Abstract
Key message
A conspicuous ‘finger-like’ branching morphology is described for three arborescent Araliaceae species with a focus on the three-dimensional vascular bundle arrangement in leaf insertion and stem–branch attachment regions during ontogenetic development.
Abstract
The central aim of this study is to gain a deeper understanding of the structure and development in leaf insertions and stem–branch attachments of the arborescent Araliaceae species: Schefflera arboricola, Fatsia japonica and Polyscias balfouriana. Therefore, the vascular bundle arrangement in the leaf insertion zone and ontogenetic development of the stem–branch attachment after decapitation were analyzed, with a special focus on their conspicuous ‘finger-like’ branching morphology that, to our knowledge, is unique to the Araliaceae. Decortication of adult ramifications allows for a morphological analysis of the woody strands in the stem–branch attachment regions. Via high-resolution microscopy of serial thin-sections and 3D reconstructions, as well as cryotome sections, anatomical analysis was carried out of the course and arrangement of vascular bundles through leaf insertions and later developing ramifications, including a comparative analysis of the different ontogenetic stages. All three species investigated present a ‘finger-like’ branching morphology with variations in the number and arrangement of the woody strands. Thin-sectioning reveals a conspicuous pattern of leaf trace emergence from the main stem, proceeding into the leaf and the early developing ramifications. Vascular bundle derivatives contribute to the vascular integration of leaves and axillary buds. The described ‘finger-like’ branching morphology in the investigated Araliaceae species represents a sophisticated mode of vascular integration in leaf insertion zones and developing ramifications. In combination with forthcoming biomechanical experiments, this analysis shall serve as a basis for biomimetic translations into textile technology (fiber-reinforced branched composite materials) and civil engineering (optimization of branched building structures).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to branch is one of the key features in many woody plants, as it enables an optimal arrangement of photosynthetic tissues and the formation of a complex stem and crown. Vascular continuity and mechanical stability of ramifications have to be ensured in all ontogenetic phases and numerous optimized stem–branch attachments have evolved to meet these challenging demands. The analysis of the morphology, anatomy and biomechanics of botanical ramifications has become of increasing interest in recent years (Mattheck 1991; Niklas 1992; Burgert and Jungnikl 2004; Achim et al. 2006; Tomlinson et al. 2005; Müller et al. 2006; Jungnikl et al. 2009; Masselter and Speck 2011; Masselter et al. 2011, 2015, 2016; Theckes et al. 2011; Müller et al. 2013; Schwager et al. 2013; Haushahn et al. 2014). Comprehending the development of botanical ramifications and identifying the central concepts of their structure also allow for biomimetic implementations into technical fiber-reinforced materials.
A functional understanding of leaf insertion zones and stem–branch attachments requires the visualization of the course and arrangement of the continuous vascular system from the main stem into the leaf, axillary bud and side branches. Conspicuous ramifications with a high potential for biomimetic implementation have been found in dicotyledons, such as columnar cacti (Schwager et al. 2013), as well as in the monocotyledon genus Dracaena (Masselter et al. 2011; Haushahn et al. 2014; Hesse et al. 2016), in which the visualization of vascular bundle progression was performed via light microscopy (Haushahn et al. 2014) and magnetic resonance imaging (MRI) of the stem–branch attachment region (Masselter et al. 2015, 2016; Hesse et al. 2016). For the first time, MRI imaging allowed analysis of living ramifications under load and comparison with the unloaded situation (Masselter et al. 2015, 2016; Hesse et al. 2016).
To the best of our knowledge, dicotyledonous leaf insertion regions, ramifications or fiber arrangements other than those of columnar cacti (Schwager et al. 2013) and Opuntia (Bouakba et al. 2013) have not been investigated in the context of biomimetic applications to fiber technology, excluding research, for example, in the clothing industry, on branched fibers in so-called ‘plant-structured textile fabrics’ for optimizing water transport properties (Fan et al. 2007; Sarkar et al. 2009; Chen et al. 2012). Lignified vascular bundles in dicotyledons are mostly arranged in a distinct vascular cylinder from which they proceed into the branch (Shigo 1985; Slater and Harbinson 2010) and are not distributed in a parenchymatous matrix, as for example, shown in Dracaena (Haushahn et al. 2014; Hesse et al. 2016; Masselter et al. 2016). Nevertheless, the morphology and ontogenetic development of a coherent vascular system from a main stem into a side axis in dicotyledons is of high interest in the context of novel structures to be used in fiber-reinforced materials, as it often involves the contribution of various vascular bundle derivatives (Zimmermann and Tomlinson 1972; Larson 1983, 1984, 1986a, b; Tomlinson et al. 2005; Schwager et al. 2015) and may result in conspicuous branching morphologies, such as in the Araliaceae genus Schefflera (Tomlinson et al. 2005). In the present study, a special focus is laid on the analysis of the branching morphology of Schefflera ramifications which has been described by Tomlinson et al. (2005), as well as two related Araliaceae species. Decorticated ramifications display a ‘finger-like’ progression of woody segments (Fig. 1c–e, h–j, m–o), representing highly lignified vascular bundles, originally indicating cortical leaf traces (Tomlinson et al. 2005). These leaf traces are arranged in the same order and position as the later developing woody strands and form ‘templates’ for their development (Tomlinson et al. 2005). Tomlinson et al. (2005) have focused on the anatomy of leaf traces and undeveloped axillary buds in Schefflera, and furthermore proposed a conclusive schematic ontogenetic scenario of vascular branch attachment, via traces of subtending leaves and accessory bundles. However, time specification and validation by actual histological samples are lacking. This study will address the following questions, which are to be clarified within the context of leaf insertion vascularity and branch development in Schefflera and related Araliaceae genera:
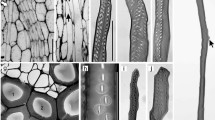
Growth habit and branching morphology of Schefflera arboricola, Fatsia japonica and Polyscias balfouriana. a Growth habit of S. arboricola. Examined ramification is highlighted with a white circle. b Ramification of S. arboricola. c Macerated and decorticated ramification of S. arboricola. d Top view of decorticated ramification of S. arboricola. e View of decorticated ramification of S. arboricola from underneath the branching. Exemplary scars of leaf traces are marked with black arrows. f Growth habit of F. japonica. Examined ramification is highlighted with a white circle. g Ramification of F. japonica. h Macerated and decorticated ramification of F. japonica. i Top view of decorticated ramification of F. japonica. j View of decorticated ramification of F. japonica from underneath the branching. Exemplary scars of leaf traces are marked with black arrows. k Growth habit of P. balfouriana. Examined ramification is highlighted with a white circle. l Ramification of P. balfouriana. m Macerated and decorticated ramification of P. balfouriana. n Top view of decorticated ramification of P. balfouriana. o View of decorticated ramification of P. balfouriana from underneath the branching. Exemplary scars of leaf traces are marked with black arrows. Scale bars a, f, k 10 cm; b–e, g–j, l–o 1 cm
-
1.
Morphological analysis: is the ‘finger-like’ ramification an exclusive trait of the genus Schefflera or is this morphology also detectable in the species Fatsia japonica and Polyscias balfouriana of the Araliaceae?
-
2.
Anatomical analysis: according to which three-dimensional pattern are the vascular bundles arranged in the region between the main stem, the leaf insertion and the axillary bud, as the origin of developing branches in Schefflera arboricola, F. japonica and P. balfouriana, 3 weeks after decapitation?
-
3.
Ontogenetic analysis: how are stem–branch attachments developing from leaf insertion regions in S. arboricola, F. japonica and P. balfouriana after branch development induced via apex decapitation? To what extent can the ontogenetic scheme of branch attachment proposed by Tomlinson et al. (2005) be substantiated by these experimental studies?
