Abstract
Key message
Our study found that birch employs different physiological pathways to tolerance salt stress in roots and leaves, and the genes closely correlated with these physiological changes were identified.
Abstract
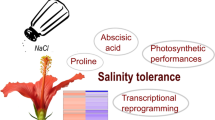
Birches are fast-growing woody plants that are adapted to adverse environments, and are widely distributed from north Europe to northeast Asia. However, the salt stress tolerance mechanism of birch has little been studied. Here, we investigated the physiological and molecular response of white birch (Betula Platyphylla) to salt stress. Long-term salt stress inhibited photosynthetic activity, and decreased stomatal conductance of birch. Abscisic acid was induced in birch during the early salt stress period, and Ca2+ level was increased slowly but maintained at a higher level for a long time. Under salt conditions, the salt-overly-sensitive pathway was activated in birch roots; reactive oxygen species (ROS) was highly accumulated, and superoxide dismutase is the main ROS scavenger in roots, while peroxidase is the main ROS scavenger in leaves. Proline plays a role in salt tolerance in both roots and leaves; however, soluble sugars and trehalose also have roles in salt stress tolerance, but mainly in leaves. Additionally, the genes that might have essential roles in controlling some of these physiological changes were identified, which represent good candidate genes to characterize the salt tolerance mechanism of birch. This study increased our understanding of the salt tolerance mechanism of birch plants.







Similar content being viewed by others
Abbreviations
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- DAB:
-
3,3′-Diaminobenzidine
- NBT:
-
Nitroblue tetrazolium
- MDA:
-
Malondialdehyde
- H2O2 :
-
Hydrogen Peroxide
- TBA:
-
Thiobarbituric acid
- Pn:
-
Net photosynthetic rate
- Tr:
-
Transpiration rate
- Gs:
-
Stomatal conductance
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- qRT-PCR:
-
Quantitative reverse transcription-polymerase chain reaction
- P5CS:
-
Delta1-pyrroline-5-carboxylate synthase
- CaM:
-
Calmodulin
- SOS:
-
Salt-overly-sensitive
- NHX:
-
Na+/H+ antiporter
- ABA:
-
Abscisic acid
- TPS:
-
Trehalose-6-phosphate synthase
- TPP:
-
Trehalose-phosphate phosphatase
References
Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63(16):5727–5740
Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, Segundo BS (2014) Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol 165:688–704
Cao WH, Liu J, He XJ (2007) Modulation of ethylene responses affects plant salt-sress responses. Plant Physiol 143:707–719
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11(2):113–116
Christmann A, Moes D, Himmelbach A, Yang Y, Tang Y, Grill E (2006) Integration of abscisic acid signalling into plant responses. Plant Biol 8(3):314–325
Czernicka M, Pławiak J, Muras P (2014) Genetic diversity of F1 and F2 interspecific hybrids between dwarf birch (Betula nana L.) and Himalayan birch (B. utilis var. jacquemontii (Spach) Winkl. ‘Doorenbos’) using RAPD-PCR markers and ploidy analysis. Acta Biochim Pol 61(2):195–199
Davenport RJ, Muñoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ 30(4):497–507
Downton WJS, Loveys BR (1981) Abscisic acid content and osmotic relations of salt-stressed grapevine leaves. Funct Plant Biol 8(5):443–452
Feki K, Quintero FJ, Khoudi H, Leidi EO, Masmoudi K, Pardo JM, Brini F (2014) A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Rep 33:277–288
Fryer MJ, Oxborough K, Mullineaux PM, Baker NR (2002) Imaging of photo-oxidative stress responses in leaves. J Exp Bot 53:1249–1254
Furlow J (1990) The genera of Betulaceae in the southeastern United States. J Arnold Arboretum 71:1–67
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics. doi:10.1155/2014/701596
He T, Cramer GR (1996) Abscisic acid concentrations are correlated with leaf area reductions in two salt-stressed rapid-cycling Brassica species. Plant Soil 179(1):25–33
James RA, Blake C, Byrt CS, Munns R (2011) Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J Exp Bot 62(8):2939–2947
Ji H, Pardo JM, Batelli G, Van Oosten MJ, Bressan RA, Li X (2013) The salt overly sensitive (SOS) pathway: established and emerging roles. Mol Plant 6:275–286
