Abstract
Background
Continuous kidney replacement therapy (CKRT) has recently become the preferred kidney replacement modality for children with acute kidney injury (AKI). We hypothesise that CKRT technical parameters and treatment settings in addition to the clinical characteristics of patients may influence the circuit lifetime in children.
Methods
The study involved children included in the EurAKId registry (NCT 02960867), who underwent CKRT treatment. We analysed patient characteristics and CKRT parameters. The primary end point was mean circuit lifetime (MCL). Secondary end points were number of elective circuit changes and occurrence of dialysis-related complications.
Results
The analysis was composed of 247 children who underwent 37,562 h of CKRT (median 78, IQR 37–165 h per patient). A total of 1357 circuits were utilised (3, IQR 2–6 per patient). MCL was longer in regional citrate anticoagulation (RCA), compared to heparin (HA) and no anticoagulation (NA) (42, IQR 32-58 h; 24, IQR 14-34 h; 18, IQR 12-24 h, respectively, p < 0.001). RCA was associated with longer MCL regardless of the patient’s age or dialyser surface. In multivariate analysis, MCL correlated with dialyser surface area (beta = 0.14, p = 0.016), left internal jugular vein vascular access site (beta = -0.37, p = 0.027), and the use of HA (beta = -0.14, p = 0.038) or NA (beta = -0.37, p < 0.001) vs. RCA. RCA was associated with the highest ratio of elective circuit changes and the lowest incidence of complications.
Conclusion
Anticoagulation modality, dialyser surface, and vascular access site influence MCL. RCA should be considered when choosing first-line anticoagulation for CKRT in children. Further efforts should focus on developing guidelines and clinical practice recommendations for paediatric CKRT.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a common condition with an increasing incidence in the paediatric population [1]. A recent meta-analysis encompassing studies from 26 countries revealed that AKI was diagnosed in 26% of hospitalised children across high-, middle-, and low-income countries [2]. In a paediatric intensive care unit setting, a large prospective multinational study corroborated a comparable incidence of AKI, with severe AKI being observed in 11.6% of cases [3]. Furthermore, AKI is linked to heightened risks of both short- and long-term adverse outcomes, including prolonged hospitalisation, chronic kidney disease, and mortality [3, 4].
Continuous kidney replacement therapy (CKRT) has become the preferred treatment modality for children with dialysis-dependent AKI [5]. CKRT holds superior appeal in critically ill children as it allows smooth fluid removal, avoids the disequilibrium syndrome seen with intermittent haemodialysis (iHD) and provides better purification efficacy and more precise fluid balance than peritoneal dialysis (PD) [6]. Nevertheless, the effectiveness of CKRT depends on the ability to preserve a circuit until it reaches its target lifetime. Premature filter clotting causes disruption and reduces the attainable ultrafiltration and solute clearance, while concurrently increasing blood loss and hemodynamic instability, workload, and treatment costs [7]. In the context of long-term therapy, effective anticoagulation strategies are of great importance. Unfortunately, up to 27% of dialyzers clot prematurely [8], necessitating unexpected circuit changes. In the adult population, RCA was proven to prolong the circuit lifetime compared to HA [9], and it is currently considered standard of care [10]. In recent years, RCA has also been successfully used in children, resulting in the creation of simplified protocols [11]. However, the absence of standardised paediatric protocols and the wide range of patient age, body mass and treatment indications, make CKRT in the paediatric population currently more challenging than in adults. Hence, it is essential to identify the modifiable factors affecting circuit survival time in order to develop clinical practice recommendations for paediatric CKRT. This study aimed to determine these factors and to evaluate the management and technicalities of CKRT procedures in children across Europe.
Materials and methods
EurAKId Registry
The EurAKId Registry (ClinicalTrials.gov NCT 02960867) was launched in September 2016 by the European Study Consortium for Chronic Kidney Disorders Affecting Paediatric Patients (ESCAPE) Network. It is a prospective, multicentre, international, observational study that collects data on acute kidney replacement therapies (KRT) in children using a web-based case report form. To date, 14 European paediatric nephrology centres have participated in the study. All participating institutions have obtained approvals from the local institutional review boards.