The analysis of ramifications in these three Araliaceae species (Fig. 1c–e, h–j, m–o) and the identification of fundamental functional principles of load-adapted fiber orientation shall also serve for a biomimetic translation of this conspicuous branching morphology. Our objective is to implement these functional principles into the development of biomimetic fiber-reinforced plastic (FRP) tubes as reinforcing coatings for branched concrete structures in architecture and constructional engineering (Born et al. 2016).
Materials and methods
Terminology
The terms ‘branching’, ‘stem–branch attachment’ and ‘(plant) ramification’ are used as synonyms to describe lignified lateral shoots, growing as subordinate axes on the main stem of a plant.
The terms ‘leaf insertion zone’ or ‘leaf insertion region’ refer to a region of the main stem with a leaf base insertion and an axillary bud at a particular node (Evert 2006; Rudall 2007). Vascular bundles in between the main stem, the leaf base and the axillary bud are named leaf traces, branch traces, bud traces or vascular bundle derivatives—depending on their origin and destination—in this study. To avoid ambiguous use of the term ‘branching’ (as used for lateral shoots), eventual bifurcation of these bundles is only referred to as ‘diverging’ or ‘splitting’. Branches developing 3 weeks after decapitation are still named leaf insertion zones, as axillary bud outgrowth and branch development are only initiated via decapitation and not advanced yet (Figs. 3, 4, 5, 6). Nevertheless, in terms of analyzing nodal regions at a time interval of more than 10 weeks after decapitation, the development of stem–branch attachments is clearly visible and, therefore, denoted as such (Fig. 8).
The terms ‘woody segments’, ‘woody strands’ or ‘lignified strands’ equally refer to highly lignified vascular bundles, which progress individually from the main axis into the side axis and are responsible for the ‘finger-like’ morphology of the stem–branch attachment region (Tomlinson et al. 2005).
Plant material
The plant taxa investigated comprise Schefflera arboricola (Fig. 1a–e), Fatsia japonica (Fig. 1f–j) and Polyscias balfouriana—variety Fabian (Fig. 1k–o). All plants originated from commercial nurseries and were further cultivated in the Botanic Garden Freiburg, Germany. S. arboricola was cultivated at an average temperature of 22 °C and humidity of 66% in the greenhouse. F. japonica was cultivated outdoors in moderate temperatures and inside the greenhouse during frost periods between October and April (average indoor cultivation conditions: 11 °C, 64% humidity). P. balfouriana was cultivated at an average temperature of 19 °C and 79% humidity in the greenhouse. The cultivation conditions were chosen in accordance with the native habits of the investigated species. Schefflera and Polyscias as pantropical and paleotropical genera (Lowry et al. 2004) favor warm and humid conditions whereas the genus Fatsia may have a temperate origin (Chiang et al. 2014) and can be cultivated in more moderate conditions concerning temperature and humidity.
For the morphological analysis, well-developed ramifications with an approximate stem diameter of 1 cm were harvested from the respective plants. For the anatomical and ontogenetic analysis of newly forming branches, apices of unbranched axes were decapitated approximately 1 cm above the insertion of a mature leaf and at least 5 cm distance from the terminal bud, to induce branching.
Morphological analysis
Harvested plant material was transferred and stored in water for overnight maceration. Decortication was performed with a knife and forceps to remove the plant cortex. Images of the plant material were taken with a Nikon D80 single-lens reflex camera with two different lenses (18–135, 28–105 mm). Image editing was performed with Adobe Photoshop CS5 Version 12.0 (Adobe Systems, San Jose, CA, USA).
Anatomical analysis
Serial sectioning and staining
Leaf insertions of S. arboricola, F. japonica and P. balfouriana were harvested 3 weeks after decapitation, following a specific sectioning system (Fig. 2). Samples were cut approximately 1 cm underneath the leaf insertion and stored in a fixative (buffered 2% glutaraldehyde) until embedding in glycol methacrylate with plasticizer. To enhance sample infiltration, which is crucial for high-quality thin-sectioning, specimens were cut longitudinally, removing half of the axis opposite the leaf insertion (Schefflera, Fig. 3) or punctuated with a needle in the pith region (Fatsia, Fig. 4 and Polyscias, Fig. 5). If punctuation of the pith was not sufficient for complete sample infiltration, longitudinal cutting was necessary (in the case of Schefflera) to facilitate sample infiltration, via maximizing the exposed surface of the parenchymatous pith region to the medium. Thin-sectioning was performed with a rotary microtome (LEICA Supercut 2065, Leica Microsystems Germany). From 100 µm plant material, three successive sections of 10 µm thickness each were retained for differential staining (Fig. 2). In the samples from F. japonica and P. balfouriana, the subsequent seven sections of 10 µm thickness each were discarded (Fig. 2). In the case of S. arboricola, additional sections of 5 µm each were collected for fluorescence microscopy, via retaining four sections of the intermediate sample region of 70 µm (Fig. 2). To achieve maximum color contrast, sections of 10 µm thickness were stained with safranin-auramine-methylene blue for light microscopy, whereas the sections of 5 µm thickness were stained with acridine orange for fluorescence microscopy.
System used for serial sectioning of Araliaceae ramifications (basal to apical). In sectioning of Fatsia japonica and Polyscias balfouriana ramifications (left side), from every 100 µm, three sections of 10 µm each were retained for differential staining and the following seven sections of 10 µm each were discarded. In sectioning of Schefflera arboricola ramifications (right side), three sections of 10 µm each were retained for differential staining and the subsequent two sections of 10 µm each were discarded. Instead of the sixth and seventh section of 10 µm, four sections of 5 µm each were prepared and retained for fluorescence microscopy and the subsequent three sections of 10 µm each were discarded
Leaf insertion zone of Schefflera arboricola in transverse sections. Thin-sections of 10 µm thickness, stained with safranin-auramine-methylene blue. The leaf insertion was examined 3 weeks after decapitation and sections are shown starting from a basal plane (a) towards three increasingly more apical planes (b–d) in the leaf insertion. e–h Detailed view of the leaf trace L1 from a basal plane (e) towards three increasingly more apical planes (f–h) in the ramification. ab axillary bud, L leaf trace to the left side of the median leaf trace, M median leaf trace, M.L leaf trace in between the median bundle and the first lateral bundle to the left side of the median bundle, M.R leaf trace in between the median bundle and the first lateral bundle to the right side of the median bundle, R lateral leaf trace to the right side of the median leaf trace. Black arrows (d, h) clarify vascular bundle designation. White arrow (a) indicates median leaf gap. Scale bars a–d 2 mm; e–h 0.5 mm
Leaf insertion zone of Fatsia japonica in transverse sections. Thin-sections of 10 µm thickness, stained with safranin-auramine-methylene blue. The leaf insertion was examined 3 weeks after decapitation and sections are shown starting from a basal plane (a) towards three increasingly more apical planes (b–d) in the leaf insertion. e–i Detailed view of the axillary bud region from a basal plane (e) towards four increasingly more apical planes (f–i) in the ramification. ab axillary bud, L leaf trace to the left side of the median leaf trace, M median leaf trace, R lateral leaf trace to the right side of the median leaf trace. White arrow (a) indicates median leaf gap. Scale bars a–d 2 mm; e–i 0.5 mm
Leaf insertion zone of Polyscias balfouriana in transverse sections. Thin-sections of 10 µm thickness, stained with safranin-auramine-methylene blue. The leaf insertion was examined 3 weeks after decapitation and sections are shown from a basal plane (a) towards three increasingly more apical planes (b–d) in the leaf insertion. e–h Detailed view of the leaf traces R7 and R8 from a basal plane (e) towards four increasingly more apical planes (f–h) in the ramification. ab axillary bud, L leaf trace to the left side of the median leaf trace, M median leaf trace, R lateral leaf trace to the right side of the median leaf trace. White arrow (a) indicates median leaf gap. Scale bars a-d 2 mm; e–h 0.5 mm
Image acquisition and editing (light microscopy)
For the analysis of the course and arrangement of the vascular bundles at intervals of 100 µm in the leaf insertion zone, the best of the three cross-sections of 10 µm thickness (for every 100 µm; Fig. 2) was photographed with a digital DP71 camera, attached to an Olympus BX61 microscope, using the control and imaging software cell^P (version 2.8—Olympus Soft Imaging Solutions GmbH, Münster, Germany). The individual light microscopic images were loaded as image stacks into the software FiJi (Schindelin et al. 2012), cropped to a ‘Region of Interest’, and manually aligned via rotation and translation operations in FiJi and Adobe Photoshop (Figs. 3, 4, 5). Individual vascular bundles were tracked image by image and their course was schematically reconstructed (Fig. 6) via a visualization method adapted from Galtier and Beck (1992). The path of leaf traces was marked in different shades of gray, getting darker towards the apical region. Where vascular bundle pathways were in close proximity (e.g., R4, R5 in Fig. 6c), coherent stages of one leaf trace were marked with a white dot. The region of the developing axillary bud was colored in blue and vascular bundle derivatives splitting off from leaf traces and proceeding either into the axillary bud or vascularizing the leaf were colored in orange (Fig. 6). Vascular bundles were labeled ‘M’ if situated in the median region with respect to the axillary bud (after Larson 1986a). Bundles on the right and left side to the bud (facing the ramification from the main stem) were labeled ‘L’ and ‘R’ and incremented starting from the median towards the lateral region.
