Abstract
Background
Extremely low birth weight (ELBW) neonates (birth weight ≤ 1000 g) are at high risk to develop drug-induced acute kidney injury (AKI). However, we lack a pragmatic detection tool to capture their time-dependent (patho)physiologic serum creatinine (Scr) patterns. Pottel et al. suggested rescaling Scr by dividing Scr with the mean Scr value of the age- and sex-specific reference population. We explored if this Pottel method can detect drug-related nephrotoxicity in ELBW neonates.
Methods
A previously reported dataset on Scr changes in ELBW neonates exposed to ibuprofen, amikacin, or vancomycin was updated to calculate Pottel scores for every available Scr value in the first 28 postnatal days. We hereby used previously published postnatal age-specific 50th centile values in an ELBW population. Linear mixed models were applied, analyzing Pottel scores as response variable and continuous time (day), drug exposure, and interaction thereof in the explanatory model.
Results
Serum creatinine (n = 3231) observations in 201 ELBW neonates were collected. A statistically significant rise of Pottel scores was observed with ibuprofen starting from postnatal day 4. In addition, a cumulative effect of treatment with mean Pottel scores on day 0 of 1.020 and on day 3 during treatment of 1.106 (95% CI 1.068–1.145, p < 0.001) was observed, corrected for effect of antibiotics. Antibiotic administrations showed a small but statistically significant difference up to postnatal day 5.
Conclusions
As rescaled Scr biomarker, the Pottel method showed a clear association with ibuprofen-exposed ELBW neonates, suggesting its applicability as a pragmatic bedside alternative tool to assess nephrotoxicity.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extremely low birth weight (ELBW) premature neonates (birth weight (BW) ≤ 1000 g) are born during active nephrogenesis [1, 2]. This population has a high risk to develop acute kidney injury (AKI) due to immature kidney physiology combined with several possible risk factors during their neonatal intensive care unit (NICU) stay, such as hemodynamic instability, late-onset sepsis (LOS), necrotizing enterocolitis (NEC), or persistent ductus arteriosus (PDA) [3,4,5,6]. These events often co-occur with nephrotoxic medication exposure, in up to 92% of ELBW neonates [6]. The recent Baby Nephrotoxic Injury Negated by Just-in-Time Action (BABY-NINJA) trial showed that identifying neonates at risk for nephrotoxic drug-induced AKI and implementing guidelines to reduce the exposure resulted in a significant decrease in AKI prevalence and intensity [5]. Consequently, early and consistent precision pharmacovigilance holds the potential to reduce or prevent AKI in ELBW neonates. However, this needs a pragmatic bedside tool.
The most currently applied definition for AKI in ELBW neonates is the neonatal modification of the KDIGO (Kidney Disease: Improving Global Outcomes, mKDIGO) definition for adults and children [7]. The mKDIGO is a grading system, based on different rises of serum creatinine (Scr), where a rise of 0.3 mg/dL or ≥ 50% compared to the previous value defines stage 1 AKI [7].
However, the pattern of physiologic Scr changes in ELBW neonates is characterized by an initial progressive Scr increase in the first days peaking on days 3–4 and a subsequent slow decrease, with interindividual variability due to maturational and non-maturational factors like drugs or fluid therapy [8, 9]. Consequently, the mKDIGO has limitations as it assumes a steady state and will not detect the absence of an appropriate, expected decline of Scr in the second part of the first week of life and beyond. It also requires consecutive Scr measurements.
The idea to explore assay-specific mean and centile Scr values to describe postnatal trends was introduced, as described by Pottel et al. [10, 11]. They suggested to normalize Scr by dividing Scr by the mean Scr value of the appropriate age- and sex-specific healthy population, rescaling it to a kidney biomarker with a normal distribution around the mean of 1 [10]. However, this effort was done from age 2 years onwards.
In previous work on Scr patterns in ELBW neonates, a modest decrease of creatinine clearance and a shift of Scr of about 1 standard deviation (SD) in ibuprofen-exposed neonates were documented, as well as a minor increase of Scr when treated with amikacin and/or vancomycin [11,12,13].
In the present study, we investigated whether the Pottel method also enables detection of drug-related nephrotoxicity in this dataset. This alternative approach would make detection more feasible bedside, is based on a single observation, and mitigates the time-dependent Scr pattern.
