Abstract
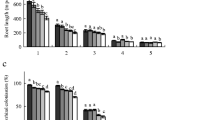
Wood structure might be altered through the physiological responses to atmospheric carbon dioxide concentration ([CO2]) and nitrogen (N) deposition. We investigated growth, water relations and wood structure of 1-year-old seedlings of two deciduous broad-leaved tree species, Quercus mongolica (oak, a ring-porous species) and Alnus hirsuta (alder, a diffuse-porous species and N2–fixer), grown under a factorial combination of two levels of [CO2] (36 and 72 Pa) and nitrogen supply (N; low and high) for 141 days in phytotron chambers. In oak, there was no significant effect of [CO2] on wood structure, although elevated [CO2] tended to decrease stomatal conductance (g s) and increased water use efficiency regardless of the N treatment. However, high N supply increased root biomass and induced wider earlywood and larger vessels in the secondary xylem in stems, leading to increased hydraulic conductance. In alder, there was significant interactive effect of [CO2] and N on vessel density, and high N supply increased the mean vessel area. Our results suggest that wood structures related to water transport were not markedly altered, although elevated [CO2] induced changes in physiological parameters such as g s and biomass allocation, and that N fertilization had more pronounced effects on non-N2-fixing oak than on N2-fixing alder.





Similar content being viewed by others
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Arp WJ (1991) Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875
Asher CJ, Edwards DG (1983) Modern solution culture techniques. In: Läuchli A, Bieleski RL (eds) Inorganic plant nutrition. Encyclopedia of plant physiology, NS. vol 15A. Springer, Berlin, pp 94–119
Atkinson CJ, Taylor JM (1996) Effects of elevated CO2 on stem growth, vessel area and hydraulic conductivity of oak and cherry seedlings. New Phytol 133:617–626
Bucher JB, Tarjan DP, Siegwolf RTW, Saurer M, Blum H, Hendrey GR (1998) Growth of a deciduous tree seedling community in response to elevated CO2 and nutrient supply. Chemosphere 36:777–782
Ceulemans R, Mousseau M (1994) Effects of elevated atmospheric CO2 on woody plants. New Phytol 127:425–446
Chaney WR, Kozlowski TT (1977) Patterns of water movement in intact and excised stems of Fraxinus americana and Acer saccharum seedlings. Ann Bot 41:1093–1100
Cooke JEK, Brown KA, Wu R, Davis JM (2003) Gene expression associated with N-induced shifts in resource allocation in poplar. Plant Cell Environ 26:757–770
Dawson JO (1983) Dinitrogen fixation in forest ecosystems. Can J Microbiol 29:979–992
Ericsson T, Rytter L, Vapaavuori E (1996) Physiology of carbon allocation in trees. Biomass Bioenergy 11:115–127
Gartner BL, Roy J, Huc R (2003) Effects of tension wood on specific conductivity and vulnerability to embolism of Quercus ilex seedlings grown at two atmospheric CO2 concentrations. Tree Physiol 23:387–395
Hättenschwiler S, Schweingruber FH, Körner Ch (1996) Tree ring responses to elevated CO2 and increased N deposition in Picea abies. Plant Cell Environ 19:1369–1378
Heath J, Kerstiens G (1997) Effects of elevated CO2 on leaf gas exchange in beech and oak at two levels of nutrient supply: consequences for sensitivity to drought in beech. Plant Cell Environ 20:57–67
Hibbs DE, Chan SS, Castellano M, Niu CH (1995) Response of red alder seedlings to CO2 enrichment and water stress. New Phytol 129:569–577
Houghton JT, Ding Y, Griggs DJ, Noguer M, van des Linden PJ, Dai X, Maskell K, Johnson CA (2001) Climate change 2001: the scientific basis: contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York
Kaakinen S, Kostiainen K, Ek F, Saranpää P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E (2004) Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Global Change Biol 10:1513–1525
Kirschbaum MUF (2004) Direct and indirect climate change effects on photosynthesis and transpiration. Plant Biol 6:242–253
Koike T (1988) Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol 3:77–87
Koike T (1995) Effects of CO2 in interaction with temperature and soil fertility on the foliar phenology of alder, birch, and maple seedlings. Can J Bot 73:149–157
Körner Ch (1995) Towards a better experimental basis for upscaling plant responses to elevated CO2 and climate warming. Plant Cell Environ 18:1101–1110
Kostiainen K, Kaakinen S, Saranpää P, Sigurdsson BD, Linder S, Vapaavuori E (2004) Effect of elevated [CO2] on stem wood properties of mature Norway spruce grown at different soil nutrient availability. Global Change Biol 10:1526–1538
Kostiainen K, Jalkanen H, Kaakinen S, Saranpää P, Vapaavuori E (2006) Wood properties of two silver birch clones exposed to elevated CO2 and O3. Global Change Biol 12:1230–1240