The registry collects data on children aged 0–18 years, treated with KRT at hospital admission or during hospitalisation, both in and outside PICU, and includes different dialysis modalities (PD, iHD, or CKRT) according to the local standards of care. The indications for acute KRT include AKI, as well as other reasons: metabolic decompensation, acute respiratory distress, sepsis, and fluid overload (FO). Children with known pre-existing chronic kidney disease are excluded. Defining AKI diagnosis, AKI staging, and exclusion of chronic kidney disease are the responsibility of on-site investigators.
Data capture involves demographic and baseline data, clinical data at the time of PICU admission and at the onset of dialysis, technical and procedural data related to the dialysis modality, and data on outcomes. All definitions used were reported previously [11].
Study population
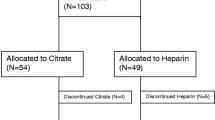
This study is a retrospective analysis of prospectively collected data focused on patients who received CKRT between September 2016 and September 2022. During this period, 443 patients were reported to the registry, of whom 301 from 11 centres received CKRT (Supplementary Fig. 1). After excluding 47 patients who underwent CKRT within an ECMO system, 5 children receiving tandem CKRT-plasma exchange, and 2 patients with significant missing data, 247 patients were included in the analysis (Fig. 1).
Patient characteristics
From the data collected in the EurAKId Registry, we selected the baseline patient characteristics to define the examined population. The demographic and anthropometric data included: age [years], sex, race, initial body mass [kg], and BSA [m2]. Among the clinical parameters, we included the presence and stage of AKI, the presence of multi-organ dysfunction syndrome (MODS) and the number of organs involved, primary disease and comorbidities, the need for vasopressors use, the need for PICU admission, and fluid overload at the start of CKRT [%]. The primary disease was defined as the patient's principal disorder at the time of hospitalisation and comprised 12 categories: kidney disease, liver disease, pulmonary disease, cardiac disease, haematologic disease/bone marrow transplant (BMT), shock (including septic shock), malignancy/tumour lysis syndrome (TLS), inborn errors of metabolism (IEM), drug intoxication, immunologic disorder, crush syndrome, and neurologic disease. We included cardiac, pulmonary, neurologic, hepato-intestinal, hematologic, immunologic, septic, metabolic, and kidney comorbidities.
Technical CKRT parameters and management
We analysed the technical aspects associated with CKRT initiation. These included the CKRT modality (CVVH, CVVHD, CVVHDF), vascular access site (right or left internal jugular vein, femoral vein, subclavian vein), dialysis membrane surface, and the administration and method of anticoagulation: no anticoagulation (NA), regional citrate anticoagulation (RCA), and heparin anticoagulation (HA). The dialysis filters were divided into three groups based on the dialysis membrane surface: small (membrane surface < 0.5m2), medium (between 0.5 and 1m2), and large (≥ 1m2). The analysed CKRT settings were: blood flow rate [ml/min], dialysate flow rate [ml/h], replacement fluid flow [ml/h], prescribed dialysis dose [ml/h], and ultrafiltration rate [ml/h]. All parameters were expressed as absolute values as well as adjusted for the patient’s body weight [ml/min/kg or ml/h/kg where applicable]. The prescribed dialysis dose was defined as the replacement flow rate (for CVVH), the dialysate flow rate (CVVHD), or the sum of both flow rates (for CVVHDF), and was expressed as absolute values as well as adjusted for the patient’s BSA [L/h/m2] and body weight [ml/h/kg]. We also assessed the total number of filters utilised and the total CKRT duration.
Endpoints
The primary endpoint was the mean circuit lifetime expressed in hours, as reported in the EurAKId registry. The secondary endpoints were the number of elective circuit changes and the occurrence of dialysis-related complications. The filter change was considered elective in case of treatment termination due to diagnostic or therapeutic procedures, reaching the circuit target lifetime, and technical issues not associated with circuit malfunction. We defined four groups of complications: catheter dislocation, bleeding, thrombosis, and other complications.
