Abstract
Acute kidney injury (AKI) in children is associated with increased morbidity, reduced health-related quality of life, greater resource utilization, and higher mortality. Improvements in the timeliness and precision of AKI diagnosis in children are needed. In this report, we highlight existing, novel, and on-the-horizon diagnostic and risk-stratification tools for pediatric AKI, and outline opportunities for integration into clinical practice. We also summarize pediatric-specific high-risk diagnoses and exposures for AKI, as well as the potential role of real-time risk stratification and clinical decision support to improve outcomes. Lastly, the key characteristics of important pediatric AKI phenotypes will be outlined. Throughout, we identify key knowledge gaps, which represent prioritized areas of focus for future research that will facilitate a comprehensive, timely and personalized approach to pediatric AKI diagnosis and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first pediatric-focused Acute Disease Quality Initiative (ADQI) (The Pediatric ADQI; pADQI) meeting was conducted in Napa, CA, USA, as the 26th meeting of the Acute Disease Quality Initiative (ADQI) group (ADQI XXVI) [1]. The current manuscript details the work performed and conclusions drawn by the Risk Assessment and Diagnosis Workgroup, one of the six a priori defined pADQI subgroups.
Acute kidney injury (AKI) is independently associated with increased morbidity and mortality [2]. Its impact may be particularly profound in children [3], who have a longer life expectancy and more time to develop long-term sequelae, including chronic kidney disease (CKD). Despite these serious consequences, AKI management remains largely supportive, and therapeutic strategies are typically instituted only after AKI has occurred. In this review, we summarize the existing literature and outline the future of AKI diagnosis in children, including ways to individualize care in real-time.
Methods
The ADQI process is described in detail elsewhere, including the pADQI parent document [1, 4]. The goal of ADQI is “to provide expert-based statements and interpretation of current knowledge for use by clinicians according to professional judgment and identify evidence care gaps to establish research priorities” [5]. In the 26th ADQI, we addressed the primary question of “What are the unique considerations for AKI risk stratification and diagnosis in children?” This question served as the foundation for the following consensus statements.
26th ADQI consensus statement (Recommendation)
Validated tools which incorporate both patient characteristics and exposures and also interface with the local health care environment should be utilized to estimate AKI risk in children, including assessment of objective measures of kidney fitness in at-risk children prior to a predictable or planned intervention [1].
High-risk diagnoses and exposures
Accurate identification of children at risk for AKI is the critical first step to improving outcomes. Children in any intensive care unit (ICU) have higher rates of AKI compared to non-ICU patients [6] with reported incidences of 27% in the pediatric ICU [3], 30% in the neonatal ICU [6], and 54% in the cardiac ICU [7]. In these populations, the risk for AKI is highest in critically ill children with sepsis, congenital heart disease, malignancies, and those receiving invasive mechanical ventilation [8,9,10]. Importantly, very low birth weight infants are especially vulnerable, with AKI rates up to 48% [11,12,13]. It is crucial that those clinicians caring for patients in neonatal, general pediatric, and cardiac ICUs maintain heightened surveillance for AKI. These risk factors are summarized in Fig. 1.
Maintaining awareness of exposures that increase the risk for AKI in hospitalized children is key to AKI prevention (Fig. 1). A common inciting risk factor for AKI in children is nephrotoxic medication exposure [14]. In particular, the combination use of certain agents such as vancomycin and piperacillin-tazobactam may be associated with an increased risk for AKI [15]. In non-critically ill children, the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) collaborative developed consensus for the classification of nephrotoxic medications [16]. Recognition of high nephrotoxic medication burden as an AKI risk factor along with enhanced monitoring in the inpatient setting can decrease the rates of nephrotoxic medication-associated AKI [14, 17, 18].