Methods
Study population and clinical characteristics
A previously reported dataset, used to define Scr trends and drug toxicity in ELBW neonates admitted to the NICU of the University Hospitals Leuven in two periods (July 2007 to August 2011 and June 2015 to March 2017), was reused [9, 12, 13]. This dataset contains retrospectively collected data on demographics, relevant clinical data (e.g., mode of delivery, exposure to inotropics), and days of treatment with ibuprofen, amikacin, or vancomycin and the available Scr for the first 42 days of life [9]. The Leuven NICU uses ibuprofen for the pharmacological PDA closure, whereas the combination of amikacin and vancomycin is the regimen for suspected LOS (late-onset sepsis, > 72-h postnatal age). Amikacin is also used as co-treatment with amoxicillin for early-onset sepsis (< 72 h) and with piperacillin-tazobactam to treat necrotizing enterocolitis.
Serum creatinine was analyzed enzymatically (Roche Diagnostics, Mannheim, Germany) during the study period, and all measurements were isotope dilution mass spectrometry (IDMS) traceable [9].
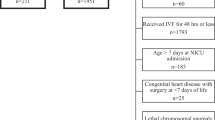
We restricted the dataset to observations obtained in the first 28 days after birth. After review of the available data, 30 patients were excluded due to insufficient baseline data, early neonatal death (< 7 days after birth), late referral (≥ postnatal age (PNA) day 15), or duplication. This resulted in 3231 Scr observations in 201 patients (Fig. 1 and Figure S1). Pottel scores for every available Scr were calculated based on 50th centile values per postnatal day in ELBW neonates (Supplementary Table 1, column 2), as previously published [11]. Ethics approval was provided (S63405, Ethics Committee Research UZ/KU Leuven).
Statistical methods
Patient characteristics were described by mean and SD or frequencies with percentages (Table 1). Descriptive summary statistics of the Pottel score were calculated, including mean and SD, based on values collected from days without influence of ibuprofen or antibiotics (i.e., no administration of any of these drugs the day before Scr measurement was performed).
To evaluate the effect of ibuprofen and antibiotics (amikacin and/or vancomycin) on the Pottel score and to determine whether the score showed a significant association, linear mixed models were used, with the Pottel score as response variable and continuous time (PNA, expressed in days), pharmacotherapy (ibuprofen, amikacin, and/or vancomycin, yes/no), and the interaction thereof in the explanatory models. Random intercept and slope in time were modeled to account for the longitudinal data structure. In addition, the combined effects of ibuprofen and antibiotics were used in multivariable models to account for possible confounding effects by estimating the effect of ibuprofen corrected for antibiotics, and vice versa.
As the effect of pharmacotherapy is expected to be seen with a 1-day delay (“lag” time), treatment was modeled as binary variable, with value 1 if the respective treatment was administered on the day before outcome evaluation and value 0 if otherwise. Log transformation was applied to the time variable to deal with non-linear trends over time, as this resulted in a better model fit (lower Akaike information criterion) compared to cubic spline models.
Results were presented graphically, presenting estimated Pottel score over time with 95% confidence bands in the presence or absence of drug exposure.
To evaluate the cumulative time effect of ibuprofen or antibiotic administration on the Pottel score, we used similar linear mixed models for data analysis, modeling the Pottel score as an effect of the number of consecutive days of drug administration.
Analyses were performed using SAS software (version 9.4, SAS Institute Inc, Cary, NC, USA) for Windows. A p-value of < 0.05 was considered statistically significant.
Results
Study population
Data from 201 ELBW neonates with a mean BW of 808 (range 370–1000, interquartile range IQR 710–900) grams and a mean gestational age (GA) of 27 (23–34, IQR 25–28) weeks were available. Most neonates (n = 171, 86.8%) received prenatal lung maturation (maternal steroids), and 136 neonates (68.3%) were born following caesarean delivery. Late neonatal death (PNA day ≥ 7) occurred in 17 patients (8.5%) (Table 1).
During neonatal life (PNA days 1–28), 124 neonates (61.7%) received ibuprofen, 151 (75.5%) amikacin, and 152 (76%) vancomycin. Ibuprofen was administered for a median of 3 (range 1–14) days, amikacin for 6 (range 1–17) days, and vancomycin for 7 (range 1–21) days. Only 29 (14.4%) patients never received any of these nephrotoxic drugs (Table 1). There were missing data for four patients concerning use of prenatal steroids, for two patients concerning mode of delivery, and for one patient concerning administration of any antibiotic respectively, which were excluded for their respective analyses. The number of available Scr observations for each day is provided in Supplementary Table 1, column 3, and the pattern in Figure S1.