Statistical analysis
Collected data were statistically analysed using Dell Statistica 13.3 software.
The normality of data was assessed using the Shapiro–Wilk test. Depending on the distribution, data were expressed as mean ± standard deviation (SD) for variables with normal distribution or median (interquartile range — IQR) for variables with distribution other than normal. The following statistical tests were applied: Mann–Whitney U test for independent groups, Spearman’s rank correlation, Kruskal–Wallis ANOVA test, and chi-squared test. Multivariate analysis was performed using the general step-wise linear regression models. The variables were introduced into the model, excluding those correlated with each other with r > 0.60 to avoid collinearity. The criterion for inclusion in the final model was p < 0.050. The results of multivariate analyses were expressed as beta, confidence interval (CI), and p-value. The results were considered statistically significant, with p values < 0.050.
Results
Patient characteristics
The general characteristics of the cohort are presented in Table 1. Median age at the start of CKRT was 4.1 (IQR 1.2–12) years. The study group comprised 23.1% of neonates and infants (up to 12 months of age, n = 57), 35.2% of young children (1–6 years, n = 87), 15.8% of children (6–12 years, n = 39), and 25.9% of adolescents (12–18 years, n = 64). The most common primary disease at hospital/PICU admission was kidney disease (19.4%, n = 48), followed closely by haematologic disease or BMT (18.5%, n = 46). The full distribution of primary diseases in the examined population is displayed in Fig. 2. The majority of patients had at least one comorbidity (79.4%, n = 196). Equal numbers of children were reported to have one, two, and three comorbidities (n = 64, 66, and 66, respectively).
Most of the patients required CKRT for AKI (78.9%, n = 195), mainly AKI stage 3 (72.3%, n = 141). Among the non-AKI patients, the three most common primary diseases were IEM (28.9%), followed by haematologic disease/BMT (21.1%), and liver disease (18.4%) (a full summary is available in Supplementary Fig. 2). Five of the non-AKI patients (11.2%) presented with fluid overload of 10% or more at CKRT initiation. There was no significant difference in fluid overload between the AKI and non-AKI patients (4.6%, IQR 0.0–11.6% vs 3.6%, IQR 0.0–8.7%, respectively, p = 0.157). Eighty-five per cent of CKRT procedures were conducted in the PICU setting. Thirty-one patients underwent another dialysis method during hospitalisation, either before or after CKRT (iHD 20 patients, PD 11 patients).
CKRT technical parameters
Two hundred and forty-seven patients underwent a total of 37,562 h of CKRT, with a median time of 78 h (IQR 37–165) per patient. A total of 1357 circuits were utilised with a median of 3 (IQR 2–6) per patient. The majority of patients (n = 192, 77.7%) underwent CKRT on Prismaflex/Prismax devices, Aquarius was used in nine (3.6%), and CARPEDIEM (Cardio-Renal Pediatric Dialysis Emergency Machine) in five (2.0%) children. In 33 children, the device was reported as “other” and not specified, and in eight patients, it was not reported. The CVVHDF modality was used in the majority of patients (n = 175, 70.9%), regardless of the applied anticoagulation (RCA – 72.6%, HA – 64.3%, NA – 82.4%). The distribution of CKRT modalities did not differ regarding anticoagulation (Table 2). Small filters were utilised in 83 patients, medium in 94 patients, and large in 60 patients. All procedures were conducted via double-lumen central venous dialysis catheters (CVC). The median CVC diameter was 8.0 French (IQR 6.0–10.0) and was equal in all anticoagulation modalities (p = 0.253). The most common vascular access site was the right internal jugular vein (n = 116, 47.0%), followed by the femoral vein (n = 74, 30.0%), left internal jugular vein (n = 34, 13.8%), and subclavian vein (n = 21, 8.5%). The most common anticoagulation modality was HA (n = 112, 45.3%), followed by NA (n = 68, 27.5%) and RCA (n = 62, 25.1%). The median prescribed dialysis dose in the whole group was 2.4 L/h/1.73 m2 (IQR 1.73–3.46) or 53.0 mL/h/kg (IQR 36.1–85.7). The prescribed parameters of CKRT by anticoagulation modality are shown in Table 2. Blood flow rate differed between the anticoagulation groups (p = 0.003) and was lower in the RCA group than in the NA group (p = 0.002 post hoc analysis), whereas other dialysis parameters did not differ.
