beginning and middle of May (DOY 124–132), T. cuspidata in the middle of May (DOY 132) and P. jezoensis between beginning and end of May (DOY 124–146) (Table 2). Based on these observations, it was verified that A. koreana (three in five trees), P. koraiensis (one in five trees), and P. jezoensis (one in five trees) required at least 73.8 d.d. to trigger the cambial activity and T. cuspidata (all five trees) 109.6 d.d. (Table 2). To completely start the cambial activity in all the trees of each species, 109.6 d.d. was required for A. koreana, P. koraiensis and T. cuspidata, and 191.2 d.d. for P. jezoensis (Table 2).
The cambium cells are vulnerable meristematic tissues under cold condition [39]. Therefore, cambium cell division in the subalpine zones starts later than the trees at low elevations [33, 48, 67]. Prior studies stated that in the Republic of Korea [50, 52, 60], conifer trees, such as Chamaecyparis pisifera, Pinus koraiensis and Pinus densiflora, growing under 600 m a.s.l. at latitudes between 36°30ʹ and 37°88ʹ begin cell division between middle of March and middle of April. This is almost half a month or one and a half month earlier than the first cambial activities of the subalpine conifers (A. koreana, P. koraiensis and P. jezoensis) used in our study. The cambial activity in T. cuspidata begins around one or 2 months later than the conifers at low elevations (Table 2). By comparing with the previous studies, we verified in the present work that a time lag exists between the cambial activities in conifers of subalpine and low altitudes. To improve our understanding of the time lag effect according to altitudes, trees at different altitudes but of the same species were studied. Additionally, tree age was also considered, because it was reported that cambial activity starts later in conifers (Larix decidua, Pinus cembra and Picea abies) older than 200 years at the Alpine timber line than in the same tree species of age 50–80 years [31].
There is only one publication from Korea on the effect of d.d. on cambial activity [50], which reported that 3 of 3 P. koraiensis and 5 of 6 C. pisifera, located at the center of the Republic of Korea (N36°50ʹ, E128°03ʹ, 520–550 m a.s.l.), initiated the cambial activity between 134 d.d. and 200 d.d, respectively. This indicates that A. koreana, P. koraiensis and T. cuspidata in the subalpine require lower d.d. to trigger the cambial activity than those at lower altitudes (Table 2). The trees growing in cold environment must complete all its growth processes within a restricted growing season to survive the winter [41] and it is essential that they adapt to the low winter temperature. Although three out of five P. jezoensis initiated the cambial activity at lower d.d. than those at lower altitudes, the other two trees initiated it at higher d.d.. Therefore, to trigger the cambial activity, P. jezoensis required a higher d.d than A. koreana, P. koraiensis and T. cuspidata even at the same altitudes. To confirm this result, the dependence of cambial activity on the age of P. jezoensis has to be considered, as done in a previous study which reported that the cambial activity started later in old (200–350 yr) conifers (Larix. decidua, Pinus cembra and Picea abies) than the adult (50–82 yr) trees [31]. In the current study, such aging effect did not seem to play any role in triggering the cambial activity of T. cuspidata. The variations in the initiation days of cambial activity in the subalpine conifers were smaller than the variations in the cessation days (Fig. 5). This result signified that the conifers in the subalpine have similar strategies for using temperature in triggering their cambial activity but not the cessation. In most publications, the mean daily temperatures between 4 and 6 °C are suggested as critical temperatures for cold-adapted trees [46, 68,69,70].
Durations of cambial activity (grey bars) in Abies koreana (AK), Pinus koraiensis (PK), Taxus cuspidata (TC) and Picea jezoensis (PJ). The boxes with horizontal and vertical lines indicate when the cell-wall thickening begun the first time and the cell division ended in the cambial zone, respectively, while the black boxes indicate when cell-wall thickening ended completely
Duration of cambial activity
The durations of cambial activity (DCA) of A. koreana, P. koraiensis, T. cuspidata, and P. jezoensis were observed as 134 (127–144), 113 (92–128), 113 (106–120), and 100 (76–128) days, respectively (Fig. 6). The difference between the longest and shortest DCAs for T. cuspidata was 14 days, followed by A. koreana (17 days), P. koraiensis (36 days), and P. jezoensis (52 days).