Assessment of kidney fitness: subclinical kidney injury and kidney functional reserve
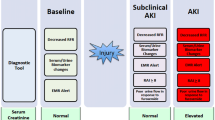
AKI diagnosis currently relies on changes in functional kidney biomarkers serum creatinine (SCr) and/or urine output, which are often delayed and imprecise markers of kidney function, particularly in patients with a rapidly changing glomerular filtration rate (GFR). A two-decade research focus has been directed at identifying novel tubular injury biomarkers to diagnose AKI sooner and more precisely, particularly after a high-risk exposure. Yet a child’s AKI risk would be assessed ideally prior to high-risk exposure. Since more than 70% of children admitted to a pediatric ICU in the USA have chronic healthcare needs [19], opportunities exist prior to hospitalization to evaluate a child’s kidney fitness. Kidney fitness refers to an adaptive ability to respond well to kidney stress and, therefore, show a decreased risk for both AKI and a decline in GFR over time [20]. Proactive assessment of kidney fitness includes standard measures of kidney function and injury obtained prior to planned events in patients with a high-risk diagnosis and/or exposure.
Kidney fitness assessment will likely move beyond traditional markers of kidney function. Quantification of kidney functional reserve has received renewed interest, with emerging evidence that adults with reduced kidney functional reserve prior to cardiac surgery are more likely to develop post-operative AKI and be at increased risk for CKD [21, 22]. This concept warrants further study in children. Pre-operative values of candidate biomarkers such as urinary DKK3 [23], uromodulin [24], and serum FGF-23 [25] show promise for predicting AKI after cardiac surgery as possible markers of kidney fitness.
Validated tools for assessing AKI risk in hospitalized children
Derived and validated specifically for critically ill pediatric patients, the Renal Angina Index (RAI) incorporates demographic characteristics and real-time patient data to predict severe AKI 72 h after ICU admission (Supplementary Fig. 1) [26], with a recent meta-analysis of over 3000 patients demonstrating a pooled AUROC of 0.88 (95% CI 0.85–0.91) [27]. Importantly, this simple and pragmatic tool has been assessed in resource-limited settings with good predictive performance [28]. Furthermore, targeted measurement of urinary biomarkers like neutrophil gelatinase-associated lipocalin (NGAL) improves risk stratification afforded by the RAI [29, 30]. The RAI has now also been modified and operationalized for early prediction of AKI in children in the emergency room [31], with sepsis [32], and after cardiac surgery [33]. Similar modifications to the RAI are needed for the neonatal ICU population, as well as for oncologic and post-transplant patients outside of the ICU setting.
However, a key limitation of the RAI is that it is obtained at a single, cross-sectional point in time from ICU or emergency department admission. It does not take into consideration changes in fluid status, SCr, and nephrotoxin exposure that are likely to occur over the course of a patient’s hospital stay. The Fluid Overload Kidney Injury Score (FOKIS) is a more recently developed score that is continuously calculated with the addition of any new data elements to the electronic health record (EHR) [34]. This four-dimensional score includes a standardized pediatric AKI assessment of changes in urine output and SCr, nephrotoxic medication exposure, and assessment of fluid overload. The score has been studied prospectively and shown to be associated with mortality and length of stay in a general pediatric ICU cohort [34] but requires validation in other patient groups.
A framework for comprehensive AKI risk assessment in children
At the time of hospital admission (or before, if planned), providers should complete a history and chart review that includes an evaluation for exposures and susceptibilities for AKI (Fig. 2A). Patients deemed to be at standard risk for AKI would undergo repeat AKI risk assessment with clinical changes during their hospital course, as their risk profile changes. Patients identified as high risk based on this assessment would undergo kidney-focused care including, but not limited to, more frequent SCr monitoring, careful attention to volume status (including close urine output monitoring, which may require foley catheter placement in some instances), and discontinuing or replacing nephrotoxic medications as soon as it is medically appropriate. Importantly, there is a crucial need for utilizing prognostic enrichment in the employment of AKI prevention and treatment strategies. However, interventions aimed at prevention of AKI in pre-identified high-risk populations have had variable success. A recent meta-analysis of 13 studies found that implementation of AKI care “bundles” in hospitalized patients reduces moderate–severe AKI, mainly driven by studies in an ICU setting [35]. In addition, there are reports of successful AKI care bundles in adults after cardiopulmonary bypass surgery [36] and non-cardiac surgery [37]. Bundle components were heterogeneous, and compliance to bundles were variable, limiting the ability to draw conclusions. There is a need for investigations exploring the impact of feasible AKI prevention bundles in pediatric patients that take into consideration the unique aspects of this patient population and their care.