Pathway of leaf traces in Schefflera arboricola, Fatsia japonica and Polyscias balfouriana. Schematic reconstruction of the pathway of leaf traces from central cylinder of the main stem towards leaf and axillary bud in a S. arboricola, b F. japonica and c P. balfouriana. Leaf traces are colored in different shades of gray, getting darker towards the apical region. The pathway of one vascular bundle was marked with a white dot in case of close proximity to other vascular bundles. Splitting vascular bundle derivatives are colored in orange and the region of the developing axillary bud in blue. L leaf trace to the left side of the median leaf trace, M median leaf trace, R lateral leaf trace to the right side of the median leaf trace. Scale bars 2 mm
Three-dimensional reconstruction of the vascular bundle progression in S. arboricola (fluorescence microscopy)
In a similar way to the light microscopy of differentially stained sections, four sections of 5 µm thickness (Fig. 2) were imaged by fluorescence microscopy (FITC filter—495 nm excitation and 519 nm emission) at intervals of 100 µm for generating a three-dimensional reconstruction (methodology adapted from Haushahn et al. 2014). The best of four captured sections was selected for each of the 100 µm segments. A series of images were loaded as stacks into the software FiJi, cropped to a ‘Region of Interest’, and aligned manually via rotation and translation operations, as well as with the registration plugin tool ‘StackReg’ in FiJi (Thévenaz et al. 1998; Schindelin et al. 2012). The color threshold plugin was applied to the images with an altering range of the RGB mode [R (0–40,255), G (0–80,255), B (0,255)]. To compensate for staining artifacts and irregularities in coloration of the individual images, red and green filter ranges were slightly adapted for every image to achieve maximum contrast. The options ‘threshold’ and ‘invert’ were selected to obtain a grayscale image with a subsequent conversion to 8-bit grayscale. The following filter settings were applied: Median filter with a pixel radius of 1.5–2 and a Maximum filter of pixel radius 2–2.5. As the staining of the image stack was not continuously of equal quality, the ranges of the filter settings ‘Median’ and ‘Maximum’ had to be adapted slightly for the individual pictures. If necessary, staining artifacts in the surrounding embedding medium were manually erased in careful comparison with the differentially stained images. Before computing the 3D image stack, pixel depth of the images was altered to a value of 20–30 times the pixel width and height to account for slice thickness and distance between the single sections, as well as for dilation by adjusting filter settings. For 3D visualization, the processed image stacks were rendered (Fig. 7) via the plugin ‘3D viewer’ (Schmid et al. 2010), as well as the ‘3D Viewer’ option from the software ‘Image Pro Plus’ (Version 6.2.0.424 for Windows 2000/XP Professional, 2005–2007 Media Cybernetics, Inc., Bethesda, USA).
3D reconstruction of vascular bundle progression in leaf insertion/developing stem–branch attachment of Schefflera arboricola. Three-dimensional reconstructions of image stacks from microscopic thin-sections of 5 µm thickness, stained with acridine orange with edited threshold and filter settings. a, b Leaf insertion 3 weeks after decapitation. c, d Stem–branch attachment 10 weeks after decapitation. ab axillary bud, BT branch trace, L leaf, LT leaf trace, P pith, x secondary xylem. Scale bars 1.5 mm
Ontogenetic analysis
The ontogenetic development of stem–branch attachments was analyzed from samples of S. arboricola, F. japonica and P. balfouriana that were harvested before decapitation, as well as at 3 weeks, between 6 and 8 weeks, and more than 10 weeks after decapitation (Fig. 8). Samples were stored in wet tissues until freezing at −22 °C and sectioning with a MEV cryostat (SLEE Medical GmbH, Mainz, Germany) into sections of 60 µm thickness. A precise comprehension of the development of vascular connections to the developing branch—as implied schematically by Tomlinson et al. (2005) also in the longitudinal plane—would require a high-resolution and complete series of thin-sections of the exact vascular bundle progression, which is almost impossible to acquire in appropriate quality, as longitudinal sections are very challenging to produce due to the fibrous character of the material. In this study, we therefore, focused on transverse sectioning. Sections were bleached in a 20% dilution of Eau de Javel (2.4 g hypochlorite in 100 g liquid, Floreal Haagen GmbH, Wadgassen, Germany) and stained with fuchsin-chryosidine-astra blue (FCA, Etzold). Light microscopy was performed as described for the anatomical analysis of serial sections (see above). To enable a description and comparison of anatomical structures during the ontogenetic development of S. arboricola, F. japonica and P. balfouriana, images at the respective time steps were selected from similar dissection planes to guarantee comparability of structures and tissues between the three species and time steps.
Anatomy of the leaf insertion/stem–branch attachment region shown in transverse section of Schefflera arboricola, Fatsia japonica and Polyscias balfouriana at different ontogenetic development stages after decapitation. Sections of 60 µm thickness were stained with fuchsin-chryosidine-astra blue. a–d S. arboricola, e–h F. japonica, i–l P. balfouriana. ab axillary bud, LT leaf trace, LTm median leaf trace, LTl lateral leaf trace, ws woody strand. Scale bars 2 mm
Results
Branching morphology of Schefflera arboricola, Fatsia japonica and Polyscias balfouriana
More than ten decorticated S. arboricola specimens display stem–branch attachments, with eight to ten ‘finger-like’ extensions as described by Tomlinson et al. (2005), which are named woody strands or lignified strands, respectively (Fig. 1c–e).The branch attachment zone is broad, i.e., the woody strands are attached to a large part of the circumference of the main stem (approx. 180°) (Fig. 1c–e). Insertion points of the woody strands in the main stem are lowest for the median (most central) woody strand, with increasing insertion height towards the lateral woody strands on the left and right. Woody strands proceed individually from the main stem basally to the branch insertion region and into the lateral shoot. Here they also merge and form a branch as can be observed in the species F. japonica and P. balfouriana.
Seven investigated F. japonica ramifications show 12–16 lignified strands that embrace the main stem circumferentially over 180° up to 360° (Fig. 1h–j). Points of woody strand insertion in the main stem are situated almost on the same level with a slightly ascending insertion starting from the median woody strands in only one specimen (Fig. 1h, j).
Four specimens of P. balfouriana display seven to nine woody strands with a varying attachment width in circumferential direction between 180° and 270° (Fig. 1m–o). Points of woody strand insertion in the main stem are almost situated at the same height (Fig. 1m, o).
In the area of fusion of the individual woody strands in the lateral shoot, scars of leaf traces can be recognized, which are highlighted with black arrows (Fig. 1e, j, o).