Circuit lifetime
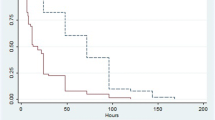
Independent of the anticoagulation, the median circuit lifetime in the entire group was 24 h (IQR 16.0–40.0). It was highest when RCA was used (42 h, IQR 32-58 h), lower with HA (24 h, IQR 14–34) and lowest when no anticoagulation was used (18 h, IQR 12–24), p < 0.001 (Fig. 3a). Circuit lifetime was longer with RCA than with HA and NA, independent of filter size (Table 3). In addition, filter size impacted mean circuit lifetimes (p < 0.001) (Fig. 3b). Small dialysers had shorter lifetimes (19 h, IQR 14–32) than both medium (26 h, IQR 16–43), (p = 0.044) and large ones (33 h, IQR 20–48), (p < 0.001) (Fig. 3b). In contrast, mean circuit lifetimes did not vary with the CKRT modality (p = 0.081).
Circuit lifetimes were significantly longer in adolescents than in young children. However, this difference was lost when we divided the group by anticoagulation modality. Moreover, in all age groups except for children aged 6–12 years, the mean circuit lifetime was the longest with RCA, with no difference between HA and NA (Table 4). Anticoagulation modality distribution did not differ between the age groups. However, in adolescents, the RCA ratio was relatively higher than in other groups (Table 5).
In univariate analysis, circuit lifetime correlated with BSA and dialysis membrane surface, but not with CVC diameter, blood, and dialysis fluid flow rates. In the centre-adjusted multivariate analysis, using the general step-wise regression model, the mean circuit lifetime was associated with dialyser surface area (beta = 0.14, 95% CI 1.3–12.6, p = 0.016), left internal jugular vein vascular access site (beta = -0.37, 95% CI -17.9 to -1.1, p = 0.027) vs. right internal jugular vein, and the use of HA (beta = -0.14, 95% CI -6.1 to -0.2, p = 0.038) or NA (beta = -0.37, 95% CI -12.3 to -5.9, p < 0.001) vs. RCA.
Elective circuit changes
A total of 441 (32.5%) circuits were exchanged electively when the maximal suggested circuit time was reached. In the RCA group, the ratio of electively changed circuits (n = 107, 39.3%) was significantly higher than in other anticoagulation modalities (HA n = 192, 35.1%; NA n = 131, 24.9%; chi-squared test, p < 0.001). The remaining 11 electively changed filters were reported in 2 patients, with no anticoagulation data provided.
Dialysis-related complications
Dialysis-related complications occurred in 86 patients (34.8%) and were related to the type of anticoagulation (p < 0.001). The lowest incidence was observed with RCA (12.9%), followed by HA (36.5%) and NA (54.4%). The incidence of complications was the lowest for RCA in young children (age 1–6 years) and adolescents. Catheter dislocation was the most common complication (37.2%). In univariate analysis, we found correlations between the occurrence of catheter dislocation and CVC diameter, as well as the femoral and left internal jugular vein vascular access site vs. the right internal jugular vein. However, in the centre-adjusted multivariate analysis, catheter dislocation was not affected by the vascular access site or the CVC diameter.