Acute kidney injury risk assessment and dynamic phenotyping. A Acute kidney injury risk assessment. A comprehensive acute kidney injury risk assessment should occur in children admitted to the hospital, prior to planned high-risk exposures, and repeated with clinical changes. This assessment allows for individual risk stratification that can focus resources and identify patients appropriate for more intensive, kidney-focused monitoring and care. B Dynamic acute kidney injury phenotyping. Combined with individual susceptibility, multiple elements contribute to discernible acute kidney injury phenotypes in affected children which may have prognostic and therapeutic implications. This phenotype is dynamic and may change over the course of illness, highlighting the importance of ongoing assessment.
Data on risks for pediatric AKI in the community or outpatient setting are scarce [6, 38]. Notably, in limited resource settings, AKI is typically community-acquired rather than hospital-acquired [39]. In contrast to focusing on AKI care bundles enacted at the time of hospital admission, efforts aimed at providing clean drinking water, sanitation, and access to healthcare are crucial in preventing AKI in resource-limited countries [39].
Making the timely diagnosis of AKI in children: expanding our diagnostic tools
The last two decades have witnessed a concerted effort to identify novel biomarkers to detect and predict AKI earlier than functional markers (i.e., SCr and urine output) alone, within a therapeutic “window of opportunity.” When available, biomarkers of kidney damage or stress may allow further insight into AKI risk and phenotype (Table 1). Ideally, therapies to prevent progression or severity of AKI would be employed before both structural and functional biomarkers are elevated. Unfortunately, despite good to excellent performance in multiple studies [40,41,42], most structural biomarkers are not routinely used clinically due to lack of biomarker assay availability or regulatory approval, or because of cost. Highlighting further the need to expand upon existing AKI diagnostic tools, in many low- and middle-income countries, even SCr testing is unavailable [43]. In these settings, point-of-care urine NGAL and salivary urea nitrogen testing have been proposed as feasible alternatives for the diagnosis of AKI in children [44].
The widespread implementation of EHRs in resource-rich settings provides an opportunity to leverage electronically accessible data to enhance risk stratification and AKI detection at the bedside to improve outcomes. Automated alerts and associated real-time clinical decision support (CDS) within the EHR can facilitate the identification of at-risk patients and notify clinicians of abnormal measurements that may predict AKI development or progression [43]. The NINJA study demonstrates that standardized AKI risk assessment and surveillance in non-critically ill hospitalized children receiving nephrotoxic medications can lead to sustained reductions in both AKI incidence and severity [45]. As part of the NINJA initiative, a CDS program was implemented to identify those at risk for AKI, directing daily SCr surveillance and medication dose adjustment or discontinuation. The initial single-center evaluation of this program demonstrated a 38% reduction in nephrotoxic medication exposure and 64% reduction in AKI [45], and it has subsequently been disseminated and implemented at more than a dozen children’s hospitals across the USA [14]. While these data are promising, it is important to note that the epidemiology of AKI and the resources available may be different in developing countries, and thus specific AKI CDS strategies should be developed and focused accordingly. Additionally, given the heterogeneity of the pediatric population, alerts should be customizable to reflect age-adjusted reference ranges and patient-specific parameters [46, 47].
While AKI alert systems may improve the timeliness of AKI diagnosis, they likely provide the greatest benefit if they also suggest targeted interventions to limit its progression and negative sequelae. For example, in a study of over 3000 hospitalized adults, AKI alerts with recommendations for a targeted nephrology consult led to a 25% decrease in severe AKI rates [48]. Similarly, a pediatric study demonstrated implementation of an AKI alert with CDS to guide patient evaluation and management resulted in a decrease in AKI stage and a higher proportion of nephrotoxic medication adjustment and changes in fluid management compared to the pre-CDS phase [49]. Though these studies highlight the potential of EHR-based AKI alerts and CDS, it is important to note that these data are balanced by a recent large, multi-center, randomized controlled trial demonstrating that an EHR AKI alert and associated CDS failed to decrease AKI progression, and even resulted in higher risk of worsening AKI or death at some centers [46]. Clearly, these conflicting study results highlight the importance of conducting further prospective clinical trials examining the role of EHR-based AKI alerts and CDS in children, ensuring the incorporation of considerations unique to the pediatric population.
26th ADQI consensus statement (Suggestion)
Unique AKI phenotypes in children may overlap and change over time. Differentiating AKI phenotype(s) informs prognosis and has the potential to guide therapeutics [1].