The mean DCA of the conifer species at low altitudes in the Republic of Korea lies approximately in the range 145–210 days [50, 52, 60]. The mean DCA of the subalpine conifers at the Deogyusan National Park was at least 45 days shorter than that of the conifers at low altitudes. However, the mean DCA of the subalpine conifers was found to be similar to the DCAs of the conifers located higher than 1280 m a.s.l. in the north-western Himalayas in India, which is around 120–150 days [33], and at 3850 m a.s.l. in the south-eastern Tibetan Plateau in China which is around 119 days [71]. Through these comparisons, it was verified that the mean DCAs higher than ~ 1300 m a.s.l. were not over 150 days.
Observations under polarized light revealed that initiation of cell wall thickening in A. koreana and T. cuspidata started on DOYs 131 and 153, respectively (Fig. 5), whereas for P. koraiensis the DOY was between 131 and 153, and for P. jezoensis, the DOY was between 153 and 160. The initiation time of the cell wall thickening showed distinct differences depending upon the species. These differences are due to phenological variations in xylem cell development depending upon the tree species and tree age [31, 51, 71].
Intra-annual variations of the number of cells in the cambium and at each phenological phase in xylem development
The intra-annual variations in the number of cells in cambium, and the cells in enlargement phase and cell-wall thickening phase showed predominantly bell-shaped curves with the exception of cambial activity and cell differentiation dynamics in P. koraiensis, which are right skewed, with delay of approximately 2–4 weeks from each other (Fig. 6). The number of accumulated mature cells showed an S-shaped curve.
According to an earlier report [60], the initiation of cell-wall thickening in P. densiflora in western (E129°10ʹ–12ʹ, 478–1005 m a.s.l.) and eastern (E126°21ʹ, 27–80 m a.s.l.) Republic of Korea was observed around 1–4 weeks later than the initiation of the cambial activity. Such restricted time delay was also reported for conifers at high altitudes [33, 48, 71]. Furthermore, the time delay between the end of cell division and cell-wall thickening, and/or lignification could be relatively larger, because increase in cell-wall thickness and lignification can occur when temperature is warm enough in autumn for xylem development [72]. Therefore, the time delay in initiations between the cambial activity and cell-wall thickening seems to rely more on a biological process than between the cessations of cambial activity and cell-wall thickening and/or lignification.
Several past studies reported that intra-annual xylem formation follows a sigmoid curve [32, 33, 36, 37, 48, 49, 53, 62, 73]. This intra-annual dynamics of wood-formation was confirmed as well in our study of coniferous tree species growing at a subalpine site in the Republic of Korea. However, due to different cambial activities along the stem circumference the number of mature xylem cells may decrease during the growing period.
Conclusions
Past studies confirmed that the area of subalpine conifers has decreased in the Republic of Korea due to climate change. To evaluate the effects of climate change on such decrease, the biological activities of the subalpine conifers were evaluated in the present study. In the current study, the first fundamental data on phenological process involved in xylem development of subalpine conifers in the Republic of Korea has been successfully presented. Based on the experimental results, our understanding on the phenological process in subalpine conifers, as well as their biological strategy to use heat sum value, the so-called degree days (d.d.), to trigger the cambial activity safely was improved. To further improve our understanding on the biological activity, studies considering age classes and altitudes are necessary. Furthermore, to obtain more reliable results, the number of sample trees should also be increased.
