Abstract
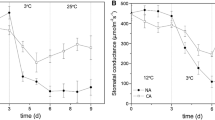
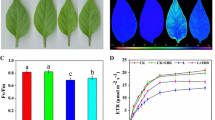
The aim of the present study was to assess the tolerance of Hevea brasiliensis to chilling temperatures since rubber production has been extended to sub-optimal environments. PB260 clone was used to analyze the responses of leaves chilled at 10°C during 96 h, as well as their recovery at 28°C. Some key parameters were used to evaluate photosynthetic apparatus functioning, membrane damage (electrolyte leakage) and oxidative stress. A short-term response versus a long-term one have been recorded, the time point of 24 h, when stomata closure was effective, being the border between the two responses. P n decreased dramatically at 1 h, and Fv/Fm was slightly affected. NPQ reached its maximal level between 4 and 7 h. Lipid peroxidation and membrane lysis were observed between 48 and 96 h. Activities of antioxidant enzymes increased, along with the induction of antioxidant gene expression. Finally, the plants were capable to recover (net photosynthetic rate, photochemical efficiency, antioxidant enzymes activities) when placed back to 28°C showing that PB260 can withstand long-term chilling.







Similar content being viewed by others
References
Alam B, Jacob J (2002) Overproduction of photosynthetic electrons is associated with chilling injury in green leaves. Photosynthetica. 40(1):91–95. doi:10.1023/A:1020110710726
Alam B, Nair DB, Jacob J (2005) Low temperature stress modifies the photochemical efficiency of a tropical tree species Hevea brasiliensis: effects of varying concentration of CO2 and photon flux density. Photosynthetica 43(2):247–252. doi:10.1007/S11099-005-0040-z
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6(1):36–42. doi:10.1016/S1360-1385(00)01808-2
Alonso A, Queiroz C, Magalhaes A (1997) Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings. Biochim Biophys Acta Biomembr 1323(1):75–84. doi:10.1016/S0005-2736(96)00177-0
Améglio T, Morizet J, Cruiziat P, Martignac M (1990) The effects of root temperature on water flux, potential and root resistance in sunflower. Agronomie 10:331–340. doi:10.1051/agro:19900407
Asada K (1996) Radical production and scavenging in chloroplasts. In: Baker NR (ed) Photosynthesis and the environment. Kluwer, Dordrecht, pp 123–150. doi:10.1007/0-306-48135-9
Assmann SM, Wang XQ (2001) From milliseconds to millions of years: guard cells and environmental responses. Curr Opin Plant Biol 4(5):421–428. doi:10.1016/S1369-5266(00)00195-3
Baek KH, Skinner D (2003) Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci 165(6):1221–1227. doi:10.1016/S0168-9452(03)00329-7
Barclay K, McKersie B (1994) Peroxidation reactions in plant membranes: effects of free fatty acids. Lipids 29(12):877–882. doi:10.1007/BF02536256
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbency changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25(3):173–185. doi:10.1007/BF00033159
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29:345–378. doi:10.1146/annurev.pp.29.060178.002021
Campos PS, Quartin Vn, Ramalho Jc, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol 160(3):283–292. doi:10.1078/0176-1617-00833
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11(2):113–116. doi:10.1007/BF02670468
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100(6):3525–3530. doi:10.1073/pnas.0635176100
Chow WS (1994) Photoprotection and photoinhibitory damage. In: Bittar EE (ed) Advanced molecular and cell biology. JAI Press Inc, Greenwich, pp 150–196
Cochard H, Martin R, Gross P, Bogeat-Triboulot MB (2000) Temperature effects on hydraulic conductance and water relations of Quercus robur L. J Exp Bot 51(348):1255–1259
Dey SK, Chandrashekar TR, Nair DB, Vijayakumar KR, Jacob J, Sethuraj MR (1998) Effect of some agro-climatic factors on the growth of rubber (Hevea brasiliensis) in a humid and dry subhumid location. Indian J Nat Rub Res 11:104–109
Drevet JR, Swevers L, Iatrou K (1995) Development regulation of a silkworm gene encoding multiple GATA-type transcription factors by alternative splicing. J Mol Biol 246(1):43–53. doi:10.1006/jmbi.1994.0064
Feng YL, Cao KF (2005) Photosynthesis and photoinhibition after night chilling in seedlings of two tropical tree species grown under three irradiances. Photosynthetica 43(4):567–574. doi:10.1007/s11099-005-0089-8
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. doi:10.1007/BF00386001