A precision medicine primer: phenotypes, endotypes, and enrichment
AKI is a heterogeneous syndrome with multiple etiologies, clinical manifestations, and biological underpinnings. Therefore, efforts are underway to identify and characterize unique subsets of AKI, in order to advance care by applying the tenets of precision medicine, as has been successfully done in other heterogeneous pediatric syndromes like sepsis and acute respiratory distress syndrome (ARDS) [50, 51]. Broadly, precision medicine refers to preventive, diagnostic and/or treatment strategies that take individual patient and/or disease factors into account [52, 53]. Key to such an approach is the identification of unique disease phenotypes, which are defined by clinically observable characteristics of the disorder of interest [54]. However, as our ability to incorporate molecular and biochemical information into these unique subsets has improved, the term endotype describes a unique phenotype defined by a distinct underlying pathobiology more appropriately [54]. Thus, the identification of endotypes represents a key step in phenotyping work, as it ties clinically defined clusters of patients to underlying biology and offers a mechanism for identifying appropriate therapies tailored to the individual patient.
This concept of identifying the right therapy for the right patient is an example of enrichment, a tenet of precision medicine, and the goal of heterogeneous disorder phenotyping [52, 53]. Enrichment strategies can be prognostic (i.e., selecting patients with a higher likelihood of having a disease-related outcome of interest, such as development of AKI or need for kidney replacement therapy (KRT)), or predictive (i.e., selecting patients more likely to respond to a therapy on the basis of biology) [52]. Thus, when defining clinically important AKI phenotypes in children, it is important to consider how a phenotyping strategy facilitates either prognostic or predictive enrichment. We propose AKI phenotyping in children focuses on the following: (1) identifying children who are more likely to suffer meaningful outcomes of interest (i.e., prognostic enrichment), and (2) identifying children with shared underlying biology who may benefit from a specific therapy (i.e., predictive enrichment). Supplementary Fig. 2 outlines these proposed priorities for pediatric AKI phenotyping.
Pediatric AKI phenotypes: current state of the art
Much of the AKI phenotyping work to date facilitates prognostic enrichment. In particular, several different strategies have been utilized to identify patients at high risk for severe AKI (i.e., ≥ Kidney Disease Improving Global Outcomes (KDIGO) Stage 2), persistent AKI (present for ≥ 48 h), and those more likely to require KRT [55,56,57,58,59,60,61,62,63]. Validation of AKI phenotypes to reliably identify at-risk patients will direct patient and family counseling, targeted implementation of intensive kidney supportive care, and clinical trial enrichment for future studies examining novel AKI therapies. The latter is of particular importance in children given the relatively small patient population compared to adults.
Urine output phenotypes
Urine output is one of two functional biomarkers in the KDIGO criteria for AKI diagnosis. Until recently, little data existed regarding its impact on AKI epidemiology and outcomes in children [64]. A post hoc analysis of the multi-center, multinational Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology (AWARE) study demonstrated nearly 20% of critically ill children with AKI were diagnosed by urine output criteria only, and that these patients had similar negative outcomes to those diagnosed only by SCr criteria [60]. Furthermore, compared to patients without AKI, meeting criteria for AKI by both SCr and urine output increased the relative risk for receiving KRT to 165.7 (95% CI, 86.3–318.2) (p < 0.01) from 9.1 (95% CI, 3.9–21.2) in those diagnosed by SCr alone [60]. A separate post hoc analysis of AWARE developed a summative AKI score that added AKI stage by SCr to AKI stage by urine output, showing increasing AKI score was associated with worse outcomes, including KRT use, prolonged length of stay, AKI non-recovery and mortality [61].
Fluid accumulation phenotypes
Excessive positive fluid balance or fluid overload (FO) has been well demonstrated to be associated with increased morbidity and mortality in critically ill children [65]. One study of 149 critically ill children combined FO with functional AKI staging by SCr or urine output to delineate unique AKI phenotypes (AKI-/FO-, AKI + /FO-, AKI-/FO + , AKI + /FO +). The 24% of patients who developed ≥ 20% fluid accumulation by ICU Day 3 experienced worse outcomes (including prolonged length of stay and mortality), irrespective of functional AKI status [58]. The concepts surrounding fluid accumulation and associated outcomes are discussed more in depth by pADQI Work Group 3.