Katschnig D, Bliek T, Rozema J, Schat H (2015) Constitutive high-level SOS1 expression and absence of HKT1;1 expression in the salt-accumulating halophyte Salicornia dolichostachya. Plant Sci 234:144–154
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37(2):300–311
Kumar D, Singh P, Sarin NB, Yusuf MA (2014) Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea seedlings. Bio-protocol. doi:10.21769/BioProtoc.1108
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Lu XH, Sun DQ, Mo YW, Xi JG, Sun G (2010) Effects of post-harvest salicylic acid treatment on fruit quality and anti-oxidant metabolism in pineapple during cold storage. J Hortic Sci Biotechnol 85:454–458
Lunn JE, Delorge I, Figueroa CM, Van Dijck P, Stitt M (2014) Trehalose metabolism in plants. Plant J 79(4):544–567
Matsushita N, Matoh T (1991) Characterization of Na+ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol Plantarum 83:170–176
Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M (2009) Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21:2163–2178
Monihan SM, Magness CA, Yadegari R, Smith SE, Schumaker KS (2016) Arabidopsis CALCINEURIN B-LIKE10 functions independently of the SOS pathway during reproductive development in saline conditions. Plant Physiol 171(1):369–379
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99:9061–9066
Rahnama A, James RA, Poustini K, Munns R (2010) Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct Plant Biol 37(3):225–263
Scharf B, Clement CC, Zolla V, Perino G, Yan B, Elci SG, Purdue E, Goldring S, Macaluso F, Cobelli N, Vachet RW, Santambrogio L (2014) Molecular analysis of chromium and cobalt-related toxicity. Sci Rep 4(4):5729
Schiop ST, Hassan MA, Sestras AF (2015) Identification of salt stress biomarkers in romanian carpathian populations of Picea abies (L.) Karst. PLoS One 10(8):980–981
Si J, Zhou T, Bo W, Xu F, Wu R (2014) Genome-wide analysis of salt-responsive and novel microRNAs in Populus euphratica by deep sequencing. BMC Genet. doi:10.1186/1471-2156-15-S1-S6
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97
Wang C, Yang A, Yin H, Zhang J (2008) Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J Integr Plant Biol 50(4):427–434
Waters S, Gilliham M, Hrmova M (2013) Plant high-affinity potassium (HKT) Transporters involved in salinity tolerance: structural insights to probe differences in ion selectivity. Int J Mol Sci 14(4):7660–7680
Yadav NS, Shukla PS, Jha A, Agarwal PK, Jha B (2012) The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol 12(1):7–15
Yamaguchi T, Hamamoto S, Uozumi N (2013) Sodium transport system in plant cells. Front Plant Sci 4(410):410
Yang CP, Wei HR (2015) Designig microarray and RNA-Seq experiments for greater system biology discovery in modern plant genomics. Mol Plant 8:196–206
Yin J, Li C, Zhan Y, Sun H, Gong Y (2016) The response of physiological characteristics, expression of OSC genes, and accumulation of triterpenoids in Betula platyphylla Suk to MeJA and SA treatment. Plant Mol Biol Rep 34:427–439
Zhang Z, Huang RF (2013) Analysis of malondialdehyde, chlorophyll, proline, soluble sugar, and glutathione content in Arabidopsis seedling. Bio-protocol. doi:10.21769/BioProtoc.817
Zhang X, Wang L, Meng H, Wen H, Fan Y, Zhao J (2011) Maize ABP9 enhances tolerance to multiple stresses in transgenic Arabidopsis by modulating ABA signaling and cellular levels of reactive oxygen species. Plant Mol Biol 75:365–378
Zhang WX, Chu YG, Su XH (2014) Transcriptome sequencing of transgenic poplar (Populus × euramericana ‘Guaruento’) expressing multiple resistance genes. BMC Genet 15(1):1–17
Zhu X, Dunand C, Snedden W, Galaud JP (2015) CaM and CML emergence in the green lineage. Trends Plant Sci 20:483–489
Acknowledgements
This work was supported by the Project of the culture of the young scientists in scientific innovation in Xinjiang Province (2013711001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Communicated by S. Chen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mijiti, M., Zhang, Y., Zhang, C. et al. Physiological and molecular responses of Betula platyphylla Suk to salt stress. Trees 31, 1653–1665 (2017). https://doi.org/10.1007/s00468-017-1576-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1576-9