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717. doi:10.1111/j.1399-3054.1994.tb03042.x
Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 50:1533–1540. doi:10.1093/jexbot/50.338.1533
Gilmore AM, Hazlett TL, Govindjee (1995) Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime. Proc Natl Acad Sci USA 92:2273–2277. doi:10.1073/pnas.92.6.2273
Govindachary S, Bukhov NG, Joly D, Carpentier R (2004) Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and -tolerant plants. Physiol Plant 121(2):322–333. doi:10.1111/j.0031-9317.2004.00305.x
Guo FX, Zhang MX, Chen Y, Zhang WH, Xu SJ, Wang JH, An LZ (2006) Relation of several antioxidant enzymes to rapid freezing resistance in suspension cultured cells from alpine Chorispora bungeana. Cryobiology 52(2):241–250. doi:10.1016/j.cryobiol.2005.12.001
Hager A (1969) Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin- → Zeaxanthin-Umwandlung; Beziehungen zur Photophosphorylierung. Planta 89:224–243. doi:10.1007/BF00385028
Herbette S, Menn AL, Rousselle P, Ameglio T, Faltin Z, Branlard G, Eshdat Y, Julien JL, Drevet JR, Roeckel-Drevet P (2005) Modification of photosynthetic regulation in tomato overexpressing glutathione peroxidase. Biochim Biophys Acta 1724(1–2):108–118. doi:10.1016/j.bbagen.2005.04.018
Herbette S, Roeckel-Drevet P, Drevet JR (2007) Seleno-independent glutathione peroxidases. More than simple antioxidant scavengers. FEBS J. doi:10.1111/j.1742-4658.2007.05774.x
Hetherington AM (2001) Guard cell signaling. Cell 107(6):711–714. doi:10.1016/S0092-8674(01)00606-7
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4):604–611. doi:10.1007/S004250050524
Holá D, Kocová M, Wilhelmová Rothová O, Na Benesová M (2007) Recovery of maize (Zea mays L.) inbreds and hybrids from chilling stress of various duration: photosynthesis and antioxidant enzymes. J Plant Physiol 164(7):868–877. doi:10.1016/j.jplph.2006.04.016
Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC (1992) Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol 98:1105–1114. doi:10.1104/pp.98.3.1105
Ivanov B, Edwards G (2000) Influence of ascorbate and the Mehler peroxidase reaction on non-photochemical quenching of chlorophyll fluorescence in maize mesophyll chloroplasts. Planta 210(5):765–774. doi:10.1007/s004250050678
Jacob J, Annmalainathan K, Alam BM, Sathick MB, Thapaliyal AP, Devakumar AS (1999) Physiological constraints for cultivation of Hevea brasiliensis in certain unfavourable agroclimatic regions of India. Indian J Nat Rub Res 12:1–16
Jung S, Steffen KL, Jae Lee H (1998) Comparative photoinhibition of a high and a low altitude ecotype of tomato (Lycopersicon hirsutum) to chilling stress under high and low light conditions. Plant Sci 134(1):69–77. doi:10.1016/S0168-9452(98)00051-X
Kotabová E, Kana R, Kyseláková H, Lípová L, Novák O, Ilík P (2008) A pronounced light-induced zeaxanthin formation accompanied by an unusually slight increase in non-photochemical quenching: a study with barley leaves treated with methyl viologen at moderate light. J Plant Physiol 165(15):1563–1571. doi:10.1016/j.jplph.2008.01.005
Krause GH, Briantais JM, Vernotte C (1983) Characterization of chlorophyll fluorescence spectroscopy at 77 KI. ∆pH-dependent quenching. Biochim Biophys Acta Bioenerg. doi:10.1016/0005-2728(83)90116-0
Leshem YY (1987) Membrane phospholipid catabolism and Ca2+ activity in control of senescence. Physiol Plant 69:551–559. doi:10.1111/j.1399-3054.1987.tb09239.x
Li XG, Meng QW, Jiang GQ, Zou Q (2003) The susceptibility of cucumber and sweet pepper to chilling under low irradiance is related to energy dissipation and water–water cycle. Photosynthetica 41(2):259–265. doi:10.1023/B:PHOT.0000011959.30746.c0
Li XG, Duan W, Meng QW, Zou Q, Zhao SJ (2004) The function of chloroplastic NAD(P)H dehydrogenase in tobacco during chilling stress under low irradiance. Plant Cell Physiol 45(1):103–108. doi:10.1093/pcp/pch011
Logan BA, Adams WW, Demmig-Adams B (2007) Avoiding common pitfalls of chlorophyll fluorescence analysis under field conditions. Funct Plant Biol 34(9):853–859. doi:10.1071/FP07113
Lu P, Sang WG, Ma KP (2007) Activity of stress-related antioxidative enzymes in the invasive plant crofton weed (Eupatorium adenophorum). J Integr Plant Biol 49(11):1555–1564. doi:10.1111/j.1774-7909.2007.00584.x
McCord J, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McKersie BD, Chen YR, De Beus M, Bowler SR, Inzé D, D’Halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol 103:1155–1163. doi:10.1104/pp.103.4.1155
Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128:970–977. doi:10.1104/pp.010924
Nualpun S, Pluang S, Russell FD, Wallie S (2005) Molecular cloning of a new cDNA and expression of 3-hydroxy-3-menthylglutaryl-CoA synthase gene from Hevea brasiliensis. Planta 221:502–512. doi:10.1007/s00425-004-1463-7
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45. doi:10.1093/nar/29.9.e45
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Priyadarshan PM, de Goncalves SP (2003) Hevea gene pool for breeding. Genet Resour Crop Evol 50:101–114. doi:1023/A:1022972320696
Pushparajah E (1983) Problems and potentials for establishing Hevea under difficult environmental conditions. Planter 59:242–251
Queiroz CGS, Alonso A, Mares-Guia M, Magalhães AC (1998) Chilling-induced changes in membrane fluidity and antioxidant enzyme activities in Coffea Arabica L. roots. Biol Plant 41(3):403–413. doi:10.1023/A:1001802528068
Raj S, Das G, Pothen J, Dey S (2005) Relationship between latex-yield of Hevea brasiliensis and antecedent environmental parameters. Int J Biometeorol 49:189–196. doi:10.1007/s00484-004-0222-6
Rao PS, Saraswathyamma CK, Sethuraj MR (1998) Studies on the relationship between yield and meteorological parameters of para rubber tree (Hevea brasiliensis). Agr Forest Meteorol 90(3):235–245. doi:10.1016/S0168-1923(98)00051-3
Schroeder JI, Kwak JM, Allen GJ (2001) Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410:327–330. doi:10.1038/35066500
Simon E (1974) Phospholipids and plant membrane permeability. New Phytol 73:377–420. doi:10.1111/j.1469-8137.1974.tb02118.x
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–69. doi:10.1016/0076-6879(90)82008-P
Sui N, Li M, Liu XY, Wang N, Fang W, Meng QW (2007) Response of xanthophyll cycle and chloroplastic antioxidant enzymes to chilling stress in tomato over-expressing glycerol-3-phosphate acyltransferase gene. Photosynthetica 45(3):447–454. doi:10.1007/s11099-007-0074-5
Szabó I, Bergantino E, Giacometti GM (2005) Light and oxygenic photosynthesis: energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep. doi:10.1038/sj.embor.7400460. Review
Tallman G (2004) Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? J Exp Bot 167(1):19–26. doi:10.1093/jxb/erh212
Tambussi E, Bartoli C, Guimet J, Beltrano J, Araus J (2004) Oxidative stress and photodamage of low temperatures in soybean (Glycine max L. Merr.) leaves. Plant Sci. doi:10.1016/j.plantsci.2004.02.018
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Ann Rev Plant Physiol Plant Mol Biol 50:571–599. doi:10.1146/annurev.arplant.50.1.571
Venkatachalam P, Priya P, Gireesh T, Amma Saraswathy, Thulaseedharan A (2006) Molecular cloning and sequencing of a polymorphic band from rubber tree [Hevea brasiliensis (Muell.) Arg.]: the nucleotide sequence revealed partial homology with praline-specific permease gene sequence. Curr Sci 90:1510–1515
Wilkinson S, Clephan AL, Davies WJ (2001) Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol 126:1566–1578. doi:10.1104/pp.126.4.1566
Wycherley PR (1992) The genus Hevea—botanical aspects. In: Sethural MR, Mathew NM (eds) Natural rubber: biology cultivation and technology. Developments in crop science, vol 23. Elsevier, Amsterdam, pp 50–66
Xu CC, Jeon YA, Lee CH (1999) Relative contributions of photochemical and non-photochemical routes to excitation energy dissipation in rice and barley illuminated at a chilling temperature. Physiol Plant. doi:10.1034/j.1399-3054.1999.100411.x
Yoshimura K, Miyao K, Gaber A, Takeda T, Kanaboshi H, Miyasaka H, Shigeoka S (2004) Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J. doi:10.1046/j.1365-313X.2003.01930.x
Acknowledgments
We are indebted to Dr. David Biron (Canadian scientist) for English grammar and syntax corrections. We are very grateful to the two anonymous referees for their insightful comments which substantially improved this paper. This work was supported in part by MICHELIN (Clermont-Ferrand, France).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Bilger.
Rights and permissions
About this article
Cite this article
Mai, J., Herbette, S., Vandame, M. et al. Effect of chilling on photosynthesis and antioxidant enzymes in Hevea brasiliensis Muell. Arg.. Trees 23, 863–874 (2009). https://doi.org/10.1007/s00468-009-0328-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-009-0328-x