Severity and duration phenotypes
The ADQI 16 outlined definitions of transient AKI (sustained reversal within 48 h of onset) versus persistent AKI (≥ 48 h in duration) in adults, recognizing that outcomes in the latter subset of patients were worse [66]. An assessment of AKI duration-based phenotypes in a cohort of children with sepsis from AWARE demonstrated patients with persistent AKI had fewer ICU-free days and more complex ICU course than patients with transient AKI [55]. Additionally, combination of AKI duration severity delineated four unique AKI phenotypes (mild–transient, mild–persistent, severe–transient, severe–persistent) that had differential outcomes [56]. Two other studies showed severity and duration phenotypes in children following cardiac surgery to be associated with worse outcomes among those with persistent AKI [59, 67].
Response to loop diuretics
A standardized assessment of diuretic responsiveness, termed the furosemide stress test (FST) predicts the progression of AKI in critically ill patients [68]. Retrospective pediatric studies have examined the association between furosemide responsiveness and AKI-related outcomes following cardiac surgery [62, 63]. Urine output response to furosemide in the first 24 h following cardiopulmonary bypass predicted cardiac surgery-associated AKI, peak FO, and other poor outcomes, including mortality [63]. Lower urine flow rates in children following cardiac surgery at 2 and 6 h after furosemide were associated with AKI and prolonged length of stay [62]. While data from these studies has been extrapolated to propose 3 ml/kg/h urine flow rate to predict AKI progression in children [69], larger, prospective studies are still needed to validate this threshold, and to fully understand the role of loop diuretic responsiveness in identifying a unique AKI phenotype in children.
Biomarker-based phenotypes
The ADQI 10 proposed a new AKI model to integrate functional markers and damage biomarkers to delineate unique biomarker-based AKI phenotypes [70]. In children after cardiopulmonary bypass, investigators showed that combining a functional biomarker like cystatin C with a tubular damage biomarker like NGAL was superior to changes in SCr for predicting the duration of AKI [56]. A single-center study derived SCr and NGAL-based AKI phenotypes (NGAL-/SCr-, NGAL + /SCr-, NGAL-/SCr + , NGAL + /SCr +) on the day of pediatric ICU admission and examined their associations with AKI outcomes at Day 3 [6]. Patients with functional AKI without evidence of tubular damage (NGAL-/SCr +) were more likely to have transient AKI (i.e., resolved by Day 3), while those with evidence of tubular damage (i.e. NGAL +) were more likely to have AKI on Day 3, regardless of functional biomarker status (i.e., SCr + or SCr-) [57]. Similar to work in adults, this study highlighted the significance of subclinical AKI (i.e. tubular injury biomarker positive without functional biomarker elevation, NGAL + /SCr-), as these patients had worse outcomes compared to biomarker negative patients [57]. Since AKI biomarkers such as NGAL remain clinically unavailable at many pediatric centers, more work is needed to validate these findings and to elucidate when and in whom to obtain tubular damage biomarkers.
Pediatric AKI phenotypes: the gaps
While the above-described frameworks outline static, one, or two-factor-based AKI phenotypes, it is likely that more than one may be present in a child with AKI at any given time, and that this unique combination of phenotypes may change over the course of illness (Fig. 2B). Thus, key knowledge gaps in our understanding of AKI phenotypes relate to delineating the synergistic and time-dependent effects of these phenotypes on patient outcomes, an important area of future research.
Finally, a second major gap in pediatric AKI phenotyping to date remains strategies that facilitate predictive enrichment (Supplementary Fig. 2). While we may be able to use some of the constructs outlined above to make therapeutic decisions (for example, a patient with NGAL elevation, who has FO and is not responsive to loop diuretics, may be an appropriate candidate for consideration of early KRT), less is known about the biological underpinnings of AKI phenotypes, which limits our ability to provide individualized, biology-informed treatments. Lessons can be learned from previous work in pediatric sepsis and ARDS, where both unique endotypes and phenotypes have been delineated that may identify those most likely to respond to a given therapy (i.e., stress corticosteroids in septic shock) [50, 51]. Ultimately, a phenotyping approach that combines prognostic enrichment (i.e., AKI risk stratification) and predictive enrichment (i.e., identification of a phenotype or endotype likely to respond to a specific therapy) will be needed to inform enrollment in future clinical trials aimed at identifying successful AKI therapies.
