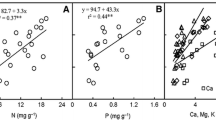
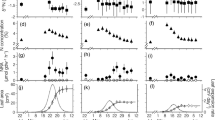
Abstract
Araucaria angustifolia (Bertol.) Kuntze is an indigenous conifer restricted to the southern region of South America. In this on-site field study, we provide a detailed description of the nitrogen compounds and sugars allocated to the different plant compartments in tall adult trees, young trees about 2–3 m tall and small seedlings at its northernmost occurrence in the mountains of Itatiaia (20°25′S; 44°50′W; 2,000 m a.s.l.), SE Brazil. We determined C and N contents, soluble sugars, soluble non-protein N-compounds and δ15 N-signatures in leaves, roots, wood of stems, xylem- and phloem- sap. We also measured chlorophyll a fluorescence of photosystem II and carbon isotope discrimination reflecting photosynthetic activity and water-use efficiency. The high C and N concentrations in fine roots suggest that they are important reservoirs of N and C. Most nitrogen taken up from the soil was metabolised in the roots. The only inorganic nitrogen form detectable in the xylem sap was a small amount of ammonium. Glutamine was the dominant transport form of nitrogen in the xylem, while glutamate and the amides glutamine and asparagine were the most abundant soluble N compounds in the phloem. Total soluble non-protein N and sugar concentrations were significantly higher in the phloem of adult trees. In this particular site, A. angustifolia was apparently not exposed to water stress, as indicated by the high values of carbon isotope discrimination. The three developmental stages were clearly separated in terms of photosynthetic performance. Indeed, effective quantum yield of photosystem II increased from seedlings to adult trees under ambient irradiance.





Similar content being viewed by others
References
Adams MA, Grierson PF (2001) Stable isotopes at natural abundance in terrestrial plant ecology and ecophysiology: an update. Plant Biol 3:299–310
Bauer GA, Persson H, Persson T, Mund M, Hein M, Kummetz E, Matteucci G, van Oen H, Scarascia-Mugnozza G, Schultze E-D (2000) Linking plant nutrition and ecosystem processes. In: Schultze E-D (ed) Carbon and nitrogen cycling in European forest ecosystems. Ecological studies, vol 142. Springer, Berlin Heidelberg New York, pp 63–98
Behling H (1998) Late Quaternary vegetation and climatic changes in Brazil. Rev Paleobot Palynol 99:143–156
Behling H (2002) South and southeast Brazilian grasslands during Late Quaternary times: a synthesis. Palaeogeogr Palaeoclimatol Palaeoecol 177:19–27
Bilger W, Schreiber U, Bock M (1995) Determination of quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 102:425–432
Blechschmidt-Schneider S, Eschrich W, Jahnke S (1997) Phloem loading, translocation, and unloading processes. In: Rennenberg H, Eschrich W, Ziegler H (eds) Trees—contributions to modern tree physiology. Backhuys, Leiden, The Netherlands, pp 139–163
Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170:489–504
Breuninger M, Einig W, Magel E, Cardoso E, Hampp R (2000) Mycorrhiza of Brazil Pine (Araucaria angustifolia [Bert.O. Ktze.]). Plant Biol 2:4–10
Broadmeadow MSJ, Griffiths H, Maxwell C, Borland A (1992) The carbon isotope ratio of plant organic material reflects temporal and spatial variations in CO28 within tropical forest formations in Trinidad. Oecologia 89:435–441
Damesin C, Lelarge C (2003) Carbon isotope composition of current-year shoots from Fagus sylvatica in relation to growth, respiration and use of reserves. Plant Cell Environ 26:207–219
Demmig-Adams B (1990) Carotenoids and photoprotection: a role for the xanthophyll zeaxanthin cycle. Biochim Biophys Acta 1020:1–24
Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:599–626
Deniro M J, Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid biosynthesis. Science 197:261–263
Duarte LD, Dillenburg LR (2000) Ecophysiological responses of Araucaria angustifolia (Araucariaceae) seedlings to different irradiance levels. Aust J Bot 48:531–537
Duarte LD, Dillenburg LR, Rosa LMG (2002) Assessing the role of light availability in the regeneration of Araucaria angustifolia (Araucariaceae). Aust J Bot 50:741–751
Einig W, Mertz A, Hampp R (1999) Growth rate, photosynthetic activity, and leaf development of Brazil pine seedlings (Araucaria angustifolia [Bert] O. Ktze). Plant Ecol 143:23–28
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:121–126
Farquhar GD, Ehleringer JR, Hubick KT (1989a) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol 40:503–537
Farquhar GD, Hubick KT, Condon AG, Richards RA (1989b) Carbon isotope fractionation and plant water-use efficiency. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotopes in ecological research. Ecological studies, vol 68. Springer, Berlin Heidelberg New York, pp 21–40
Fotelli MN, Rennenberg H, Holst T, Mayer H, Geßler A (2003) Carbon isotope composition of various tissues of beech (Fagus sylvatica) is indicative of recent environmental conditions within the forest understorey. New Phytol 159:229–244
Franco AC, Lüttge U (2002) Midday depression in savanna trees: coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 131:356–365
Geßler A, Schneider S, Weber P, Hanemann U, Rennenberg H (1998) Soluble N compounds in trees exposed to high loads of N: a comparison between the roots of Norway spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol 138:385–399