Anticoagulation-related complications (thrombosis/bleeding) occurred in 24 patients (27.9%). Thrombosis was reported in 16 patients (18.6% of all complications), with no significant difference between anticoagulation modalities (p = 0.356). Bleeding occurred in 8 of the patients (9.3% of all complications) who were undergoing CKRT with HA (n = 5) and NA (n = 3), respectively. No cases of bleeding were reported in patients on RCA-CKRT. In NA, the haemorrhagic complications were probably caused by coagulation defects related to the primary disease (liver disease, haematologic disease, and malignancy). In 30 patients the occurrence of complications was reported but not specified.
Discussion
Paediatric CKRT presents a considerable number of challenges due to a wide variety of patient anthropometric characteristics, resulting in the necessity to use different-sized membranes and sets when compared to the adult population. Over the last years, CKRT has evolved into the leading dialysis technique in the paediatric intensive care setting [11]. Therefore, it is crucial to seek solutions to optimise the efficacy of paediatric CKRT procedures, which can be achieved, for example, by providing the maximum possible circuit longevity. In this study, we identified several independent factors affecting the circuit lifetime.
RCA superiority over HA in maintaining circuit survival was proven in an adult randomised control trial [9]. In the paediatric population, there is still no consensus regarding anticoagulation modality. Access to different anticoagulants varies worldwide. In our cohort, at least 77.7% of patients underwent CKRT on a device that includes the RCA software. In a recent European survey [12], RCA was the first-choice anticoagulation in 35% of centres. A global international study [13], as well as a worldwide systematic review [14], reported citrate as the most commonly used anticoagulant. Nafamostat mesylate [15] and prostacyclin [16] have also been applied with satisfactory results. Our current study, one of the largest paediatric CKRT cohorts to date, demonstrates clear advantages of RCA compared to HA and NA. RCA resulted in longer circuit lifetime independent of filter sizes and in different age groups, with no significant difference between HA and NA. This finding aligns with previous smaller observational studies [17,18,19,20], and a small crossover trial [21]. In contrast, a relatively large study from Australia [22] did not show a difference in circuit lifetime between RCA and HA, however, the results could have been influenced by the inclusion of patients on ECMO-related CKRT. A recent meta-analysis [23] and the latest systematic literature review [24] confirmed the superiority of RCA over HA in children. In addition, in our cohort, RCA showed the lowest incidence of dialysis-related complications thanks to the complete absence of bleeding complications. Our findings are in keeping with randomised controlled trial results in adults, where HA was associated with a higher risk of haemorrhage [25] and with higher transfusion rates [26] compared to RCA. It is of note that in our cohort, HA, while fraught with bleeding complications, did not prevent thrombotic complications.
The mean circuit lifetime was higher in adolescents than in children below the age of 6. This difference may be related to the use of larger circuits, as in our study, the dialyser membrane surface correlated positively with longer circuit survival. However, the difference between the age groups disappeared when we considered each anticoagulation modality separately. This discrepancy might be related to the relatively higher use of RCA in the group of adolescents. We also observed that RCA (compared to HA and NA) was associated with the lowest incidence of CKRT complications in all age groups. A study by Raymakers-Jansen et al. [20] showed that RCA significantly prolonged the circuit lifetime in children < 15 kg. Thus, RCA might be safe and effective even in the youngest children.
Another factor influencing the circuit lifespan identified in the present study is the filter membrane surface, with larger filters being associated with longer circuit survival in both uni- and multivariate analyses. The use of membranes below 0.5 m2 was associated with a shorter lifetime, which is consistent with a study by Cortina et al. [22]. Similarly, a study comparing HA and NA (but not RCA) [27] showed that filters larger than 0.4 m2 had higher longevity. Interestingly, in the present study, membrane size did not affect the mean circuit lifetime when RCA or NA was applied. A study by Miklaszewska et al. [28] including eight patients treated with RCA showed that the filter size influenced the circuit lifespan with no impact on anticoagulation modality. In our present analysis, we discovered that RCA is associated with significantly longer filter maintenance than both HA and NA, regardless of the filter size.