Vascular bundle arrangement in leaf insertion zones of S. arboricola, F. japonica and P. balfouriana
The course and arrangement of vascular bundles starting from the basal part of the leaf insertion region (Figs. 3a, 4a, 5a) towards its apical part (Figs. 3d, 4d, 5d) are visualized. The image series through the leaf insertion zones of S. arboricola, F. japonica and P. balfouriana are additionally displayed as videos and provided as electronic supplementary material with the online version of this article.
In S. arboricola, the leaf gap of the median vascular bundle is already closed at the beginning of the image series (white arrow in Fig. 3a), whereas the median leaf gap is still open at the beginning of the image series in F. japonica and P. balfouriana (white arrows in Figs. 4a, 5a), indicating a further distance of the investigated specimen from the leaf base insertion in Schefflera compared to Fatsia and Polyscias. From the base to the top of the leaf insertion in S. arboricola, a median leaf trace (already split in the median plane into the two bundles M.1 and M.2 in Fig. 3a, b) exits the central cylinder most basally with lateral bundles (L1, L2, L3, R1, R2, R3 in Fig. 3a–c) to the left and right, and subsequently in increasingly more apical positions. This is coherent with the hierarchical insertion of woody strands into the main stem in well-developed stem–branch attachments (Fig. 1c). The outermost lateral bundles (L4, R4, R5 in Fig. 3a–d) can be found completely emerged from the central cylinder in a lower part of the leaf insertion region. Apically to that, vascular bundles in between the median bundle and the first lateral bundles (named M.L as to the left side of the median bundle and M.R as to the right side of the median bundle) and lateral vascular bundles, situated underneath the region of the axillary bud, exit the central cylinder almost perpendicularly (M.L, M.R in Fig. 3b), whereas lateral bundles progress in a slightly curved way from their lateral origin in the main stem towards the median region of the leaf (R2 in Fig. 3c; R3 in Fig. 3d; L3, R3 in Fig. 6a). Median and lateral vascular bundles split after complete emergence from the central cylinder and proceed into the leaf as well as into the developing axillary bud, respectively (M.1, M.2 in Fig. 3a, b; L1.1, L1.2 in Fig. 3b; L1.1, L1.2 in Fig. 3f–h; L2, R2 in Fig. 6a). Additionally, vascular bundle divergence can also be observed in the apical part, with all daughter bundles (R1, daughter bundles indicated with arrows, on Fig. 3d) contributing to the vascular integration of the leaf (L1.1, R1, R2 in Fig. 3d; L1 in Fig. 6a).
Similar to S. arboricola, median leaf traces exit the central cylinder first in basal parts of F. japonica (M in Fig. 4a). Lateral traces emerge in an initially asymmetric pattern (in the section shown) with bundles on the right side (R1, R2 in Fig. 4a) and on the left side (L1 in Fig. 4b) of the median bundle as the axillary bud already develops. A slightly asymmetric emergence pattern of leaf traces with respect to the median strand can be observed in all investigated individuals (Figs. 3a, 4a, 5b). Leaf traces in F. japonica follow an increasingly curved pathway in the lateral regions (R2, R3, R4, L3, L4 in Fig. 6b). Divergence of leaf traces occurs with daughter bundles vascularizing the leaf (M1, M2 in Fig. 4c; L1.1, L1.2 in Fig. 4d), as well as with bundles progressing into the leaf and axillary bud region, respectively (R2, L2 in Fig. 6b). In contrast to the split of the median bundle in S. arboricola (M.1, M.2 in Fig. 3a), resulting in one bundle proceeding in between its mother bundle and the main stem, the median bundle in F. japonica splits transversally, in a lateral direction (M.1, M.2 in Fig. 4c), resulting in two bundles running in parallel next to each other. The axillary bud is at this stage of development basally discontinuous to the vascular system of the main stem (ab in Fig. 4b) and only connected via lateral traces, exiting the stem apically to the axillary bud origin (L2 in Fig. 4c). A detailed view of the axillary bud region from a basal (Fig. 4e) to a more apical part of the ramification (Fig. 4i) demonstrates that right-sided leaf traces (R1, R2, R3 in Fig. 4e–i) are at this developmental stage not connected to the developing axillary bud region (R1 in Fig. 4f; R2 in Fig. 4g; R3 in Fig. 4i).
Serial thin-sections of a leaf insertion from P. balfouriana show a broad emergence zone of leaf traces from the main stem of more than 270° circumference (Fig. 5a, b), with all vascular bundles exiting the main stem at the same level (Fig. 5b). Lateral leaf traces follow a curved pathway in an apical region (R6, R7, R8, L5, L6 in Fig. 6c). Vascular bundle divergence takes place underneath the region of the developing axillary bud and differentiating derivative vascular bundles contribute to the vascular integration of the bud (R7.1 in Fig. 5c; R7.1 in Fig. 5f–h; R7 in Fig. 6c). Additionally, vascular bundles split in the leaf in apical parts of the leaf insertion zone (R4, R7, R8 in Fig. 5d).
In all three investigated species, a similar vascular organization of the stem-leaf-bud complex can be summarized: whereas median bundles (M in Fig. 6a–c) proceed linearly into the leaf and start to split in the region of the leaf—with the exception of the median bundle in P. balfouriana (M in Fig. 6c), which also connects to the axillary bud—lateral bundles follow a curved pathway as they exit the main stem and diverge for a better vascular integration of both the leaf and the axillary bud (L3 in Fig. 6a; L2–L5, R2–R5 in Fig. 6b; L2–L6, R5–R8 in Fig. 6c).
For S. arboricola, the circumferential vascular bundle arrangement in the leaf insertion regions is visualized 3 weeks (Fig. 7a, b; Electronic supplementary information 4) and 10 weeks (Fig. 7c, d; Electronic supplementary information 5) after decapitation. Leaf traces (LT in Fig. 7a–d) exit the central cylinder of secondary xylem in the main axis (x in Fig. 7a–d) which surrounds the pith region (P in Fig. 7a–d). Vascular connection to an establishing axillary bud is missing in the younger specimen with leaf traces (LT in Fig. 7a, b) propagating exclusively in the leaf (L in Fig. 7a, b). Ten weeks after decapitation, both the leaf (L in Fig. 7c, d) as well as the developing axillary bud (ab in Fig. 7c, d) show a vascular connection via leaf traces (LT in Fig. 7c, d) and vascular bundle derivatives, which are named branch traces (BT in Fig. 7c, d). Vascular integration of the bud is formed via lateral vascular bundles, whereas median and inner lateral bundles proceed into the associated leaf (ab, L in Fig. 7c, d).
Ontogenetic development of stem–branch attachments in S. arboricola, F. japonica and P. balfouriana
Before decapitation, leaf insertions in S. arboricola possess an undeveloped axillary bud (ab in Fig. 8a) and numerous vascular leaf traces (LT in Fig. 8a) proceed into the leaf. Three weeks after decapitation, an initial proliferation of the axillary bud (ab in Fig. 8b) region can be observed with leaf traces (LT in Fig. 8b) encompassing the axillary bud. The development of the axillary bud is strongly advanced at a later stage in development with a distinct formation of circular woody structures (ws in Fig. 8c) originating at the transition to the axillary bud region (ab in Fig. 8c). After more than 10 weeks of development, individual woody strands (ws in Fig. 8d) start to merge in the median region. The vascular integration of the leaf undergoes reorganization via leaf bundle divisions and rearrangements (LT in Fig. 8a, c) during branch development, before the leaf is finally shed when the respective ramification is fully established (Fig. 8d).