Abbreviations
- LULUCF:
-
land use change and by the forestry
- DBH:
-
diameter at breast height
- a.s.l.:
-
above see level
- P:
-
pith
- nP:
-
near pith
- d.d.:
-
degree-days
- DOY:
-
day of year
- DCA:
-
duration of cambial activity
- AK:
-
Abies koreana
- PK:
-
Pinus koraiensis
- TC:
-
Taxus cuspidata
- PJ:
-
Picea jezoensis
- Py:
-
xylem in the previous year
- MC:
-
mature cells
- WTC:
-
cells in cell-wall thickening phase
- EC:
-
cells in cell enlargement phase
- CA:
-
cambial cells
- Ph:
-
phloem
References
Greenhouse Gas Inventory and Research Center of Korea (2019) National greenhouse gas inventory report of Korea. (in Korean)
Lee N-Y (2010) Carbon cycle in terrestrial ecosystems – Net ecosystem production (NEP) in a forest. J National Park Res 1(3):163–168 (in Korean with English abstract)
Kim K-M, Lee J-B, Kim E-S, Park H-J, Roh Y-H, Lee S-H, Park K-H, Shin H-S (2011) Overview of research trends in estimation of forest carbon stocks based on remote sensing and GIS. J KAGIS 14(3):236–256 (in Korean with English abstract)
Rebetez M, Dobbertin M (2004) Climate change may already threaten Scots pine stands in the Swiss Alps. Theor Appl Climatol 79:1–9
Lindner M, Maroschek M, Netherer S, Kremer A, Barbati A, Garcia-Gonzalo J, Seidl R, Delzon S, Corona P, Kolström M, Lexer MJ, Marchetti M (2010) Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For Ecol Manage 259:698–709
Koo KA, Kong W-S, Nibbelink NP, Hopkinson CS, Lee JH (2015) Potential effects of climate change on the distribution of cold-tolerant evergreen broadleaved woody plants in the Korean Peninsula. PLoS ONE 10(8):e0134043
Park H-C, Moon G-S, Lee H, Lee NY (2020) A study on the spatial information and location environment of dead coniferous tree in subalpine zone in Jirisan National Park. Korean J Environ Ecol 34(1):42–54 (in Korean with English abstract)
Gomez-Pineda E, Sáenz-romero C, Ortega-Rodríguez JM, Blance-garcía A, Madrigal-Sánchez X, Lindig-Cisneros R, Lopez-Toledo L, Pedraza-Santos ME, Rehfeldt GE (2020) Suitable climatic habitat changes for Mexican conifers along altitudinal gradients under climatic change scenarios. Ecol Appl 30(2):e02041
Bisht VK, Kuniyal CP, Bhandari AK, Nautiyal BP, Prasad P (2014) Phenology of plants in relation to ambient environment in a subalpine forest of Uttarakhand, western Himalaya. Physiol Mol Biol Plants 20(3):399–403
Maruta E, Kubota M, Ikeda T (2020) Effects of xylem embolism on the winter survival of Abies veitchii shoots in an upper subalpine region of central Japan. Sci Reps 10:6594
Kimmins JP (2004) Forest ecology. In: Northcote TG, Harman GF (eds) Fishes and forestry: Worldwide watershed interactions and management. Wiley-Blackwell, New York
Park H-C, Lee H-Y, Lee N-Y, Lee H, Song J-Y (2019) Survey on the distribution of evergreen conifers in the major national park – a case study on Seoraksan, Odaesan, Taebaeksan, Sobaeksan, Deogyusan, Jirisan National Park. J National Park Res 10(2):1–8 (in Korean with English abstract)
Kong W-S (2004) Species composition and distribution of native Korean conifers. J Korean Geographical Society 39(4):528–543 ((in Korean with English abstract))
Kong W-S, Kim K, Lee S, Park H, Cho S-H (2014) Distribution of high mountain plants and species vulnerability against climate change. J Environ Impact Assess 23(2):119–136 (in Korean with English abstract)
Park H-C, Lee J-H, Lee G-G, Um G-J (2015) Environmental features of the distribution areas and climate sensitivity assessment of Korean fir and Khinghan fir. J Environ Impact Assess 24(3):260–277 (in Korean with English abstract)
