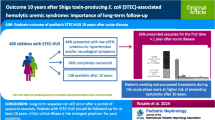
Abstract
Background
The gastrointestinal (GI) tract represents one of the main targets of typical hemolytic uremic syndrome (HUS) in children. In this observational study, we tried to establish (1) the main features of GI complications during STEC-HUS and (2) the relationship between Escherichia coli serotypes and Shiga toxin (Stx) variants with hepatopancreatic involvement.
Methods
A total of 79 STEC-HUS patients were admitted to our pediatric nephrology department between January 2012 and June 2021. Evidence of intestinal, hepatobiliary, and pancreatic involvements was reported for each patient, alongside demographic, clinical, and laboratory features. Frequency of gastrointestinal complications across groups of patients infected by specific E. coli serotypes and Stx gene variants was evaluated.
Results
Six patients developed a bowel complication: two developed rectal prolapse, and four developed bowel perforation which resulted in death for three of them and in bowel stenosis in one patient. Acute pancreatitis was diagnosed in 13 patients. An isolated increase in pancreatic enzymes and/or liver transaminases was observed in 41 and 15 patients, respectively. Biliary sludge was detected in three, cholelithiasis in one. Forty-seven patients developed direct hyperbilirubinemia. Neither E. coli serotypes nor Shiga toxin variants correlated with hepatic or pancreatic involvement.
Conclusions
During STEC-HUS, GI complications are common, ranging from self-limited elevation of laboratory markers to bowel perforation, a severe complication with a relevant impact on morbidity and mortality. Hepatopancreatic involvement is frequent, but usually short-lasting and self-limiting.
Graphical abstract

A higher resolution version of the Graphical abstract is available asSupplementary information
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hemolytic uremic syndrome (HUS) is a thrombotic microangiopathy (TMA) characterized by thrombocytopenia, non-immune hemolytic anemia and secondary organ damage, and primarily acute kidney injury (AKI). The disease predominantly affects children, with a peak incidence in children under age 5, and is rare in adults [1,2,3]. We commonly refer to “STEC-HUS” when a GI infection by Shiga-like toxin-producing strains of Escherichia coli (STEC) is detected in the setting of established HUS [1, 4].
After STEC-induced bowel infection, children usually develop bloody diarrhea, often associated with abdominal pain, fever, and vomiting. HUS develops in approximately 15% of infected children, mostly younger than 10 and predominantly during summer and autumn. In Italy, O26 is the most commonly identified serotype (38% of cases), while others, such as O157, O145, O111, and O103, are responsible for a minority of cases, different than in other areas of the world [1, 2, 5].
Shiga toxins are encoded by Stx genes, the most common of which are Stx1a, Stx2a, and Stx2c. The toxin (Stx) is produced in the intestine by E. coli and translocates across the epithelium through multiple mechanisms [6, 7]. Systemic dissemination follows, leading to platelet aggregation, formation of small thrombi, and intravascular hemolysis due to shear stress to erythrocytes [8, 9]. The kidneys are by far the most severely affected organs during the acute phase of the disease, and HUS is among the leading causes of AKI in children.
Approximately half of affected patients develop additional extrarenal manifestations, and in more than one third of them, the GI tract is involved [10]. Abdominal symptoms such as bloody diarrhea, vomiting, and abdominal pain are expected during STEC-induced gastroenteritis (GE-STEC) [11, 12], while the incidence of other GI complications and their effect on clinical outcome is still poorly documented and is probably underestimated.
This study has two main objectives: (1) Describe the main clinical, instrumental, and laboratory features of intestinal, pancreatic, and liver complications retrospectively reported in a large cohort of STEC-HUS patients admitted over a 10-year period in a single tertiary referral center and (2) establish whether microbiological features (E. coli serotype and Stx variants) are associated with hepatic and/or pancreatic involvement.