Conclusions
Given that AKI is associated with a higher risk of morbidity, mortality, and health care resource utilization, establishing a timely and accurate diagnosis is essential. It is necessary that those caring for children admitted to the hospital have a knowledge of high-risk diagnoses and exposures that are associated with pediatric AKI, and that community-based providers are given tools to identify and assess at-risk patients prior to hospitalization. It has become evident that we cannot solely rely on traditional markers of kidney function, such as urine output and SCr, for diagnosing AKI. The use of novel biomarkers incorporated with a validated AKI risk assessment tool like the RAI, can improve our ability to predict AKI. Additionally, tubular damage biomarkers along with factors such as fluid status, diuretic response, AKI severity, and duration may help us to identify unique pediatric patient AKI phenotypes. Ultimately, knowledge of these dynamic phenotypes show tremendous promise to guide clinicians in making prognostic and therapeutic decisions for children with AKI.
References
Goldstein SL, Akcan-Arikan A, Alobaidi R, Askenazi DJ, Bagshaw SM, Barhight M, Barreto E, Bayrakci B, Bignall ONR, Bjornstad E, Brophy PD, Chanchlani R, Charlton JR, Conroy AL, Deep A, Devarajan P, Dolan K, Fuhrman DY, Gist KM, Gorga SM, Greenberg JH, Hasson D, Ulrich EH, Iyengar A, Jetton JG, Krawczeski C, Meigs L, Menon S, Morgan J, Morgan CJ, Mottes T, Neumayr TM, Ricci Z, Selewski D, Soranno DE, Starr M, Stanski NL, Sutherland SM, Symons J, Tavares MS, Vega MW, Zappitelli M, Ronco C, Mehta RL, Kellum J, Ostermann M, Basu RK; Pediatric ADQI Collaborative (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open 5:e2229442
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning, Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–212
Kellum JA, Bellomo R, Ronco C (2008) Acute Dialysis Quality Initiative (ADQI): methodology. Int J Artif Organs 31:90–93
Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, Ling XB (2013) AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8:1661–1669
Alten JA, Cooper DS, Blinder JJ, Selewski DT, Tabbutt S, Sasaki J, Gaies MG, Bertrandt RA, Smith AH, Reichle G, Gist KM, Banerjee M, Zhang W, Hock KM, Borasino S; Neonatal and Pediatric Heart and Renal Outcomes Network (NEPHRON) Investigators (2021) Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med 49:e941–e951
Chang JW, Jeng MJ, Yang LY, Chen TJ, Chiang SC, Soong WJ, Wu KG, Lee YS, Wang HH, Yang CF, Tsai HL (2015) The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int 87:632–639
Hui-Stickle S, Brewer ED, Goldstein SL (2005) Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45:96–101
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ; Neonatal Kidney Collaborative (NKC) (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194
Carmody JB, Swanson JR, Rhone ET, Charlton JR (2014) Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol 9:2036–2043
Mwamanenge NA, Assenga E, Furia FF (2020) Acute kidney injury among critically ill neonates in a tertiary hospital in Tanzania; prevalence, risk factors and outcome. PLoS One 15:e0229074
Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, Askenazi DJ, Henderson T, Dill L, Somers MJG, Kerr J, Gilarde J, Zaritsky J, Bica V, Brophy PD, Misurac J, Hackbarth R, Steinke J, Mooney J, Ogrin S, Chadha V, Warady B, Ogden R, Hoebing W, Symons J, Yonekawa K, Menon S, Abrams L, Sutherland S, Weng P, Zhang F, Walsh K (2020) A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97:580–588
Downes KJ, Cowden C, Laskin BL, Huang YS, Gong W, Bryan M, Fisher BT, Goldstein SL, Zaoutis TE (2017) Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr 171:e173219
Goswami E, Ogden RK, Bennett WE, Goldstein SL, Hackbarth R, Somers MJG, Yonekawa K, Misurac J (2019) Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm 76:1869–1874