Kim E-S, Lee J-S, Park G-E, Lim J-W (2019) Change of subalpine coniferous forest area over the last 20 years. J Korean Soc For Sci 108(1):10–20 ((in Korean with English abstract))
Li M-H, Yang J (2004) Effects of microsite on growth of Pinus cembra in the subalpine zone of the Austrian Alps. Ann For Sci 61:319–325
Kong W-S (2005) Selection of vulnerable indicator plants by global warming. Asia-Pac J Atmos Sci 41(2–1):263–273 (in Korean with English abstract)
Petitpierre B, Mcdougall K, Seipel T, Broennimann O, Guisan A, Kueffer C (2016) Will climate change increase the risk of plant invasion into mountains? Ecol Appl 26(2):530–544
Korea national park research institute (2012) Deogyusan National park natural resources survey (in Korean)
Gruber A, Zimmermann J, Wieser G, Oberhuber W (2009) Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann For Sci 66:503
Rossi S, Anfodillo T, Čufar K, Cuny H, Deslauriers A, Fonti P, Frank D, Gričar J, Gruber A, Huang J-G, Jyske T, Kašpar J, King G, Krause C, Liang E, Mäkinen H, Morin H, Nöjd P, Oberhuber W, Prislan P, Saracino RCBK, A, Swidrak I, Treml V, (2016) Pattern of xylem phenology in conifers of cold ecosystems at the Northern hemisphere. Glob Chang Biol 22:3804–3813
Begum S, Kudo K, Rahman M-H, Nakaba S, Yamagishi Y, Nabeshima E, Nugroho W-D, Oribe Y, Kitin P, Jin H-O, Funada R (2017) Climate change and the regulation of wood formation in trees by temperature. Trees. https://doi.org/10.1007/s00468-017-1587-6
Lanner R-M (2002) Why do trees live so long? Aging Res Rev 1:653–671
Prislan P, Čufar K, Koch G, Schmitt U, Gričar J (2013) Review of cellular and subcellular changes in the cambium. IAWA J 34(4):391–407
Seo J-W, Aalto T, Jalkanen R, Eckstein D, Schmitt U, Fromm J (2012) Bud break and intra-annual height growth dynamics of saplings and pole-stage trees of Scots Pine: Case study for a bereal forest in Northern Finland. Balt For 18(1):34
Seo J-W, Eckstein D, Olbrich A, Jalanen R, Salminen H, Schmitt U, Fromm J (2013) Climate control of wood formation: illustrated for Scots pine at its northern distribution limit. In: Fromm J (ed) Cellular aspects of wood formation. Springer, Berlin
Plomion C, Leprovost G, Stokes A (2001) Wood formation in trees. Plant Physiol 127:1513–1523
De Micco V, Carrer M, Rathgever CBK, Camarero JJ, Voltas J, Cherubini P, Battipaglia G (2019) From xylogenesis to tree rings: wood traits to investigate tree response to environmental changes. IAWA J 40(2):155–182
Seo J-W, Eckstein D, Schmitt U (2007) The pinning method: From pinning to data preparation. Dendrochronologia 25:79–86
Rossi S, Deslauriers A, Anfodillo T, Carrer M (2008) Age-dependent xylogenesis in timberline conifers. New Phytol 177:199–208
Cuny HE, Rathgeber CBK, Lebourgeois F, Fortin M, Fournier M (2012) Life strategies in intra-annual dynamics of wood formation: example of three conifer species in a temperate forest in north-east France. Tree Physiol 32:612–625
Malik R, Rossi S, Sukumar R (2020) Cambial phenology in Abies pindrow (Pinaceae) along an altitudinal gradient in northwestern Himalaya. IAWA J 41(2):186–201
Wilson JW (1970) The growing tree. The university of Massachusetts Press, Amherst MA
Denn MP, Dodd RS (1981) The environmental control of xylem differentiation. In: Barnett JR (ed) Xylem cell development. Tunbridge wells, London
Rossi S, Deslauriers A, Anfodillo T (2006) Assesment of cambial activity and xylogenesis by microsampling tree species: an example at the alpine timberline. IAWA J 27(4):383–394
Balducci L, Deslauriers A, Giovannelli A, Rossi S, Rathgeber CBK (2013) Effects of temperature and water deficit on cambial activity and woody ring features in Picea mariana saplings. Tree Physiol 33(10):1006–1017