Pottel scores
To explore Pottel score distribution without impact of drug exposure, scores collected on days without drug administration on the previous days were used to obtain summary statistics. This analysis revealed a normal distribution with a mean of 1.01 (SD 0.257).
Association with drug administration over postnatal age (Supplementary Table 2)
Ibuprofen
Pottel scores, calculated in function of ibuprofen exposure on the previous day, showed statistically significant differences starting from PNA day 4 with higher Pottel scores after exposure compared to no exposure (on PNA day 4 mean difference in Pottel score 0.040 [0.017; 0.063], p = 0.0005). The magnitude of differences increased with PNA, with larger confidence intervals reflecting more dropout cases, reflecting a sample size effect. Results of ibuprofen corrected for administration of antibiotics were comparable (Fig. 2).
Mean (standard deviation) Pottel score per postnatal day (days 1–28) in function of administration of ibuprofen the day before or not, corrected for administration of amikacin and/or vancomycin. The estimated curves are presented within the range of observations: days on which (almost) no influence of ibuprofen was present (defined as administration to none or maximal one newborn on the day before) are not shown. Estimated means are presented with 95% confidence intervals
Antibiotics
Effects for amikacin alone, vancomycin alone, and a combination of both were calculated, with comparable results for these three analyses. Antibiotic administration resulted in a small but statistically significant difference in Pottel scores up to PNA day 5 (on PNA day 5 for amikacin mean difference 0.070 [0.035; 0.106], p = 0.0001; for vancomycin mean difference 0.080 [0.045; 0.114] p ≤ 0.0001; for antibiotics combined mean difference 0.069 [0.036; 0.102], p ≤ 0.0001). In contrast, starting from PNA day 25, we observed a statistically significant difference resulting in a lower Pottel score with amikacin or vancomycin administration alone, but not with both combined (on PNA day 25 for amikacin mean difference − 0.030 [− 0.054; − 0.006], p = 0.0159; for vancomycin mean difference − 0.034 [− 0.058; − 0.010], p = 0.0057; for antibiotics combined mean difference − 0.022 [− 0.044; 0.001], p = 0.0610). When correcting for ibuprofen, again, similar results were obtained with statistically significant differences up to PNA day 5 (Fig. 3).
Both antibiotics (amikacin and vancomycin) were combined for this analysis. Mean (standard deviation) Pottel score per day (postnatal, days 1–28) in function of administration of antibiotics the day before or not, corrected for administration of ibuprofen. The estimated curves are presented within the range of observations: days on which (almost) no influence of antibiotics was present (as there was administration to none or one child on the day before) are not shown. Estimated means are presented with 95% confidence intervals
Cumulative effect during treatment over consecutive days (Supplementary Table 3)
Ibuprofen
A clear cumulative effect of ibuprofen on the Pottel score was observed, with significant differences starting from day 1 after administration (mean Pottel score on day 0 1.018 [0.994; 1.042], on day 1 of administration 1.048 [1.012; 1.085], p = 0.0393). A significant further rise of scores in the following days of exposure was seen, with a subsequent flattening of the mean scores (mean Pottel score on day 3 1.101 [1.063; 1.140], p ≤ 0.0001 and on day ≥ 5 1.132 [1.079; 1.185], p ≤ 0.0001). When corrected for antibiotics there is a similar trend (mean Pottel score on day 0 1.020 [0.995; 1.044], on day 3 1.106 [1.068; 1.145], p ≤ 0.0001, and on day ≥ 5 1.135 [1.082; 1.187], p ≤ 0.0001) (Fig. 4).
Antibiotics
A small statistically significant difference was observed only on ≥ 5 days of treatment (mean Pottel score on day 0 1.027 [1.002; 1.052], on day 3 of administration 1.034 [0.997; 1.071], p = 0.6399, and on day ≥ 5 of administration 1.055 [1.022; 1.088], p = 0.0194), with similar results when corrected for ibuprofen (mean Pottel score on day 0 1.024 [0.999; 1.048] and on day ≥ 5 of administration 1.060 [1.027; 1.093], p = 0.0021) (Fig. 5).