Benoit SW, Goldstein SL, Dahale DS, Haslam DB, Nelson A, Truono K, Davies SM (2019) Reduction in nephrotoxic antimicrobial exposure decreases associated acute kidney injury in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant 25:1654–1658
Young J, Dahale D, Demmel K, O’Brien M, Geller J, Courter J, Haslam DB, Danziger-Isakov L, Goldstein SL (2020) Reducing acute kidney injury in pediatric oncology patients: an improvement project targeting nephrotoxic medications. Pediatr Blood Cancer 67:e28396
Murphy Salem S, Graham RJ (2021) Chronic illness in pediatric critical care. Front Pediatr 9:686206
Fuhrman DY, Kellum JA (2016) Biomarkers for diagnosis, prognosis and intervention in acute kidney injury. Contrib Nephrol 187:47–54
Husain-Syed F, Ferrari F, Sharma A, Danesi TH, Bezerra P, Lopez-Giacoman S, Samoni S, de Cal M, Corradi V, Virzi GM, De Rosa S, Mucino Bermejo MJ, Estremadoyro C, Villa G, Zaragoza JJ, Caprara C, Brocca A, Birk HW, Walmrath HD, Seeger W, Nalesso F, Zanella M, Brendolan A, Giavarina D, Salvador L, Bellomo R, Rosner MH, Kellum JA, Ronco C (2018) Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg 105:1094–1101
Husain-Syed F, Emlet DR, Wilhelm J, Danesi TH, Ferrari F, Bezerra P, Lopez-Giacoman S, Villa G, Tello K, Birk HW, Seeger W, Giavarina D, Salvador L, Fuhrman DY, Kellum JA, Ronco C; IRRIV-AKI Study Group (2022) Effects of preoperative high-oral protein loading on short- and long-term renal outcomes following cardiac surgery: a cohort study. J Transl Med 20:204
Schunk SJ, Zarbock A, Meersch M, Kullmar M, Kellum JA, Schmit D, Wagner M, Triem S, Wagenpfeil S, Grone HJ, Schafers HJ, Fliser D, Speer T, Zewinger S (2019) Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet 394:488–496
Bennett MR, Pyles O, Ma Q, Devarajan P (2018) Preoperative levels of urinary uromodulin predict acute kidney injury after pediatric cardiopulmonary bypass surgery. Pediatr Nephrol 33:521–526
Volovelsky O, Terrell TC, Swain H, Bennett MR, Cooper DS, Goldstein SL (2018) Pre-operative level of FGF23 predicts severe acute kidney injury after heart surgery in children. Pediatr Nephrol 33:2363–2370
Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL (2014) Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 85:659–667
Abbasi A, Mehdipour Rabori P, Farajollahi R, Mohammed Ali K, Ataei N, Yousefifard M, Hosseini M (2020) Discriminatory precision of renal angina index in predicting acute kidney injury in children; a systematic review and meta-analysis. Arch Acad Emerg Med 8:e39
Kaur R, Dhooria GS, Pooni PA, Bhat D, Bhargava S, Kakkar S, Arora K, Bansal N (2018) Utilization of the renal angina index in PICU of a developing country for prediction of subsequent severe acute kidney injury. Pediatr Nephrol 33:2185–2191
Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, Arnold P, Bennett MR, Basu RK (2016) Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant 31:586–594
Goldstein SL, Krallman KA, Kirby C, Roy JP, Collins M, Fox K, Schmerge A, Wilder S, Gerhardt B, Chima R, Basu RK, Chawla L, Fei L (2022) Integration of the renal angina index and urine neutrophil gelatinase-associated lipocalin improves severe acute kidney injury prediction in critically ill children and young adults. Kidney Int Rep 7:1842–1849
Hanson HR, Carlisle MA, Bensman RS, Byczkowski T, Depinet H, Terrell TC, Pitner H, Knox R, Goldstein SL, Basu RK (2021) Early prediction of pediatric acute kidney injury from the emergency department: a pilot study. Am J Emerg Med 40:138–144
Stanski NL, Wong HR, Basu RK, Cvijanovich NZ, Fitzgerald JC, Weiss SL, Bigham MT, Jain PN, Schwarz A, Lutfi R, Nowak J, Allen GL, Thomas NJ, Grunwell JR, Quasney M, Haileselassie B, Chawla LS, Goldstein SL (2021) Recalibration of the renal angina index for pediatric septic shock. Kidney Int Rep 6:1858–1867
Gist KM, SooHoo M, Mack E, Ricci Z, Kwiatkowski DM, Cooper DS, Krawczeski CD, Alten JA, Goldstein SL, Basu RK (2022) Modifying the renal angina index for predicting AKI and related adverse outcomes in pediatric heart surgery. World J Pediatr Congenit Heart Surg 13:196–202