Villalba R, Veblen TT, Ogden J (1994) Climatic influences on the growth of subalpine trees in the colorado front range. Ecology 75(5):1450–1462
Häkkinen R, Linkosalo T, Hari P (1995) Methods for combining phenological time series: application to bud brust in Birch (Betula pendula) in central Finland for the period 1896–1955. Tree Physiol 15:721–726
Splechtna BE, Dobry J, Klinka K (2000) Tree-ring characteristics of subalpine fir (Abies lasiocarpa (Hook.) Nutt.) in relation to elevation and climatic fluctuations. Ann For Sci 57:89–100
Seo J-W, Eckstein D, Jalkanen R, Rickebusch S, Schmitt U (2008) Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiol 28:105–112
Harrington CA, Gould PJ (2010) Modeling the effects of winter environment or dormancy release of Douglas-fir. For Ecol Manage 259:798–808
Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R (2013) Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiol Plant 147:46–54
Réaumur RA (1735) Observations du thermomètre faites à Paris pendant l’année 1735 comparées avec celles qui ont été faites sous la ligne, à l’Isle de France, à Alger, et en quelques-unes de nos îles de l’Amérique. Mémorie de I’Acad Roy des Sci 9: 545–576 (in French)
Deslauriers A, Morin H, Begin Y (2003) Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can J For Res 33:190–200
Rossi S, Deslauriers A, Gricar J, Seo J-W, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R (2008) Critical temperatures for xylogenesis in conifers of cold climates. Glob Ecol Biogeogr 17:696–707
Liang E, Eckstein D, Shao X (2009) Seasonal cambial activity of relict Chinese Pine at the northern limit of its natural distribution in North China – exploratory results. IAWA J 30(4):371–378
Moser L, FontiP BU, Esper J, LuterbacherJ FranzenJ, Frank D (2010) Timing and duration of European Larh growing season along altitudinal gradients in the Swiss Alps. Tree Physiol 30:225–233
Prislan P, Shmitt U, Koch G, Gričar J, Čufar K (2011) Seasonal ultrastructural changes in the cambial zone of beech (Fagus sylvatica) grown at two different altitudes. IAWA J 32(4):443–459
Seo J-W, Choi E-B, Ju J-D, Shin C-S (2017) The association of intra-annual cambial activities of Pinus koraiensis and Chamaecyparispisifera planted in Mt Worak with climatic factors. J Korean Wood Sc. Technol 45(1):43–52 (in Korean with English abstract)
Zeng Q, Rossi S, Yang B (2018) Effects of age and size on xylem phenology in two conifers of northwestern China. Front Plant Sci 8:2664
Kwon SM, Kim NH (2005) Annual ring formation of major wood species growing in Chuncheon, Korea (Ι) -The period of cambium activity. J Korean Wood Sci Technol 33(4):1–8 (in Korean with English abstract)
Thibeault-Martel M, Krause C, Morin H, Rossi S (2008) Cambial activity and intra-annual xylem formation in roots and stems of Abies balsamea and Picea mariana. Annal Bot 102:667–674
Kim H-S, Lee S-M, Song H-K (2011) Actual vegetation distribution status and ecological succession in the Deogyusan National Park. Korean J Environ Ecol 25(1):37–46
Han SH, Han SH, Yun CW (2016) Classification and characteristics of subalpine forest vegetation at Hyangjeukbong and Jungbong in Mt. Deogyusan J Korean Soc For Sci 105(1):48–62
Jang Y-S, Yang D-C, Chung D-J (2004) Vegetation structure of natural Taxus cuspidata forests in Mt. DuckYoo Korean J Plant Res 17(1):58–66 (in Korean with English abstract)
Rathgeber CBK, Rossi S, Bontemps JD (2011) Cambial activity related to tree size in a mature silver-fir plantation. Ann Bot 108:429–438