The last circuit lifetime predictor identified in the multifactorial analysis was the vascular access site. In line with clinical practice recommendations, the internal jugular vein was the most common vascular access site. However, our analysis showed that the left internal jugular vein access was associated with worse circuit longevity compared to the right site. Literature on this aspect is inconsistent. Data from the ppCRRT registry suggested superior circuit survival with internal jugular access [29]. Another study in a paediatric cohort showed no influence of the vascular access site on circuit longevity [22]. However, a meta-analysis of adult studies [30] showed a trend toward better filter survival with the femoral site compared to both jugular and subclavian accesses. Due to the divergent reported results, this aspect requires further research in the paediatric population before the optimal vascular CKRT access in children is established.
Interestingly, in our study, CKRT modality, dialysis dose, and blood flow did not correlate with circuit lifetime. The currently available data are inconsistent for all these points, and further research is required, especially in the paediatric population. Some studies reported that CVVHD [31], or CVVHDF [32, 33] may increase filter longevity, compared to CVVH. Higher blood flow rates may prolong circuit longevity by reducing clotting [34], and a recent meta-analysis [30] indeed suggested better circuit survival with higher blood flow rates. However, several paediatric observational studies showed no association of blood flow rate with circuit longevity [27, 35]. Interestingly, in our cohort, the blood flow rate was lower in patients receiving RCA. Hence, the lack of blood flow correlation with circuit lifetime may be partially related to the better efficacy of RCA even with low blood flow compared to the other modalities.
While our study represents one of the largest analyses of factors affecting CKRT function in children, it has several limitations. The multicentre character of the study limited our access to data that might have been useful but was not included in the EurAKId registry. Our study does not involve other anticoagulation modalities, such as nafamostat mesylate (currently unavailable in Europe) or prostacyclin. A high proportion of patients included in the registry come from the leading centre that initially designed the registry (see Supplementary Fig. 1). The mentioned leading centre has developed a regional protocol for RCA [11], which was described as safe and effective. This can cause bias, the risk of which we reduced by adjusting the multivariate analysis for centre, but also leads us to the conclusion that the development of unified protocols could improve the efficacy of CKRT in children. Furthermore, lack of data on individual circuit function precluded time-to-event analyses.
Conclusions
Our prospective, multicentre, international registry study identified several factors influencing circuit lifetime in paediatric CKRT, in particular, anticoagulation modality, vascular access site, and dialyzer surface area. The present study demonstrates the superiority of RCA in maintaining CKRT functionality regardless of patient age, dialyser size, and blood flow rate. Hence, we conclude that RCA should be considered when choosing first-line anticoagulation for CKRT in children. Furthermore, the present study provides new data on aspects that are not yet clear and deserve to be extensively discussed to improve circuit survival and CKRT treatment in general. Further efforts should focus on developing guidelines and clinical practice recommendations for paediatric CKRT.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Raina R, Chakraborty R, Tibrewal A, Sethi SK, Bunchman T (2022) Advances in pediatric acute kidney injury. Pediatr Res 91:44–55. https://doi.org/10.1038/s41390-021-01452-3
Meena J, Mathew G, Kumar J, Chanchlani R (2023) Incidence of Acute Kidney Injury in Hospitalized Children: A Meta-analysis. Pediatrics 151:e2022058823. https://doi.org/10.1542/peds.2022-058823