Methods
We reviewed the clinical and autoptic records of 79 consecutive patients admitted or transferred to our pediatric nephrology unit between 1 January 2012 and 30 June 2021, with a diagnosis of STEC-induced HUS. The Giovanni XXIII Pediatric Hospital is one of the largest pediatric hospitals in Southern Italy and is a tertiary regional referral center for pediatric nephrology, covering an estimated population of 700,000 children.
We included all patients with a confirmed STEC-HUS diagnosis aged between 0 and 18 years.
STEC-HUS was defined by the presence of thrombocytopenia (platelet count < 150,000/mm3 or sharp drop > 50%), hemolytic anemia (schistocytes and/or elevated LDH levels), and evidence of kidney involvement (serum creatinine level > normal limit for age and/or hematuria and/or proteinuria) in a patient with polymerase chain reaction (PCR)-confirmed STEC infection [13].
For each patient, demographic, microbiological, clinical, and instrumental findings were collected in a database. These included age, race, gender, length of hospital stay, STEC serotype, isolated Stx variant, GI co-pathogens detected (if any), symptoms, and both GI and extra-GI complications including acute kidney failure requiring kidney replacement therapy (KRT) and central nervous system (CNS) involvement (defined as new onset of alteration of consciousness, seizures, hypotonia, hypertonia, verbal disorders, vegetative symptoms [14]). All laboratory reference values were related to patient’s age and gender [15]. In our cohort, when KRT was required, continuous venovenous hemofiltration (CVVH) was the modality of choice.
All patients tested positive for STEC in their stools during their hospital stay. PCR was used for microbiological diagnosis. All positive samples were screened for Stx1, Stx2, and eae virulence genes according to Loconsole et al. [3]. Additionally, positive samples were sent to the Italian National Institute of Health (Istituto Superiore di Sanità, ISS, Rome) for confirmation [11].
Gastrointestinal complications
Bowel complications
We defined bowel complication as any de novo event arising during the course of the disease, including rectal prolapse, bowel perforation, and colonic stenosis. Common presenting symptoms of STEC-associated gastroenteritis such as vomiting, diarrhea, or hematochezia were not labeled as “bowel complications.”
Pancreas involvement
We included among pancreatic complications both isolated elevations in serum pancreatic enzymes and overt acute pancreatitis. Hyperamylasemia and hyperlipasemia were classified as mild or severe in presence of values below or above three times the upper limit of normal (ULN), respectively. “Acute pancreatitis” (ACPAN) was diagnosed according to the INSPPIRE (International Study Group of Paediatric Pancreatitis: In search of a cuRE) criteria [16] (modified after the Atlanta criteria) in the presence of at least two of the following: (1) abdominal pain compatible with ACPAN, (2) increase of serum amylase and/or lipase values ≥ 3 times upper limits of normality (ULN), (3) imaging findings consistent with ACPAN [16, 17].
Liver and biliary tract involvement
We defined liver involvement as the presence of markers of cytolytic damage: an increase of serum aspartate transaminase (AST) and/or alanine transaminase (ALT) levels ≥ 5 times the ULN was considered moderate, while an increase of ≥ 10 times the ULN was considered severe [18].
Direct hyperbilirubinemia, gamma-glutamyltransferase (γGT) elevation, or ultrasound evidence of gallstones/biliary sludge was included among signs of biliary tract involvement (Table 3). Abdominal ultrasound was obtained only in some patients, at the discretion of the treating clinician.
Statistical analysis
All normally distributed variables are reported as mean ± SD. Median is indicated for not normally distributed variables. Ranges and interquartile ranges (IQR) are also reported when relevant.
Descriptive statistics to test for differences between subgroups of patients included t tests for continuous, normally distributed variables and chi-square (χ2) tests for categorical variables. Statistical analysis was performed using IBM SPSS26.
Results
Patients
Over 10 years, 79 patients were treated in our unit for STEC-HUS. All patients were of White/Caucasian origin, and 43 (54.4%) were females. The mean age was 2.8 years [± 2.5 (range, 0.6–16.4)]. The average length of stay (LoS) was 20.4 ± 14.6 days (range, 8–121), irrespective of gender and STEC serotypes (p = NS). Clinical and biochemical descriptives for our cohort of patients are provided in Tables 1 and 2.