Geßler A (1999) Untersuchungen zum N-Haushalt der Buche (Fagus sylvatica) in einem N-übersättigten Waldökosystem. PhD thesis, University of Freiburg, Germany
Geßler A, Rennenberg H, Keitel C (2004) Stable isotope composition of organic compounds transported in the phloem of European beech—evaluation of different methods of phloem sap collection and assessment of gradients in carbon isotope composition during leaf-to-stem transport. Plant Biol (in press)
Glad C, Regnard JL, Querou Y, Brun O, Morot-Gaudry JF (1992) Fluxes and chemical composition of xylem exudates from Chardonnay grapevines—temporal evolution and effect of recut. Am J Enol Vinicult 43:275–282
Hampp R, Mertz A, Schaible R, Schwaigerer M, Nehls U (2000) Distinction of Araucaria angustifolia seeds from different locations in Brazil by a specific DNA sequence. Trees 14:429–434
Heizmann U, Kreuzwieser J, Schnitzler J-P, Brüggemann N, Rennenberg H (2001) Assimilate transport in the xylem sap of pedunculate oak (Quercus robur) saplings. Plant Biol 3:132–138
Hobbie EA, Werner RA (2004) Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol 161:371–385
Horton P, Ruban AV, Walters RG (1994) Regulation of light-harvesting in green plants–indication by nonphotochemical quenching of chlorophyll fluorescence. Plant Physiol 106:415–420
Hueck K (1972) As florestas da America do Sul: ecologia, composicao e importancia economica (a translation by Hans Reichardt from the original Die Waelder Sudamerikas, 1966, Gustav Fischer.). Editora Universidade de Brasilia, Brasilia
Keitel C, Adams MA, Holst T, Matzarakis A, Mayer H, Rennenberg H, Geßler A (2003) Carbon and oxygen isotope composition of organic compounds in the phloem sap provides a short-time measure for stomatal conductance of European beech (Fagus sylvativa). Plant Cell Environ 26:1157–1168
Kershaw P, Wagstaff B (2001) The southern conifer family Araucariaceae: history, status and value for paleoenvironmental reconstruction. Annu Rev Ecol Syst 32:397–414
Ledru MP, Salgado-Labouriau ML, Lorscheiter ML (1998) Vegetation dynamics in southern and central Brazil during the last 10 000 yr BP. Rev Paleobot Palynol 99:131–142
Martinelli LK, Piccolo MC, Townsend AR, Vitousek PM, Cuevas E, Mc Dowell W, Robertson GP, Santos OC, Treseder K (1999) Nitrogen stable isotope composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46:45–65
Morselli MF, Marvinn JW, Laing FM (1978) Image-analysing computer in plant science: more and larger vascular rays in sugar maples of high sap and sugar yield. Can J Bot 56:983–986
Osmond CD, Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46:1351–1362
Pfündel E, Bilger W (1994) Regulation and possible function of the violaxanthin cycle. Photosynth Res 42:89–109
Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23:1397–1405
Rennenberg H, Schneider S, Weber P (1996) Analysis of uptake and allocation of nitrogen and sulphur compounds by trees in the field. J Exp Bot 47:1491–1498
Rizzini CT (1997) Tratado de fitogeografia do Brasil, 2nd edn. Âmbito Cultural Edições, Rio de Janeiro
Sauter JJ (1980) Seasonal variations of sucrose content in the xylem sap of Salix. Z Pflanzenphysiol 98:377–391
Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic rain forest. Ann Bot 90:517–524
Scarano FR, Duarte HM, Ribeiro KT, Rodrigues PJFP, Barcellos EMB, Franco AC, Brulfert J, Deléens E, Lüttge U (2001) Four sites with contrasting environmental stress in southeastern Brazil: relations of species, life form diversity, and geographic distribution to ecophysiological parameters. Bot J Linn Soc 136:345–364
Schindler C, Lichtenthaler HK (1996) Photosynthetic CO2-assimilation, chlorophyll fluorescence and zeaxanthin accumulation in field grown maple trees in the course of a sunny and a cloudy day. J Plant Physiol 148:399–412
Schmidt S, Stewart GR (1998) Transport, storage and mobilisation of nitrogen by trees and shrubs in wet/dry tropics of Northern Australia. Tree Physiol 18:403–410
Schneider S, Geßler A, Weber P, v. Sengbusch D, Hanemann U, Rennenberg H (1996) Soluble N compounds in trees exposed to high loads of N: a comparison of spruce (Picea abies) and beech (Fagus sylvatica) grown under field conditions. New Phytol 134:103–114
Segadas-Vianna F, Dau L. (1965) Ecology of the Itatiaia range, southeastern Brazil. II—Climates. Arq Mus Nac 53:31–53
Winter H, Lohaus G, Heldt W (1992) Phloem transport of amino acids in relation to their cytosolic levels in barley leaves. Plant Physiol 99:996–1004
Zandavalli R, Dillenburg LR, de Souza PVD (2004) Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with the mycorrhizal fungus Glomus clarum. Appl Soil Ecol 25:245–255
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall, Upper Saddle River, N.J.
Acknowledgements
This investigation was supported by grants from PROBRAL (CAPES-DAAD), Pronex I and II (CNPq/FINEP), CAPES (Brazilian Education Council) and CNPq (Brazilian Research Council) and a grant to A.C.F. from the David Rockefeller Centre for Latin American Studies at Harvard University
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franco, A.C., Duarte, H.M., Geβler, A. et al. In situ measurements of carbon and nitrogen distribution and composition, photochemical efficiency and stable isotope ratios in Araucaria angustifolia. Trees 19, 422–430 (2005). https://doi.org/10.1007/s00468-004-0401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-004-0401-4