Discussion
In this retrospective study, the applicability of the Pottel method—a rescaled Scr marker by dividing Scr with the mean Scr value of the age- and sex-specific healthy population (Qcrea)—to detect drug-induced nephrotoxicity in ELBW neonates was assessed.
AKI is common in ELBW neonates, with incidences up to 60% [3, 14,15,16]. ELBW neonates already have a decreased kidney reserve due to their disrupted kidney development, with evidence showing smaller kidney size and abnormal kidney-related outcomes in childhood compared to healthy controls [1, 2, 17]. Experiencing neonatal AKI likely further imposes an increased risk of long-term kidney dysfunction [17, 18]. These patients are often exposed to nephrotoxic drugs, which co-occurs with several known risk factors such as hemodynamic instability, LOS, NEC, or PDA [3,4,5,6]. Consequently, timely detection of AKI during the use of nephrotoxic drugs could reduce AKI rates in ELBW neonates and improve long-term kidney outcomes. However, accurate detection of AKI remains challenging. Several definitions are available, such as the predominantly used mKDIGO, the pRIFLE (pediatric Risk, Injury, Failure, Loss, End-stage kidney disease), or AKIN (Acute Kidney Injury Network), all showing similar AKI incidences in ELBW neonates [16]. These definitions are all based on defined changes in urine output (UOP) or Scr, assuming a steady-state situation and needing at least two observations. ELBW neonates, however, exhibit a physiologic postnatal Scr rise from the day of birth (day 1) to approximately day 3 with a subsequent decline, delayed more with increasing immaturity [8]. The Scr rise over the first days of life noted in ELBW in many instances crosses the thresholds to diagnose stage 1 AKI by the modified, neonatal KDIGO definition (Figure S1). However, in these neonates, this may be consistent with a normal, physiologic phenomenon rather than a pathophysiologic state. The use of current definitions is therefore limited in preterm neonates due to their time-dependent Scr physiology as well as challenges in accurately measuring UOP [19].
This scenario serves as an example of the utility of a rescaled Scr marker, such as the Pottel method, which may be better for detection of truly abnormal Scr than the present KDIGO criteria in circumstances such as these. Pottel et al. analyzed the use of their rescaled Scr marker with a multicohort study in a population > 2 years of age demonstrating a distribution of Scr/Qcrea around 1, with the 2.5th and 97.5th percentile at 0.67 and 1.33, respectively, showing specificity and sensitivity for impaired kidney function close to 90% over all age groups when using 1.33 as the cut-off value [10, 20].
We hypothesized that the Pottel method could be useful to explore (drug-related) nephrotoxicity in ELBW cases. An existing cohort, previously used to describe Scr patterns in ELBW neonates during ibuprofen, amikacin, or vancomycin exposure, was used as we knew this cohort showed an association between drug exposure and kidney impairment [12, 13].
In ibuprofen-exposed neonates, a clear association was observed with a statistically significant increase in Pottel scores during ibuprofen treatment starting from PNA day 4. There was a progressive difference in scores, increasing with advancing age, suggesting a more robust nephrotoxic AKI effect, confirming previous analyses [11, 12]. Exploring the cumulative effect during ibuprofen exposure revealed a rising Pottel score until day 3 of exposure followed by a relatively stable value thereafter. This emphasizes the importance of well-timed assessments during drug treatment before drawing definitive conclusions about potential kidney damage. Early measurements might not fully capture the full nephrotoxic effect.
In contrast, we could not show a significant association beyond PNA day 5 when evaluating the Pottel score during the use of amikacin and/or vancomycin. This seems to reflect a less pronounced nephrotoxic effect of these antibiotics in our study population. In our prior analysis, there was also a more modest change in Scr dynamics during amikacin and vancomycin treatment compared to ibuprofen [13]. Unexpectedly, we even noticed a decreased Pottel score in the latter half of the first month of life. As this study was explorative, we describe this finding, but fail to explain it. Among other causes, this could be due to less robust Q reference values in the third and fourth week in the currently available dataset as one of the limitations of the current analysis.