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med 376:11–20. https://doi.org/10.1056/nejmoa1611391
Macedo E, Cerdá J, Hingorani S et al (2018) Recognition and management of acute kidney injury in children: the ISN 0by25 global snapshot study. PLoS One 13:e0196586. https://doi.org/10.1371/journal.pone.0196586
De Galasso L, Picca S, Guzzo I (2020) Dialysis modalities for the management of pediatric acute kidney injury. Pediatr Nephrol 35:753–765. https://doi.org/10.1007/s00467-019-04213-x
Sutherland SM, Alexander SR (2012) Continuous renal replacement therapy in children. Pediatr Nephrol 27:2007–2016. https://doi.org/10.1007/s00467-011-2080-x
Raina R, Chakraborty R, Davenport A et al (2022) Anticoagulation in patients with acute kidney injury undergoing kidney replacement therapy. Pediatr Nephrol 37:2303–2330. https://doi.org/10.1007/s00467-021-05020-z
Mann L, Ten Eyck P, Wu C et al (2023) CVVHD results in longer filter life than pre-filter CVVH: Results of a quasi-randomized clinical trial. PLoS One 18:e0278550. https://doi.org/10.1371/journal.pone.0278550
Zarbock A, Küllmar M, Kindgen-Milles D et al (2020) Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury: A Randomized Clinical Trial. JAMA 324:1629–1639. https://doi.org/10.1001/jama.2020.18618
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group (2012) Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2:1–138
Cappoli A, Labbadia R, Antonucci L et al (2023) A simplified protocol of regional citrate anticoagulation with phosphate-containing solutions in infants and children treated with continuous kidney replacement therapy. Pediatr Nephrol 38:3835–3844. https://doi.org/10.1007/s00467-023-05994-y
Guzzo I, de Galasso L, Bayazit AK et al (2022) Acute paediatric kidney replacement therapies in Europe: demographic results from the EurAKId Registry. Nephrol Dial Transplant 37:770–780. https://doi.org/10.1093/ndt/gfab280
Daverio M, Cortina G, Jones A et al (2022) Continuous Kidney Replacement Therapy Practices in Pediatric Intensive Care Units Across Europe. JAMA Netw Open 5:e2246901. https://doi.org/10.1001/jamanetworkopen.2022.46901
Starr MC, Gist KM, Zang H et al (2024) Continuous Kidney Replacement Therapy and Survival in Children and Young Adults: Findings From the Multinational WE-ROCK Collaborative. Am J Kidney Dis. https://doi.org/10.1053/j.ajkd.2023.12.017
Fuhrman DY, Gist KM, Akcan-Arikan A (2023) Current practices in pediatric continuous kidney replacement therapy: a systematic review-guided multinational modified Delphi consensus study. Pediatr Nephrol 38:2817–2826. https://doi.org/10.1007/s00467-022-05864-z
Miyaji MJ, Ide K, Takashima K et al (2022) Comparison of nafamostat mesilate to citrate anticoagulation in pediatric continuous kidney replacement therapy. Pediatr Nephrol 37:2733–2742. https://doi.org/10.1007/s00467-022-05502-8
Goonasekera CD, Wang J, Bunchman TE, Deep A (2015) Factors affecting circuit life during continuous renal replacement therapy in children with liver failure. Ther Apher Dial 19:16–22. https://doi.org/10.1111/1744-9987.12224
Buccione E, Guzzi F, Colosimo D et al (2021) Continuous Renal Replacement Therapy in Critically Ill Children in the Pediatric Intensive Care Unit: A Retrospective Analysis of Real-Life Prescriptions, Complications, and Outcomes. Front Pediatr 9:696798. https://doi.org/10.3389/fped.2021.696798
Sık G, Demirbuga A, Annayev A, Citak A (2020) Regional citrate versus systemic heparin anticoagulation for continuous renal replacement therapy in critically ill children. Int J Artif Organs 43:234–241. https://doi.org/10.1177/0391398819893382
Rico MP, Fernández Sarmiento J, Rojas Velasquez AM et al (2017) Regional citrate anticoagulation for continuous renal replacement therapy in children. Pediatr Nephrol 32:703–711. https://doi.org/10.1007/s00467-016-3544-9
Raymakers-Janssen PAMA, Lilien M, van Kessel IA et al (2017) Citrate versus heparin anticoagulation in continuous renal replacement therapy in small children. Pediatr Nephrol 32:1971–1978. https://doi.org/10.1007/s00467-017-3694-4