Microbiological features
STEC infection was confirmed in all 79 patients. Serotypes were identified in 72 patients (91%). The two most common serotypes were O26 in 37 (46.8%) and O111 in 14 patients (17.7%). We found a co-infection by two STEC serotypes in two cases (O26 + O111 and O111 + O145) (Table 2). Stx variants could be isolated in 51 patients. Relative frequencies of Stx variants and eae genes are reported in Table 2. No significant correlations could be found between E. coli serotypes O26 and O111 and likelihood of hepatic and pancreatic complications (p = NS). Similarly, Stx1, Stx2, and Stx1Stx2 positive E. coli strains were not associated with a greater risk of developing hepatic and pancreatic complications (p = NS). Among co-pathogens, Clostridia was the most common (9%), followed by Salmonella enterica, Salmonella typhimurium, Citrobacter freundii, and Adenovirus, detected in one patient each. Neither co-pathogens nor specific STEC serotypes were found to have an association with specific bowel, pancreatic, or liver complications (p = NS).
Gastrointestinal involvement
Fifty-eight patients (73.4%) presented one or more GI complications, as summarized in Table 3.
Bowel involvement
Two patients developed rectal prolapse followed by spontaneous resolution with no relevant sequelae. Four patients developed perforation (patients 1–4). Patients 1 and 3 died 3 days and 12 h after admission, respectively. In both, perforation was confirmed on autopsy, which revealed hemorrhagic colitis with an intraluminal collection of hemorrhagic material and massive necrosis of the intestinal wall (Fig. 1). Patient 3 presented intraluminal pseudomembranous colitis. Patient 2 suddenly developed an intestinal perforation with acute abdominal distension and hemoperitoneum. Patient 4 suffered from bowel perforation 18 days after admission, undergoing emergency surgical resection and temporary sigmoidostomy. Post-operatively, the patient developed segmental stenosis of the colon, requiring surgical resection. The colostomy was reversed by colocolic anastomosis 2 months later. He was the only patient who survived intestinal perforation in our cohort. Fifteen patients (19.5%) presented ultrasound evidence of peritoneal effusion (Table 3).
Pancreatic involvement
Thirteen patients (16.5%) met the INSPPIRE criteria for diagnosing ACPAN. In 41 patients (68.4%), we observed an isolated increase in amylase, lipase, or both. This was severe in 32 patients (40.5%). In particular, 4 patients had a severe increase in both, 28 only a severe increase in lipase, and one only increased amylase (Table 3). Serum levels of pancreatic enzymes in affected patients are reported in Table 4. When present, pancreatic enzyme elevation was longer lasting for lipase than it was for amylase. No patient developed endocrine pancreas insufficiency, nor required pancreatic enzymes replacement.
Hepatic and biliary tract involvement
Fifteen patients had at least a moderate increase in AST and/or ALT. AST elevation was moderate in 8 patients and severe in 4 patients, while ALT elevation was moderate in 9 patients and severe in 4 patients (Table 3). Serum levels of AST and ALT in affected patients, as well as duration of elevation, are reported in Table 4.
Indirect hyperbilirubinemia developed in most patients: 47 patients (59.5%) presented increased conjugated bilirubin, and 15 had jaundice on admission. Fourteen patients (17.7%) showed γGT elevation, one had ultrasound evidence of cholelithiasis, and three had biliary sludge.
We found no differences in terms of age and/or sex between patients presenting with pancreatic (acute pancreatitis or increase of amylase and/or lipase) and/or liver involvement compared to those who did not develop any of those (p = NS).
Discussion
Our study shows a high prevalence of gastrointestinal involvement in children with STEC-HUS ranging within a broad spectrum of clinical manifestations and severities. Hepatobiliary and pancreatic involvements are especially frequent. The clinical relevance of GI complications should not be underestimated. Indeed, in contrast to kidney function, which can be temporarily replaced with KRT (i.e., peritoneal or extra-corporeal dialysis), when other organs are affected, this might worsen the prognosis.