There are obvious limitations. Likely most relevant, the p50 values used for calculating the Pottel scores were derived from the same cohort in a previous analysis, influencing statistical outcomes [11]. However, our main intention was to introduce the concept. We do not claim that the p50 Scr values applied (Supplementary Table 1, column 2) to convert absolute Scr values to Pottel scores are ready reference values as they were derived from a single-center, small dataset (Table 1) [11]. Our primary goal was to illustrate the potential strength of this approach, as Scr displays extensive time-dependent variability in the first weeks of postnatal age, not yet at steady state. We do not claim that this approach is already a robust substitute for the currently used AKI definitions. A next obvious step would be to determine p50 values for Scr for a diversity of subpopulations commonly admitted to NICUs and to validate the approach used. Preferably, these datasets are generated from different units to reflect diversity in practices and settings.
We did not account for prolonged medication effects after cessation of treatment, nor did we study the impact of other drugs as we did not have sufficient granularity in our current dataset. The AWAKEN study showed that diuretics, vasopressors, and methylxanthines were associated with a lower risk for early onset AKI (postnatal days 2–7), whereas some medications were associated with increased odds for late AKI (> 7 days after birth) in neonates [4, 21]. The impact on the Pottel score could be different based on the type and duration of drug exposure. We neither accounted for other known variables in ELBW neonates that may have impacted kidney health, e.g., prenatal factors such as maternal hypertension or certain drug exposure or postnatal factors such as hypotension or elevated mean airway pressure, as we did not have sufficient data available in our retrospective dataset [22].
Considering these limitations, our findings underscore the potential value of the Pottel method in Scr-based bedside pharmacovigilance for ELBW neonates as an alternative technique. This approach enables bedside assessment based on a single Scr value and captures the time-dependent pattern. In addition, it could provide a way to standardize AKI definitions in clinical trials, improving comparability in this population. It is important to note that the established upper limit of 1.33 (for chronic kidney disease and in children > 2 years) does not seem to be applicable in ELBW neonates. Our initial findings hint towards a considerably lower threshold. Scr remains a widely recognized and measured biomarker, and the application of the Pottel method moderates certain inherent limitations linked to its use in this population. Additional steps, with comprehensive observational large-scale multicentre studies, are needed to further explore and develop this approach, to ascertain solid p50 values, to make the current findings more generalizable, and to compare this tool to the currently used AKI definitions.
Conclusion
As rescaled Scr biomarker, the Pottel method showed a clear association with ibuprofen-exposed ELBW neonates, suggesting its applicability as a pragmatic bedside alternative tool to assess nephrotoxicity. We suggest further exploration and development of this alternative approach.
Data availability
The corresponding author can be contacted. Data availability will be considered, if based on a reasonable study proposal.
References
Hoogenboom LA, Wolfs TGAM, Hütten MC et al (2021) Prematurity, perinatal inflammatory stress, and the predisposition to develop chronic kidney disease beyond oligonephropathy. Pediatr Nephrol 36:1673–1681. https://doi.org/10.1007/s00467-020-04712-2
Gilarska M, Raaijmakers A, Zhang ZY et al (2019) Extremely low birth weight predisposes to impaired renal health: a pooled analysis. Kidney Blood Press Res 44:897–906. https://doi.org/10.1159/000502715
Jetton JG, Boohaker LJ, Sethi SK et al (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/S2352-4642(17)30069-X
Charlton JR, Boohaker L, Askenazi D et al (2019) Late onset neonatal acute kidney injury: results from the AWAKEN Study. Pediatr Res 85:339–348. https://doi.org/10.1038/S41390-018-0255-X
Stoops C, Stone S, Evans E et al (2019) Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr 215:223-228.e6. https://doi.org/10.1016/J.JPEDS.2019.08.046
Mohamed TH, Abdi HH, Magers J et al (2022) Nephrotoxic medications and associated acute kidney injury in hospitalized neonates. J Nephrol 35:1679–1687. https://doi.org/10.1007/S40620-022-01264-6