Zaoral T, Hladík M, Zapletalová J, Trávnícek B, Gelnarová E (2016) Circuit Lifetime With Citrate Versus Heparin in Pediatric Continuous Venovenous Hemodialysis. Pediatr Crit Care Med 17:e399–e405. https://doi.org/10.1097/pcc.0000000000000860
Cortina G, McRae R, Chiletti R, Butt W (2020) The Effect of Patient- and Treatment-Related Factors on Circuit Lifespan During Continuous Renal Replacement Therapy in Critically Ill Children. Pediatr Crit Care Med 21:578–585. https://doi.org/10.1097/pcc.0000000000002305
Raina R, Agrawal N, Kusumi K et al (2022) A Meta-Analysis of Extracorporeal Anticoagulants in Pediatric Continuous Kidney Replacement Therapy. J Intensive Care Med 37:577–594. https://doi.org/10.1177/0885066621992751
Kutsogiannis DJ, Gibney RT, Stollery D, Gao J (2005) Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney Int 67:2361–2367. https://doi.org/10.1111/j.1523-1755.2005.00342.x
Monchi M, Berghmans D, Ledoux D et al (2004) Citrate vs. heparin for anticoagulation in continuous venovenous hemofiltration: a prospective randomized study. Intensive Care Med 30:260–265. https://doi.org/10.1007/s00134-003-2047-x
del Castillo J, López-Herce J, Cidoncha E et al (2008) Circuit life span in critically ill children on continuous renal replacement treatment: a prospective observational evaluation study. Crit Care 12:R93. https://doi.org/10.1186/cc6965
Miklaszewska M, Korohoda P, Zachwieja K et al (2017) Filter Size Not the Anticoagulation Method is the Decisive Factor in Continuous Renal Replacement Therapy Circuit Survival. Kidney Blood Press Res 42:327–337. https://doi.org/10.1159/000477609
Hackbarth R, Bunchman TE, Chua AN et al (2007) The effect of vascular access location and size on circuit survival in pediatric continuous renal replacement therapy: a report from the PPCRRT registry. Int J Artif Organs 30:1116–1121. https://doi.org/10.1177/039139880703001212
Brain M, Winson E, Roodenburg O, McNeil J (2017) Non anti-coagulant factors associated with filter life in continuous renal replacement therapy (CRRT): a systematic review and meta-analysis. BMC Nephrol 18:69. https://doi.org/10.1186/s12882-017-0445-5
Ricci Z, Ronco C, Bachetoni A et al (2006) Solute removal during continuous renal replacement therapy in critically ill patients: convection versus diffusion. Crit Care 10:R67. https://doi.org/10.1186/cc4903
Saudan P, Niederberger M, De Seigneux S et al (2006) Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 70:1312–1317. https://doi.org/10.1038/sj.ki.5001705
Tsujimoto Y, Miki S, Shimada H et al (2021) Non-pharmacological interventions for preventing clotting of extracorporeal circuits during continuous renal replacement therapy. Cochrane Database Syst Rev 9:CD013330. https://doi.org/10.1002/14651858.CD013330.pub2
Davies H, Leslie G (2006) Maintaining the CRRT circuit: non-anticoagulant alternatives. Aust Crit Care 19:133–138. https://doi.org/10.1016/s1036-7314(06)80026-3
Mottes T, Owens T, Niedner M et al (2013) Improving delivery of continuous renal replacement therapy: impact of a simulation-based educational intervention. Pediatr Crit Care Med 14:747–754. https://doi.org/10.1097/PCC.0b013e318297626e
Funding
The EurAKId registry was initially supported by an investigator-initiated research grant from Baxter Healthcare (Deerfield, IL, USA). Clinical-Trials.gov identifier: NCT02960867; registration date November 10, 2016 (www.clinicaltrials.gov). The sponsor did not participate in the study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose. The results in this article have not been published previously in whole or part, including not being published in abstract form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deja, A., Guzzo, I., Cappoli, A. et al. Factors influencing circuit lifetime in paediatric continuous kidney replacement therapies – results from the EurAKId registry. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06459-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00467-024-06459-6