While cardiac [19,20,21] and neurological [14, 22] involvements in STEC-HUS have been previously described, we could only find a few publications having extraintestinal GI involvement as their primary focus [10]. The pancreas may be affected in multiple ways, potentially leading to acute pancreatitis and pancreatic necrosis, and if the damage is persistent and becomes chronic, can lead to insulin-dependent diabetes [11, 20]. In our cohort, more than half of patients presented evidence of pancreatic involvement, which was severe in 57% of our patients. Lipase elevation appears to be more frequent as well as longer lasting compared to amylase elevation (Table 4). Some degree of amylase and/or lipase elevation in the setting of acute or chronic kidney failure is to be expected, as pancreatic enzymes are partially cleared by the kidneys [23]. This implies that some patients with a mild to moderate increase in pancreatic enzymes possibly do not in fact experience any pancreatic damage, and serum levels of pancreatic enzymes were instead the consequence of reduced renal clearance. In our cohort, ACPAN was self-limited, and no affected patient required specific therapy (i.e., pancreatic enzyme replacement therapy). Diabetes development is a rare complication of STEC-HUS (far less common than acute pancreatitis) and is usually transient [10]. In our cohort of patients, none developed diabetes or sustained hyperglycemia requiring insulin administration. Similarly, despite 13 patients presenting acute pancreatitis, none resulted in exocrine pancreas insufficiency (thus pancreatic enzymes replacement therapy was not indicated).
Hepatic involvement was also common, similarly to what has been previously reported [3, 11, 24], and elevation of liver transaminases was the most frequent manifestation. In most cases, it was self-limited and relatively short lasting, with transaminases returning within the range of normality after approximately 1 week (Table 4).
Unconjugated hyperbilirubinemia, which affected most of our patients, is due to the rapid rate of intravascular hemolysis. In contrast, conjugated (direct) hyperbilirubinemia results from bilirubin buildup and can lead to cholestasis, biliary sludge, and cholelithiasis. In our cohort, three patients were found to have biliary sludge and one had gallstones. However, only 39 patients (49%) underwent abdominal ultrasound, possibly underestimating the prevalence of these among STEC-HUS patients.
The secondary aim of our study was to assess whether some microbiological features, including E. coli serotypes and Stx variants, were associated with a greater risk of developing hepatopancreatic involvement. Some Stx variants (i.e., Stx2a) have been associated with greater virulence, carrying both a greater risk of causing HUS, as well as resulting more frequently as AKI and KRT [25, 26].
In our cohort, however, neither serotypes (O26 or O111) nor Stx variants were associated with statistically significant differences in the prevalence of hepatic and pancreatic involvement.
The precise mechanism of bowel damage is multifactorial. Two distinct pathological processes have been hypothesized: a vascular mechanism characterized by a microangiopathic thrombosis and a Stx toxin-mediated pro-apoptotic mechanism [27]. These are not mutually exclusive, and both probably contribute to cell injury. The involvement of the intestine begins as mucosal ulcerations with some areas of necrosis and progresses to pseudomembrane formation and transmural infarction. If not promptly treated, this can evolve into toxic megacolon, perforation, and hemoperitoneum [20, 27]. Hemorrhagic colitis represents one of the most threatening complications of typical HUS, with a reported mortality as high as 33% according to some authors [28]. In our experience, all three patients who died had developed bowel perforation possibly secondary to hemorrhagic colitis. In all three patients, diagnosis and hospital admission were delayed. “Pain” can be a problematic symptom to interpret, especially in young children or when there is an altered state of consciousness. The threshold for repeat radiological investigations (two projections abdominal X-ray or upright chest radiography to detect subdiaphragmatic air) should be low, and surgical consultation should be pursued early in any patient with severe abdominal pain, acute abdomen, or sudden clinical deterioration. Prompt clinical recognition can increase the chance of survival, as happened with Patient 4.