As with S. arboricola, the axillary bud (ab in Fig. 8e) in F. japonica is undeveloped before inducing outgrowth of the axillary bud via decapitation with vascular bundles (LT in Fig. 8e) in the leaf starting to split. After a slight lateral expansion of the axillary bud (ab in Fig. 8f) 3 weeks after decapitation, the bud undergoes distinct outgrowth, forming a median bulge (ab in Fig. 8g). This rather restricted radial development is different to the broad circumferential development of the axillary bud (ab in Fig. 8b) in S. arboricola. In F. japonica, lignification of the lateral vascular strands is noticeable only in a late stage of development (>10 weeks) with individual woody strands (ws in Fig. 8h) starting to merge in the median region and encircling the main stem by more than 270°. Vascular leaf traces (LT in Fig. 8f) supplying the respective leaf undergo multiple divisions before the leaf is shed after approximately 6 weeks in the investigated specimens (Fig. 8g).
The axillary bud (ab in Fig. 8i) in P. balfouriana is rudimentally developed before decapitation. Broad enlargement of the bud (ab in Fig. 8j) can be observed after 3 weeks, with median leaf traces (LT in Fig. 8j) splitting up to enhance vascular integration in the leaf, which is still attached at this point of development. Extension of the axillary bud (ab in Fig. 8k) proceeds with increasing time after decapitation, whereas median leaf traces (LTm in Fig. 8k) are reduced in number and size, as the associated leaf is shed in the course of branch development. In contrast, lateral leaf traces (LTl in Fig. 8k) propagate and are arranged into distinct circular sections. Via divergence and reorganization, these lateral leaf traces develop into the ‘finger-like’ woody vascular strands (ws1 in Fig. 8l), clearly recognizable after more than 10 weeks of branch development, with several ‘fingers’ already partially merged together (ws3 in Fig. 8l). The ontogenetic development of a stem–branch attachment in P. balfouriana (Fig. 8i–l) proceeds differently from Schefflera and Fatsia, involving multiple divergences of lateral leaf traces (LTl in Fig. 8k). ‘Finger-like’ vascular strands (ws2, ws3 in Fig. 8l) are formed via circular reorganization of those lateral leaf traces (LTl in Fig. 8k), instead of the secondary development of individual leaf traces and/or of accessory bundles to lignified branch traces as in Schefflera and Fatsia (ws in Fig. 8d, h). This leads to primarily hollow woody strands in Polyscias (ws2 in Fig. 8l) in the course of vascular bundle rearrangement, which merge together and form woody strands with reduced parenchymatous ‘cores’ (ws3 in Fig. 8l).
Discussion
Branching morphology of Schefflera arboricola, Fatsia japonica and Polyscias balfouriana
The ‘finger-like’ branching morphology, described in S. arboricola and S. actinophylla ramifications by Tomlinson et al. (2005), can be confirmed for S. arboricola (Fig. 1c–e) and is furthermore present in the Araliaceae species, F. japonica (Fig. 1h–j) and P. balfouriana (Fig. 1m–o).
Schefflera arboricola and P. balfouriana are comparable in their number of woody strands and the degree of main stem encirclement, whereas F. japonica exhibits a higher number of woody strands and also a higher degree of stem encirclement. We assume that a large encompassing of the stem and a high number of woody strands correlate with an increased mechanical stiffness and possibly a higher water-conductance of the ramification in F. japonica, a development which might be driven by the following two constraints:
-
1.
In adult plants, F. japonica shoots have approximately 1.5-times larger stem and branch diameters in comparison to S. arboricola and P. balfouriana, so that higher forces could act on Fatsia ramifications, requiring a well-established mechanical support (Fig. 1f, g).
-
2.
Leaves of F. japonica are significantly larger with more than twice the leaf surface compared to leaves of S. arboricola and P. balfouriana (Fig. 1a, f, k). Therefore, they require higher mechanical support and better hydraulic supply, assumedly facilitated by a higher number of lignified vascular bundles (woody strands) being distributed around the stem circumference.
A specimen of Schefflera actinophylla with 17 woody strands was shown by Tomlinson et al. (2005). Similar to Fatsia, with comparable large leaves and stem diameters, S. actinophylla was described as being in general larger than the species S. arboricola. This is probably associated with larger leaves and stem diameters, and therefore presumably also requires a broad vascular integration of the side axis—mechanically and hydraulically—via numerous lignified strands (Tomlinson et al. 2005).
The hierarchical insertion of woody strands in the main stem is most conspicuous in S. arboricola (Fig. 1c) and is also described by Tomlinson et al. (2005). Hypothesizing that a more basal and hierarchically arranged insertion of woody strands is associated with an increased mechanical stiffness of the ramification, it remains uncertain why Schefflera presents this particular morphology, whereas Fatsia reacts to high mechanical loads with an increased number of woody strands. Even if these two different strategies to enhance branch stiffness exist, the need for such a reinforced system in the case of Schefflera is not obvious, as its leaves are comparably small (Fig. 1a, f, k) and therefore external bending loads are not prominent. It is conceivable that the basal and hierarchical woody strand insertion in Schefflera can be attributed originally to its hemiepiphytic growth form, which even lead to the assumption that the woody strands represent aerial roots, though this could not be confirmed (Tomlinson et al. 2005). We assume that the more basal insertion of woody strands at different levels in the main stem contributes to the stiffness of Schefflera ramifications.
The temporarily hollow strands in P. balfouriana are observable in the ontogenetic series (ws2, ws3 in Fig. 8l), but are likely to change into completely lignified woody strands during progressive development (compare difference between ws2 and ws3 in Fig. 8l). Presumably the presence of hollow woody strands in Polyscias is a consequence of the anatomical reorganization during branch development and not beneficial in terms of functional morphology.
All tested specimens had a self-supporting habit even though the naturally occurring climbing habit of S. arboricola needs to be taken into account for the discussion of morphological adaptations. It is conceivable that ramifications in Araliaceae species with different growth habits might reveal variations in branching morphology. For example, morphological and anatomical analyses of stem–branch attachments of prostrate, climbing and self-supporting axes in the species Hedera helix exhibit diverse ‘finger-like’ branching morphologies, with profound variability in the basic pattern (data not shown).
The Araliaceae family has been subject to many changes in its systematics during the last few decades, with insecure classification of many genera and species (Wen et al. 2001; Lowry et al. 2004; Frodin et al. 2010; Plunkett and Lowry 2010). The ‘finger-like’ branching morphology has until now been identified in four genera of the Araliaceae, Schefflera, Fatsia, Polyscias and Hedera (data not shown), with Schefflera and Polyscias being the two largest genera in the family (Lowry et al. 2004) and to the best of our knowledge has not been described in other plant families. Forthcoming studies shall clarify if this character is exclusive to the Araliaceae.
Vascular bundle arrangement in leaf insertion zones of S. arboricola, F. japonica and P. balfouriana
Median leaf traces are often the most prominent vascular bundles (M.1 in Fig. 3a; M in Fig. 4b) and also the first ones (i.e., the most basal ones and the first ones developed during ontogeny) to vascularize the leaf, and later form the distinct midvein in the developing leaf venation system (Beck 2010). In the case of the investigated multilacunar Araliaceae species, lateral vascular bundles occur subsequently to contribute to the leaf vascularization and establish the venation network of the leaf lamina or, respectively, to vascularize the axillary bud if its outgrowth is initiated. A possible reason why apical median bundles, being in-plane with the developing branch, do not contribute to the vascularization of the correlating bud might be their disposition to vascularize the subsequent leaf in an apical node, as described for Polyscias quilfoylei (Larson 1986a, b) and S. arboricola (Tomlinson et al. 2005).
As leaf traces serve as ‘templates’ for woody strand development (as described by Tomlinson et al. (2005) for S. arboricola), the amount, exit position and curvature of vascular bundles are closely correlated with the amount, insertion and circumferential arrangement of woody strands in well-established ramifications, as discussed in detail above.
Vascular connection to a leaf, axillary bud or a developing ramification are often initiated several nodes beneath the leaf insertion zone, with the exit of the median vascular bundle from the main stem, followed consecutively by the lateral bundles as described for S. arboricola (Tomlinson et al. 2005) and P. balfouriana (Larson 1986a, b). In the present study, such a hierarchical arrangement is well-established in Schefflera (Fig. 3), whereas it has been only observed rudimentarily in Fatsia (Fig. 4), and is entirely absent in Polyscias (Fig. 5). These patterns are very conspicuous in decorticated ramifications (Fig. 1c–e, h–j, m–o).