Jetton JG, Askenazi DJ (2012) Update on acute kidney injury in the neonate. Curr Opin Pediatr 24:191–196. https://doi.org/10.1097/MOP.0B013E32834F62D5
George I, Mekahli D, Rayyan M et al (2011) Postnatal trends in creatinemia and its covariates in extremely low birth weight (ELBW) neonates. Pediatr Nephrol 26:1843–1849. https://doi.org/10.1007/S00467-011-1883-0
van Donge T, Allegaert K, Gotta V et al (2021) Characterizing dynamics of serum creatinine and creatinine clearance in extremely low birth weight neonates during the first 6 weeks of life. Pediatr Nephrol 36:649–659. https://doi.org/10.1007/S00467-020-04749-3
Pottel H, Dubourg L, Schaeffner E et al (2017) The diagnostic value of rescaled renal biomarkers serum creatinine and serum cystatin C and their relation with measured glomerular filtration rate. Clin Chim Acta 471:164–170. https://doi.org/10.1016/J.CCA.2017.06.005
Allegaert K, Smits A, van Donge T et al (2020) Renal precision medicine in neonates and acute kidney injury: how to convert a cloud of creatinine observations to support clinical decisions. Front Pediatr 8:366. https://doi.org/10.3389/FPED.2020.00366
van Donge T, Allegaert K, Pfister M et al (2021) Creatinine trends to detect ibuprofen-related maturational adverse drug events in neonatal life: a simulation study for the ELBW newborn. Front Pharmacol 11:610294. https://doi.org/10.3389/FPHAR.2020.610294
van Donge T, Smits A, van den Anker J, Allegaert K (2021) Amikacin or vancomycin exposure alters the postnatal serum creatinine dynamics in extreme low birth weight neonates. Int J Environ Res Public Health 18:662. https://doi.org/10.3390/IJERPH18020662
Lee CC, Chan OW, Lai MY et al (2017) Incidence and outcomes of acute kidney injury in extremely-low-birth-weight infants. PLoS One 12:e0187764. https://doi.org/10.1371/journal.pone.0187764
Askenazi DJ, Heagerty PJ, Schmicker RH et al (2020) Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr Nephrol 35:1737–1748. https://doi.org/10.1007/s00467-020-04563-x
Chowdhary V, Vajpeyajula R, Jain M et al (2018) Comparison of different definitions of acute kidney injury in extremely low birth weight infants. Clin Exp Nephrol 22:117–125. https://doi.org/10.1007/s10157-017-1430-9
Hingorani S, Schmicker R, Ahmad KA et al (2022) Prevalence and risk factors for kidney disease and elevated BP in 2-year-old children born extremely premature. Clin J Am Soc Nephrol 17:1129–1138. https://doi.org/10.2215/CJN.15011121
Harer MW, Pope CF, Conaway MR, Charlton JR (2017) Follow-up of Acute kidney injury in Neonates during Childhood Years (FANCY): a prospective cohort study. Pediatr Nephrol 32:1067–1076. https://doi.org/10.1007/S00467-017-3603-X
Branagan A, Costigan CS, Stack M et al (2022) Management of acute kidney injury in extremely low birth weight infants. Front Pediatr 10:867715. https://doi.org/10.3389/fped.2022.867715
Pottel H, Hoste L, Martens F (2010) New insights in glomerular filtration rate formulas and chronic kidney disease classification. Clin Chim Acta 411:1341–1347. https://doi.org/10.1016/j.cca.2010.05.031
Charlton J, Boohaker L, Askenazi D et al (2019) Incidence and risk factors of early onset AKI. Clin J Am Soc Nephrol 14:184–195. https://doi.org/10.2215/CJN.03670318
Viswanathan S, Manyam B, Azhibekov T, Mhanna MJ (2012) Risk factors associated with acute kidney injury in extremely low birth weight (ELBW) infants. Pediatr Nephrol 27:303–311. https://doi.org/10.1007/s00467-011-1977-8
Acknowledgements
The research activities of A. Smits are supported by a Senior Clinical Investigatorship of the Research Foundation–Flanders (FWO) (18E2H24N).
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Material preparation, data collection, and analysis were performed by M. Dumoulin, K. Allegaert, and A. Laenen. The first draft of the manuscript was written by M. Dumoulin and K. Allegaert. All authors commented on the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Previously provided ethical approval covered the analysis and no additional data search in patient records was needed (internal study number S63405, Ethics Committee Research UZ/KU Leuven).
Consent to participate/publish
Due to the nature of this study, no additional consent was needed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dumoulin, M., Pottel, H., Mekahli, D. et al. Pharmacovigilance of nephrotoxic drugs in neonates: the Pottel method for acute kidney injury detection in ELBW neonates. Pediatr Nephrol 39, 2525–2532 (2024). https://doi.org/10.1007/s00467-024-06335-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-024-06335-3