This study has several strengths: it includes one of the largest cohorts in the literature for any study focusing on gastrointestinal complications in children affected by STEC-HUS. Additionally, it provides a description of the main features of liver and pancreatic involvement, including prevalence, severity of elevation of serum biomarkers, and duration.
We were unfortunately unable to determine how these complications affect prognosis, and this, we believe, represents the greatest limitation of our study. A significantly larger sample of patients would be required in order to establish the contribution that each individual GI complication has on mortality, length of stay, and other relevant prognostic indicators.
Conclusions
Gastrointestinal complications are common in children affected by STEC-HUS and can involve any organ. Patients with GI involvement should be distinguished from those with common gastroenteritis symptoms that characterize the prodromal phase of STEC infection. Pancreatic and hepatic involvements are common; however, they are often self-limited and resolve in a few days to weeks.
Although rare, intestinal perforation is potentially associated with significant morbidity and mortality. The rapidly evolving nature of the disease, associated with its unpredictability, underlines the need for frequent clinical re-evaluation that could significantly benefit from a multidisciplinary approach (gastroenterologist, nephrologist, radiologist, surgeon, and intensivist). Instrumental investigations (ultrasonography, abdominal X-ray, and CT scan) to identify signs of perforation of intra-abdominal collections must not be procrastinated in patients with sudden clinical deterioration, in order to promptly identify surgical conditions.
More studies are needed to identify the prognostic impact of GI involvement on STEC-HUS patients and to investigate the potential role of laboratory markers such as fecal calprotectin [29] or lactoferrin [30], which could potentially predict intestinal involvement.
Data Availability
All data regarding this study are included in this published aricle.
References
Corrigan JJ, Boineau FG (2001) Hemolytic-uremic syndrome. Pediatr Rev 22:365–369
Marina Noris GR (2005) Hemolytic uremic syndrome. J Am Soc Nephrol 16:1035–1050
Loconsole D, Giordano M, Centrone F et al (2020) Epidemiology of Shiga toxin-producing Escherichia coli infections in Southern Italy after implementation of symptom-based surveillance of bloody diarrhea in the pediatric population. Int J Environ Res Public Health 17:5137
Bruyand M, Mariani-Kurkdjian P, Gouali M et al (2018) Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med Mal Infect 48:167–174
Istituto Superiore di Sanità (2022) Italian haemolytic uraemic syndrome registry. Report / 1 December 2020 – 30 November 2021. https://www.epicentro.iss.it/en/hus/epidemiology-italy
Malyukova I, Murray KF, Zhu C et al (2009) Macropinocytosis in Shiga toxin 1 uptake by human intestinal epithelial cells and transcellular transcytosis. Am J Physiol Gastrointest Liver Physiol 296:78–92
Croxen MA, Law RJ, Scholz R et al (2013) Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880
Canpolat N (2015) Hemolytic uremic syndrome. Turk Pediatri Arsivi 50:73–82
Obrig TG, Karpman D (2012) Shiga toxin pathogenesis: kidney complications and renal failure. Curr Top Microbiol Immunol 357:105–136
De Buys Roessingh AS, De Lagausie P, Baudoin V et al (2007) Gastrointestinal complications of post-diarrheal hemolytic uremic syndrome. Eur J Pediatr Surg 17:328–334
Loconsole D, Giordano M, Laforgia N et al (2020) Case-management protocol for bloody diarrhea as a model to reduce the clinical impact of Shiga toxin-producing Escherichia coli infections. Experience from Southern Italy. Eur J Clin Microbiol Infect Dis 39:539–547
Giordano M, Baldassarre ME, Palmieri V et al (2019) Management of stec gastroenteritis: is there a role for probiotics? Int J Environ Res Public Health 16:1649