Even though the leaf traces are described to serve as ‘templates’ for the subsequent development of lignified woody strands, the amount of leaf traces observable in the cortical area of leaf insertion regions 3 weeks after decapitation (Figs. 3, 4, 5) does not necessarily reflect the amount of woody strands observed later in well-established ramifications (Fig. 1c, h, m). It is conceivable that at an early stage of branch initiation, the emergence of vascular bundles from the main stem is not at a final stage and more bundles might follow in ongoing branch development. In the case of Polyscias (Fig. 5), the high amount of vascular bundles already in this early stage of development supports the observation from the ontogenetic analysis, that lateral leaf traces in this species are arranged circularly and merge together to form woody strands (Fig. 8k), so that not every leaf trace develops into an individual woody strand. Otherwise Polyscias would possess a very high number of woody strands which is not the case (Fig. 1m–o). In S. arboricola, the outermost lateral bundles leave the main stem before the other lateral vascular bundles (Figs. 3a, 6a), whereas this is not observed in the arrangement of woody strands (Fig. 1c–e), implying that not every leaf trace serves as a ‘template’ for the development of woody strands.
The length and degree of the curvature of lateral vascular bundles correlates with the degree of circumferential encompassing of the woody strands around the main stem. In Fatsia (Fig. 6b) and Polyscias (Fig. 6c) the vascular traces have to circuit almost the whole stem circumference, whereas woody strands in Schefflera only show a circumference of about 180° (Figs. 1d, 6a) and vascular bundles are only slightly curved in the case of the outermost lateral bundles.
Uni-, tri- and multilacunar nodes with mostly odd numbers of up to seven leaf traces are common in many plant families (Neubauer 1971, 1979, 1981, 1984a, b; Napp-Zinn 1973). The development of complex leaf trace patterns due to multiple divergence of a single leaf trace in unilacunar nodes as in the Gentianaceae or Rubiaceae (Neubauer 1981, 1984a, b), or variations in lacunarity within a family, species or even single individual due to variable fusion or divergence of vascular bundles is not uncommon (Napp-Zinn 1973), complicating the assignment of a specific lacunarity to a plant family or species. A ‘plurilacunar’ node with more than ten leaf traces and leaf gaps being circumferentially distributed around the main stem between 180° and almost 360°, as in the described Araliaceae species, raises the question of its function and advantage compared to plants with fewer lacunae. The abundant splitting of leaf traces in the nodal regions in many plant families allows for the assumption, that ‘plurilacunarity’ is not a necessity for establishing complex leaf venation systems and ensuring vascular and mechanical integration of the leaf. It is conceivable that ‘plurilacunar nodes’, as in the Araliaceae, are associated with the vascularization of broad sheathing leaf bases protecting the axillary bud, or the complex vascularization of not only the leaf base, but also the (outgrowing) axillary bud via vascular bundle derivatives diverging from leaf traces, eventually leading to a conspicuous branching morphology as described in this study.
Referring to the presence of accessory bundles as described by Tomlinson et al. (2005) in S. arboricola, the following amendments are proposed based on the present study. Two types of vascular bundle derivatives, in addition to leaf traces, connect the vascular system of the main stem with the leaf, axillary bud/developing branch, respectively, in S. arboricola, F. japonica and P. balfouriana (Fig. 6a–c): (1) ‘real’ derivative bundles, used as a generic term for structures similar to satellite bundles (Zimmermann and Tomlinson 1972), subsidiary bundles (Larson 1975, 1980, 1983) or accessory bundles (Tomlinson et al. 2005), and (2) sub-bundles that are described as ‘daughter’ vascular bundles (described by Larson 1986a, b) splitting off from vascular traces in the leaf. Median leaf traces mainly split into sub-bundles in the leaf (with the exception of Polyscias, with a ‘real’ derivative bundle diverging from the median bundle), whereas derivative bundles split off from lateral bundles for vascular integration of the axillary bud (Fig. 6a–c). From these analyses, it can be concluded that vascular connections to axillary buds/developing side branches are primarily established by derivatives from lateral vascular bundles of the main stem, rather than exhibiting an ‘independent’ vascular connection to the main stem, as for instance described for Begonia (Neubauer 1979) or Pelargonium (Neubauer 1971). As observed, the vascularization of the leaf and the axillary bud are closely linked in the described Araliaceae leaf insertions. This might resemble the vascular situation in a recaulescent bud, where the axillary bud and the subtending leaf are relocated onto a common side axis produced by the axillary meristem (Weberling 1989). However, recaulescent buds have not been observed in the investigated Araliaceae and the vascular connection to the axillary bud via the leaf traces, instead of a direct connection to the main stem, might be an anatomical peculiarity in the analyzed species. This may be linked to the conspicuous branching morphology of the Araliaceae and its ontogenetic development with leaf traces serving as ‘templates’, as described in our study as well as by Tomlinson et al. (2005). Understanding axillary bud vascularization allows for reconstructing the initial linkage to a lateral shoot in a yet unlignified state, which is established not only for hydraulic connectivity with the axillary bud, but to our understanding also as a predisposition for subsequent mechanical integration of the developing branch.
Ontogenetic development of stem–branch attachments in S. arboricola, F. japonica and P. balfouriana
Evaluating the ontogenetic development of stem–branch attachments via the outgrowth of the axillary bud in the three investigated species, it can be stated that the leaf is always established first, with the growth of the axillary bud—origin of the developing branch—following, and the leaf is shed as the development of the bud advances. This order of organ initiation allows for the outgrowth of the axillary bud that often begins retrospectively (e.g., via decapitation), with the establishing side branch presumably needing existing leaf traces as ‘templates’ to form vascular connections to the main stem. The latter is the case in F. japonica and P. balfouriana—similar to S. arboricola as described by Tomlinson et al. (2005)—and has not been shown before. Leaves on the other hand are necessary for photosynthetic activity and are continuously established.
Nevertheless, how the woody strands develop and from which vascular tissues their secondary development originates seems to be different for Schefflera, Fatsia and Polyscias.
Despite the seemingly confined growth of the axillary bud in Fatsia japonica (ab in Fig. 8f, g), the broadly circumferential arrangement of leaf traces in an early stage (LT in Fig. 8e) and of woody strands in a later stage (ws in Fig. 8h) allow for concluding that leaf traces provide the origin for the development of woody strands. The merging of the woody strands with the developing vascular strands of the axillary bud (Fig. 8h) underlines the fluent transition between the woody strands and the bud as the vascular connection establishes.
The contribution of vascular bundle derivatives in Polyscias for the development of woody strands is even more distinct in comparison to Schefflera and Fatsia, as it involves complex divisions, rearrangements and fusions of derivative bundles (LTl in Fig. 8k), which is not the case in Schefflera and Fatsia. The conspicuous vascular bundle arrangement during the ontogenetic development of woody strands in Polyscias represents the main difference between the three investigated species and might be explained by the following:
-
1.
Additional species of the genus Polyscias, such as P. quilfoylei, which have been analyzed in previous studies (Larson 1986a, b), possess ‘well-developed’ multilacunar nodes. As Polyscias displays a comparatively more intensive vascularization with a higher number of vascular bundles contributing to the leaf, axillary bud and branch, this might be correlated with a complex venation system of the leaves.
-
2.
The Polyscias specimen from which the samples of this study originate is big (approx. 2 m high) in comparison to the Schefflera (approx. 1 m high) and Fatsia (approx. 0.5 m high) specimens. The increasing size of the plant might lead to the formation of more intensive vascularization via leaf traces and derivative bundles, contributing to the development of woody strands. In Fatsia (as described above), a larger stem diameter seems to result in a greater quantity and bigger circumference of woody strands. Polyscias, on the other hand, seems to react to increasing plant height and mechanical requirements not via a higher amount of woody strands, but with a different strategy for their establishment.