Luo ZB, Langenfeld-Heyser R, Calfapietra C, Polle A (2005) Influence of free air CO2 enrichment (EUROFACE) and nitrogen fertilisation on the anatomy of juvenile wood of three poplar species after coppicing. Trees 19:109–118
Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomäki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149:247–264
Norby RJ, Wullschleger SD, Gunderson CA, Nietch CT (1995) Increased growth efficiency of Quercus alba trees in a CO2-enriched atmosphere. New Phytol 131:91–97
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol 162:253–280
Oh HK, Choung Y (2005) Does elevated CO2 affect the physiology and growth of Quercus mongolica under different nitrogen conditions? Key Eng Mat 277–279:528–535
Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schäfer KVR, McCarthy H, Hendrey G, McNulty SG, Katul GG (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enrichment atmosphere. Nature 411:469–472
SAS Institute (2003) JMP: statistics and graphics guide, version 5.1. SAS Institute, Cary
Savill PS, Mather RA (1990) A possible indicator of shake in oak: relationship between flushing dates and vessel sizes. Forestry 63:355–362
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
Sperry JS, Ikeda T (1997) Xylem cavitation in roots and stems of Douglas-fir and white fir. Tree Physiol 17:275–280
Telewski FW, Swanson RT, Strain BR, Burns JM (1999) Wood properties and ring width responses to long-term atmospheric CO2 enrichment in field-grown loblolly pine (Pinus taeda L.). Plant Cell Environ 22:213–219
Tjepkema JD (1985) Utilization of photosynthate for nitrogen fixation in seedlings of Myrica gale and Alnus rubra. In: Ludden PW, Burris JE (eds) Nitrogen fixation and CO2 metabolism. Elsevier, New York, pp 183–192
Tobita H, Kitao M, Koike T, Maruyama Y (2005) Effects of elevated CO2 and nitrogen availability on nodulation of Alnus hirsuta Turcz. Phyton 45:125–131
Tognetti R, Johnson JD (1999) Responses of growth, nitrogen and carbon partitioning to elevated atmospheric CO2 concentration in live oak (Quercus virginiana Mill.) seedlings in relation to nutrient supply. Ann For Sci 56:91–105
Tyree MT, Alexander JD (1993) Plant water relations and the effects of elevated CO2: a review and suggestions for future research. Vegetatio 104/105:47–62
Urban O (2003) Physiological impacts of elevated CO2 concentration ranging from molecular to whole plant responses. Photosynthetica 41:9–20
Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J (1998) Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol 117:1463–1471
Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J (1999) The progression of cavitation in earlywood vessels of Fraxinus mandshurica var japonica during freezing and thawing. Plant Physiol 121:897–904
Vogel CS, Curtis PS, Thomas RB (1997) Growth and nitrogen accretion of dinitrogen-fixing Alnus glutinosa (L.) Gaertn. under elevated carbon dioxide. Plant Ecol 130:63–70
Wallace ZP, Lovett GM, Hart JE, Machona B (2007) Effects of nitrogen saturation on tree growth and death in mixed-oak forest. For Ecol Manage 243:210–218
Yazaki K, Funada R, Mori S, Maruyama Y, Abaimov AP, Kayama M, Koike T (2001) Growth and annual ring structure of Larix sibirica grown at different carbon dioxide concentrations and nutrient supply rates. Tree Physiol 21:1223–1229
Yazaki K, Ishida S, Kawagishi T, Fukatsu E, Maruyama Y, Kitao M, Tobita H, Koike T, Funada R (2004) Effects of elevated CO2 concentration on growth, annual ring structure and photosynthesis in Larix kaempferi seedlings. Tree Physiol 24:941–949
Yazaki K, Maruyama Y, Mori S, Koike T, Funada R (2005) Effects of elevated carbon dioxide concentration on wood structure and formation in trees. In: Omasa K, Nouchi I, De Kok LJ (eds) Plant responses to air pollution and global change. Springer, Tokyo, pp 89–97
Acknowledgments
We thank Dr. S. Kitaoka and Ms. H. Taoka for their help in sampling and Mr. M. Tanaka, Mr. K. Mima and Ms. N. Morii for technical assistance. This study was supported in part by a Grant-in-Aid for Research Revolution 2002 (RR2002) Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a Grant-in-Aid for Scientific Research (A) from Japan Society for the Promotion of Science (17,208,013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Linder.
Rights and permissions
About this article
Cite this article
Watanabe, Y., Tobita, H., Kitao, M. et al. Effects of elevated CO2 and nitrogen on wood structure related to water transport in seedlings of two deciduous broad-leaved tree species. Trees 22, 403–411 (2008). https://doi.org/10.1007/s00468-007-0201-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-007-0201-8