Balestracci A, Battaglia L, Toledo I et al (2021) Diagnostic sensitivity of extended renal and hematologic criteria to define hemolytic uremic syndrome. Arch Argent Pediatr 119:238–244
Giordano P, Netti GS, Santangelo L et al (2019) A pediatric neurologic assessment score may drive the eculizumab-based treatment of Escherichia coli-related hemolytic uremic syndrome with neurological involvement. Pediatr Nephrol 34:517–527
The Johns Hopkins Hospital (2021) The Harriet lane handbook, 32nd edn. Elsevier, Philadelphia. Chapter 19, Nephrology II:472–477)
Morinville VD, Lowe ME, Ahuja M et al (2014) Design and implementation of INSPPIRE. J Pediatr Gastroenterol Nutr 59:360–364
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG; Vege SS, Acute Pancreatitis Classification Working Group (2013) Classification of acute pancreatitis - - 2012: revision of the Atlanta classification and definitions by international consensus. Gut 62:102–111
Vajro P, Maddaluno S, Veropalumbo C (2013) Persistent hypertransaminasemia in asymptomatic children: a stepwise approach. World J Gastroenterol 19:2740–2751
Sanders E, Brown CC, Blaszak RT et al (2021) Cardiac manifestation among children with hemolytic uremic syndrome. J Pediatr 235:144-148.e4
Khalid M, Andreoli S (2019) Extrarenal manifestations of the hemolytic uremic syndrome associated with Shiga toxin-producing Escherichia coli (STEC HUS). Pediatr Nephrol 34:2495–2507
Filip C, Nicolescu A, Cinteza E et al (2020) Cardiovascular complications of hemolytic uremic syndrome in children. Maedica (Bucur) 15:305–309
Santangelo L, Netti G, Torres D et al (2021) Peripheral nervous system manifestations of Shiga toxin-producing E. coli-induced haemolytic uremic syndrome in children. Ital J Pediatr 47:181
Vidal E, Alberici I, Verrina E (2019) Acute pancreatitis in children on chronic maintenance dialysis. Pediatr Nephrol 34:1501–1512
Joseph A, Cointe A, Kurkdjian PM et al (2020) Shiga toxin-associated hemolytic uremic syndrome: a narrative review. Toxins 12:67
Tarr GA, Stokowski T, Shringi S et al (2019) Contribution and interaction of shiga toxin genes to Escherichia coli O157:H7 Virulence. Toxins 11:607
Ardissino G, Possenti I, Vignati C et al (2020) Is Shigatoxin 1 protective for the development of Shigatoxin 2-related hemolytic uremic syndrome in children? Data from the ItalKid-HUS Network. Pediatr Nephrol 35:1997–2001
Békássy ZD, Toledo CC, Leoj G et al (2011) Intestinal damage in enterohemorrhagic Escherichia coli infection. Pediatr Nephrol 26:2059–2071
Rahman RC, Cobeñas CJ, Drut R et al (2012) Hemorrhagic colitis in postdiarrheal hemolytic uremic syndrome: retrospective analysis of 54 children. Pediatr Nephrol 27:229–233
van Hoffen E, Mercenier A, Vidal K et al (2021) Characterization of the pathophysiological determinants of diarrheagenic Escherichia coli infection using a challenge model in healthy adults. Sci Rep 11:6060
Greenberg D, Jiang Z, Steffen R et al (2002) Markers of inflammation in bacterial diarrhea among travelers, with a focus on enteroaggregative Escherichia coli pathogenicity. J Infect Dis 185:944–949
Funding
Partial financial support was received from “Michela, l’Angelo Farfalla” volunteer organization, Lecce, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with our Ethics Committee which determined that our study did not need ethical approval.
Competing interests
Five authors of this publication are members of the European Reference Network for Rare Kidney Diseases (ERKNet). The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giordano, M., Iacoviello, O., Santangelo, L. et al. Gastrointestinal involvement in STEC-associated hemolytic uremic syndrome: 10 years in a pediatric center. Pediatr Nephrol 39, 1885–1891 (2024). https://doi.org/10.1007/s00467-023-06258-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06258-5