-
3.
As described by Tomlinson et al. (2005) for Schefflera, various origins for the development of secondary xylem can lead to branch establishment, which allows for the assumption that no standard scenario for the development of woody strands can be distinguished and that different amounts and types of vascular bundles—as in Polyscias—can be involved. This might lead to the assumption that the ontogenetic development of woody strands is highly individual depending on the respective size, growth condition and habit of each plant—not only in between different species but also within a species similar to various scenarios of lacunarity, vascular bundle arrangement and axillary bud vascularization within plant families, species and individuals, as elaborated earlier and described in Napp-Zinn (1973) and Neubauer (1979, 1981, 1984a, b).
Conclusion
The morphological, anatomical and ontogenetic analyses allow for the following conclusions:
A ‘finger-like’ branching morphology is not exclusive to Schefflera, but seems to be a common character in the Araliaceae—a family which is originally native to tropical areas (Hallé et al. 1978). In their natural habitat, Araliaceae are part of dense understory vegetation and grow sometimes even as hemiepiphytes (Tomlinson et al. 2005). The need for high axial growth in competition for light, but also rare exposure to stress such as wind, snow or heavy fruits, might imply that a completely merged stem–branch attachment with a very high mechanical stiffness is not necessary for Araliaceae plants in their natural habitat, and therefore, can be reduced to the existing ‘finger-like’ morphology for the benefit of material saving. Some ramifications—intraspecific as well as interspecific—exhibit higher degrees of coalescence of woody strands than others or variations such as different amounts of woody strands or a greater circumference. These morphological differences might be individual responses to respective mechanical requirements in the specific environment of a particular specimen or even a single ramification. Biomechanically, the circumferential arrangement and hierarchical insertion of woody strands in Araliaceae ramifications are hypothesized to be beneficial for the respective plants due to several reasons: (1) they establish appropriate mechanical stability and vascular supply with a relatively small amount of lignified material, (2) the arrangement of the woody strands in the stem–branch attachment region is probably well adapted for bearing compressive loads at the abaxial side and tensile forces at the adaxial side of the branch so that it is well supported mechanically, (3) the woody strands could fail consecutively via gradual fracture events under load instead of a sudden fracture of the complete branch. Deformation and stress distribution in the loaded ramification shall be analyzed in future biomechanical experiments supported with finite element simulations (Born et al. 2016), to clarify the mechanical relevance of the vascular strands for branch integration and to gain further insights in its functionality.
A general ontogenetic scenario of branch development in Araliaceae remains challenging, as only a high-resolution three-dimensional understanding of the stem–branch attachment region, at short-interval time steps after decapitation, might resolve the exact reorganization and contribution of different leaf traces and their derivatives. As such a three-dimensional reconstruction of plant ramifications is nearly impossible with invasive methods including embedding and sectioning (Brodersen and Roddy 2016), we aim to gain further knowledge of Araliaceae ramifications with µCT imaging to improve our understanding of the three-dimensional vascular bundle distribution in the stem–branch attachments.
Based on biological concept generators, such as Araliaceae ramifications, we aim to develop novel bio-inspired fiber-reinforced branched plastic hulls for concrete filling in building constructions (Born et al. 2016), similar to the manufacture of other hollow branched fiber-reinforced structures (Milwich et al. 2006; Masselter and Speck 2011; Masselter et al. 2016; Schwager et al. 2013). The biomimetic abstraction of the arrangement, number and insertion points of fibers emerging from a main into a subordinate axis of biological concept generators, such as the investigated Araliaceae genera, offer a high potential application for optimizing fiber-reinforced branched braiding structures in architecture and other fields of technology (Born et al. 2016).
Author contribution statement
KB planned and executed the study, acquired, edited and evaluated all images and wrote the manuscript. SF conducted embedding of samples, as well as preparation and staining of the serial sections. TS initiated the study and contributed to the improvement of the manuscript. TM initiated the study and contributed to the planning and execution of the study, as well as the improvement of the manuscript.
References
Achim A, Gardiner B, Leban JM, Daquitaine R (2006) Predicting the branching properties of sitka spruce grown in Great Britain. New Zeal J For Sci 36(2/3):246–264
Beck CB (2010) An introduction to plant structure and development: plant anatomy for the twenty-first century, 2nd edn. Cambridge University Press, Cambridge
Born L, Jonas FA, Bunk K, Masselter T, Speck T, Knippers J, Gresser GT (2016) Branched structures in plants and architecture. In: Knippers J, Nickel K, Speck T (eds) Biomimetic research for architecture and building construction: Biological design and integrative structures, biologically-inspired systems, vol 9. Springer International Publishing, Switzerland, pp 195–215
Bouakba M, Bezazi A, Boba K, Scarpa F, Bellamy S (2013) Cactus fibre/polyester biocomposites: manufacturing, quasi-static mechanical and fatigue characterization. Compos Sci Technol 74:150–159
Brodersen CR, Roddy AB (2016) New frontiers in the three-dimensional visualization of plant structures and function. Am J Bot 103(2):184–188
Burgert I, Jungnikl K (2004) Adaptive growth of gymnosperm branches-ultrastructural and micromechanical examinations. J Plant Growth Regul 23(2):76–82
Chen Q, Fan JT, Sarkar MK (2012) Biomimetics of branching structure in warp knitted fabrics to improve water transport properties for comfort. Text Res J 82(11):1131–1142
Chiang TY, Chen SF, Kato H, Hwang CC, Moore SJ, Hsu TW, Hung KH (2014) Temperate origin and diversification via southward colonization in Fatsia (Araliaceae), an insular endemic genus of the West Pacific Rim. Tree Genet Genomes 10:1317–1330
Evert RF (2006) Esau’s plant anatomy. John Wiley & Sons Inc, New Jersey
Fan JT, Sarkar MK, Szeto YC, Tao XM (2007) Plant structured textile fabrics. Mater Lett 61(2):561–565
Frodin DG, Lowry PP II, Plunkett GM (2010) Schefflera (Araliaceae): taxonomic history, overview and progress. Plant Divers Evol 128(3):561–595
Galtier J, Beck CB (1992) Triichnia, a new eustelic calamopityacean from the lower carboniferous of France. Palaeontogr Abt B 224:1–16
Hallé F, Oldeman RAA, Tomlinson PB (1978) Tropical trees and forests. Springer, Heidelberg
Haushahn T, Speck T, Masselter T (2014) Branching morphology of decapitated arborescent monocotyledons with secondary growth. Am J Bot 101(5):754–763
Hesse L, Masselter T, Leupold J, Spengler N, Speck T, Korvink JG (2016) Magnetic resonance imaging reveals functional anatomy and biomechanics of a living dragon tree. Sci Rep 6:32685
Jungnikl K, Goebbels J, Burgert I, Fratzl P (2009) The role of material properties for the mechanical adaptation at branch junctions. Trees Struct Funct 23(3):605–610
Larson PR (1975) Development and organization of the primary vascular system in Populus deltoides according to phyllotaxy. Am J Bot 62(10):1084–1099
Larson PR (1980) Interrelations between phyllotaxis, leaf development and the primary-secondary vascular transition in Populus deltoides. Ann Bot 46(6):757–769