Rossi S, Anfodillo T, MenardiR, (2006) Trephor: a new tool for sampling microcores from stems. IAWA J 27(1):89–97
Anfodillo T, Deslauriers A, Menardi R, Tedoldi L, Petit G, Rossi S (2012) Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. J Exp Bot 63(2):837–845
Park S-Y, Eom C-D, Seo J-W (2015) Seasonal change of cambium activity of pine trees at different growth sites. J Korean Wood Sci Technol 43(4):411–420 (in Korean with English abstract)
Hubbe MA, Chandra RP, Dogu D, Van Velzen STJ (2019) Analytical staining of cellulosic materials: a review. BioResources 14(3):7387–7464
Vieira J, Rossi S, Campelo F, Nabais C (2014) Are neighboring trees in tune? wood formation in Pinus pinaster. Eur J For Res 133:41–50
Abraham Y, Elbaum R (2013) Quantification of microfibril angle in secondary cell walls at subcellular resolution by means of polarized light microscopy. New Phytol 197:1012–1019
Baldacci-Cresp F, Spriet C, Twyffels L, Blervacq A-S, Neutelings G, Baucher M, Hawkins S (2020) A rapid and quantitative safranin-based fluorescent microscopy method to evaluate cell wall lignification. Plant J 102:1074–1089
Skene DS (1969) The period of time taken by cambial derivatives to grow and differentiate into tracheids in Pinus radiata D. Don Annal Bot 33(2):253–262
Sarvas R (1972) Investigation on the annual cycle of development of forest trees. Active Period Communicationes Instituti Forestalis Fenniae 76:1–110
Ziaco E, Biondi F (2016) Tree growth, cambial phenology, and wood anatomy of limber pine at a Great Basin (USA) mountain observatory. Trees 30:1507–1521
Rossi S, Deslauriers A, Anfodillo T, Carraro V (2007) Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152:1–12
Kirdyanov A, Hughes M, Vaganov E, Schweingruber F, Silkin P (2002) The importance of early summer temperature and date of snow melt for tree growth in the Siberian Subarctic. Trees 17:61–69
Swidrak I, Gruber A, Kofler W, Oberhuber W (2011) Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiol 31:483–493
Li X, Liang E, Gričar J, Prislan P, Rossi S, Čufar K (2013) Age dependence of xylogenesis and its climatic sensitivity in Smith fir on the south-eastern Tibetan Plateau. Tree Physiol 33:48–56
Gričar J, Čufar K, Oven P, Schmitt U (2005) Differentiation of terminal latewood rracheids in silver fir trees during autumn. Ann Bot 95:959–965
Palombo C, Fonti P, Lasserre B, Cherubini P, Marchetti M, Tognetti R (2018) Xylogenesis of compression and opposite wood in mountain pine at a Mediterranean treeline. Annal For Sci 75:93
Acknowledgements
This study was supported by the National Park Research Institute (NPRI), Korea National Park Service (KNPS), Ministry of Environment (MOE) of the Republic of Korea, as the “The monitoring project of Ecosystem in National Park according to climate change”. We also appreciate the Deogyusan National Park Office for supporting the sampling.
Author information
Authors and Affiliations
Contributions
J-HP was a major contributor to write the manuscript and perform all statistical analyses as well as design the experiment. E-BC contributed to collect samples and prepare the samples for microscopic observation. H-CP and N-YL contributed to collect samples. J-WS managed the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing inteterest
The authors declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Park, JH., Choi, EB., Park, HC. et al. Intra-annual dynamics of cambial and xylem phenology in subalpine conifers at Deogyusan National Park in the Republic of Korea. J Wood Sci 67, 22 (2021). https://doi.org/10.1186/s10086-021-01950-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10086-021-01950-2