Larson PR (1983) Primary vascularization and the siting of primordia. In: Dale JE, Milthorpe FL (eds) The growth and functioning of leaves. Cambridge University Press, Cambridge, pp 25–51
Larson PR (1984) The role of subsidiary trace bundles in stem and leaf development of the dicotyledoneae. In: White RA, Dickison WC (eds) Contemporary problems in plant anatomy. Academic Press Inc., London, pp 109–129
Larson PR (1986a) Vascularization of a multilacunar species: Polyscias quilfoylei (Araliaceae). I. The stem. Am J Bot 73(11):1620–1631
Larson PR (1986b) Vascularization of a multilacunar species: Polyscias quilfoylei (Araliaceae). II. The leaf base and rachis. Am J Bot 73(11):1632–1641
Lowry PP II, Plunkett G, Wen J (2004) Generic relationships in Araliaceae: looking into the crystal ball. S Afr J Bot 70(3):382–392
Masselter T, Speck T (2011) Biomimetic fiber-reinforced compound materials. In: George A (ed) Adv Biomi. InTech, Rijeka, pp 185–210
Masselter T, Eckert S, Speck T (2011) Functional morphology, biomechanics and biomimetic potential of stem–branch connections in Dracaena reflexa and Freycinetia insignis. Beilstein J Nanotechnol 2:173–185
Masselter T, Hesse L, Böhm H, Gruhl A, Schwager H, Leupold J, Gude M, Milwich M, Neinhuis C, Speck T (2016) Biomimetic optimization of branched fibre-reinforced composites in engineering by detailed analyses of biological concept generators. Bioinspir Biomim 11(5):055005
Masselter T, Hesse L, Leupold J, Spengler N, Korvink JG, Speck T (2015) Using MRI for analyzing the anatomy and biomechanics of monocotyledons. In: Abstracts of the 8th plant biomechanics international conference, Nagoya, pp 230–234
Mattheck C (1991) Trees: the mechanical design. Springer, Heidelberg
Milwich M, Speck T, Speck O, Stegmaier T, Planck H (2006) Biomimetic in technical textiles: solving engineering problems with the help of nature’s wisdom. Am J Bot 93(10):1455–1465
Müller L, Milwich M, Gruhl A, Böhm H, Gude M, Haushahn T, Masselter T, Schwager H, Neinhuis C, Speck T (2013) Biomimetically optimized branched fiber composites as technical components of high load capacity. Tech Text 56:231–235
Müller U, Gindl W, Jeronimidis G (2006) Biomechanics of a branch–stem junction in softwood. Trees Struct Funct 20(5):643–648
Napp-Zinn K (1973) Anatomie des Blattes II. Blattanatomie der Angiospermen A. Entwicklungsgeschichtliche und topographische Anatomie des Angiospermenblattes. Gebrüder Borntraeger Berlin, Stuttgart
Neubauer HF (1971) Über Blattgrund und Stammknoten von Pelargonien. Österr Bot Z 119:141–153
Neubauer HF (1979) Knotenbau, Blattgrund- und Achselknospenvaskularisation bei Begonien. Flora 168:329–343
Neubauer HF (1981) Der Knotenbau einiger Rubiaceae. Plant Syst Evol 139:103–111
Neubauer HF (1984a) Knotenbau und Blattgrundvaskularisation bei einigen Gentianaceae. Plant Syst Evol 144:1–7
Neubauer HF (1984b) Über Knotenbau und Blattgrundvaskularisation einiger Melastomataceae. Plant Syst Evol 147:119–123
Niklas K (1992) Plant biomechanics: an engineering approach to plant form and function. University of Chicago Press, London
Plunkett GM, Lowry PP II (2010) Paraphyly and polyphyly in Polyscias sensu lato: molecular evidence and the case for recircumscribing the ‘pinnate genera’ of Araliaceae. Plant Divers Evol 128(1):23–54
Rudall P (2007) Anatomy of flowering plants. Cambridge University Press, Cambridge
Sarkar M, Fan J, Szeto Y, Tao X (2009) Biomimetics of plant structures in textile fabrics for the improvement of water transport properties. Text Res J 79(7):657–668
Schindelin J, Aranga-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S et al (2012) FiJi: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682
Schmid B, Schindelin J, Cardona A, Longair M, Heisenberg M (2010) A high-level 3D visualization API for Java and ImageJ. BMC Bioinf 11(1):274
Schwager H, Masselter T, Speck T, Neinhuis C (2013) Functional morphology and biomechanics of branch-stem junctions in columnar cacti. Proc R Soc Lond [Biol]. doi:10.1098/rspb.2013.2244
Schwager H, Neinhuis C, Mauseth JD (2015) Secondary growth of the leaf and bud traces in Hylocereus undatus (Cactaceae) during the formation of branches or flowers. Int J Plant Sci 176(8):762–769
Shigo AL (1985) How tree branches are attached to trunks. Can J Bot 63(8):1391–1401
Slater D, Harbinson C (2010) Towards a new model of branch attachment. Arboric J 33(2):95–105
Theckes B, de Langre E, Boutillon X (2011) Damping by branching: a bioinspiration from trees. Bioinspir Biomim. doi:10.1088/1748-3182/6/4/046010
Thévenaz P, Ruttiman UE, Unser M (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7(1):27–41
Tomlinson PB, Fisher JB, Hallé F, Villalobos R (2005) Development of woody branch attachments in Schefflera (Araliaceae or Apiaceae). Am J Bot 92(11):1765–1773
Weberling F (1989) Morphology of flowers and inflorescences. Cambridge University Press, Cambridge
Wen J, Plunkett GM, Mitchell AD, Wagstaff SJ (2001) The evolution of Araliaceae: a phylogenetic analysis based on ITS sequences of nuclear ribosomal DNA. Syst Bot 26(1):144–167
Zimmermann MH, Tomlinson PB (1972) The vascular system of monocotyledonous stems. Bot Gaz 133(2):141–155
Acknowledgements
The authors thank the German Research Foundation (DFG) for funding this study within the Collaborative Research Center TRR141 ‘Biological Design and Integrative Structures—Analysis, Simulation and Implementation in Architecture’—Project A06. They acknowledge the support of Sabine Diener and Susanne Röske from the Department of Forest Botany, University of Freiburg in preparation of serial sections, as well as of Sandra Eckert from the Plant Biomechanics Group, University of Freiburg in preparation of cryotome sections. They also thank Johanna Maria Seitz for valuable help in image acquisition and editing, Alex Chepstow-Lusty for linguistic editing, as well as the two anonymous reviewers for their contributions, which significantly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by E. Beck.
Electronic supplementary material
Below is the link to the electronic supplementary material.
468_2017_1585_MOESM1_ESM.avi
Supplemental Information 1 Schefflera arboricola—video clip of propagation of vascular bundles in a leaf insertion region, examined 3 weeks after decapitation, from a basal plane towards more apical planes (transversal thin-sections of 10 µm thickness, stained with safranin-auramine-methylene blue). Scale bar 2 mm (AVI 4977 kb)
468_2017_1585_MOESM2_ESM.avi
Supplemental Information 2 Fatsia japonica—video clip of propagation of vascular bundles in a leaf insertion region, examined 3 weeks after decapitation, from a basal plane towards more apical planes (transversal thin-sections of 10 µm thickness, stained with safranin-auramine-methylene blue). Scale bar 2 mm (AVI 4843 kb)
468_2017_1585_MOESM3_ESM.avi
Supplemental Information 3 Polyscias balfouriana—video clip of propagation of vascular bundles in a leaf insertion region, examined 3 weeks after decapitation, from a basal plane towards more apical planes (transversal thin-sections of 10 µm thickness, stained with safranin-auramine-methylene blue). Scale bar 2 mm (AVI 4444 kb)
468_2017_1585_MOESM4_ESM.avi
Supplemental Information 4 Schefflera arboricola—three-dimensional z-stack of edited serial thin-sections (fluorescence microscopy) to track vascular bundle progression through leaf insertion region 3 weeks after decapitation (AVI 4431 kb)
468_2017_1585_MOESM5_ESM.avi
Supplemental Information 5 Schefflera arboricola—three-dimensional z-stack of edited serial thin-sections (fluorescence microscopy) to track vascular bundle progression through stem–branch attachment region 10 weeks after decapitation (AVI 4674 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bunk, K., Fink, S., Speck, T. et al. Branching morphology, vascular bundle arrangement and ontogenetic development in leaf insertion zones and ramifications of three arborescent Araliaceae species. Trees 31, 1793–1809 (2017). https://doi.org/10.1007/s00468-017-1585-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1585-8