Akcan-Arikan A, Gebhard DJ, Arnold MA, Loftis LL, Kennedy CE (2017) Fluid overload and kidney injury score: a multidimensional real-time assessment of renal disease burden in the critically ill patient. Pediatr Crit Care Med 18:524–530
Schaubroeck HAI, Vargas D, Vandenberghe W, Hoste EAJ (2021) Impact of AKI care bundles on kidney and patient outcomes in hospitalized patients: a systematic review and meta-analysis. BMC Nephrol 22:335
Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, Zarbock A (2017) Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 43:1551–1561
Gocze I, Schlitt HJ, Bergler T (2018) Biomarker-guided Intervention to prevent AKI or KDIGO care bundle to prevent AKI in high-risk patients undergoing major surgery? Ann Surg 268:e68–e69
Greenberg JH, Coca S, Parikh CR (2014) Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol 15:184
Kashani K, Macedo E, Burdmann EA, Hooi LS, Khullar D, Bagga A, Chakravarthi R, Mehta R (2017) Acute kidney injury risk assessment: differences and similarities between resource-limited and resource-rich countries. Kidney Int Rep 2:519–529
Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P (2007) Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care 11:R127
Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX; TRIBE-AKI Consortium (2011) Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22:1748–1757
Meersch M, Schmidt C, Van Aken H, Rossaint J, Gorlich D, Stege D, Malec E, Januszewska K, Zarbock A (2014) Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One 9:e110865
Sandokji I, Yamamoto Y, Biswas A, Arora T, Ugwuowo U, Simonov M, Saran I, Martin M, Testani JM, Mansour S, Moledina DG, Greenberg JH, Wilson FP (2020) A time-updated, parsimonious model to predict AKI in hospitalized children. J Am Soc Nephrol 31:1348–1357
Calice-Silva V, Vieira MA, Raimann JG, Carter M, Callegari J, Levin NW, Kotanko P, Pecoits-Filho R (2014) Saliva urea nitrogen dipstick - a novel bedside diagnostic tool for acute kidney injury. Clin Nephrol 82:358–366
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES (2016) A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90:212–221
Wilson FP, Martin M, Yamamoto Y, Partridge C, Moreira E, Arora T, Biswas A, Feldman H, Garg AX, Greenberg JH, Hinchcliff M, Latham S, Li F, Lin H, Mansour SG, Moledina DG, Palevsky PM, Parikh CR, Simonov M, Testani J, Ugwuowo U (2021) Electronic health record alerts for acute kidney injury: multicenter, randomized clinical trial. BMJ 372:m4786
Greenberg JH, Parikh CR (2017) Biomarkers for diagnosis and prognosis of AKI in children: One Size Does Not Fit All. Clin J Am Soc Nephrol 12:1551–1557
Park S, Baek SH, Ahn S, Lee KH, Hwang H, Ryu J, Ahn SY, Chin HJ, Na KY, Chae DW, Kim S (2018) Impact of electronic acute kidney injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: a quality improvement study. Am J Kidney Dis 71:9–19
Menon S, Tarrago R, Carlin K, Wu H, Yonekawa K (2021) Impact of integrated clinical decision support systems in the management of pediatric acute kidney injury: a pilot study. Pediatr Res 89:1164–1170
Dahmer MK, Yang G, Zhang M, Quasney MW, Sapru A, Weeks HM, Sinha P, Curley MAQ, Delucchi KL, Calfee CS, Flori H; RESTORE and BALI study investigators, Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network (2022) Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis. Lancet Respir Med 10:289–297
Wong HR, Atkinson SJ, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Checchia PA, Meyer K, Quasney M, Hall M, Gedeit R, Freishtat RJ, Nowak J, Raj SS, Gertz S, Lindsell CJ (2016) Combining prognostic and predictive enrichment strategies to identify children with septic shock responsive to corticosteroids. Crit Care Med 44:e1000-1003
Stanski NL, Wong HR (2020) Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol 16:20–31
Wong HR (2017) Intensive care medicine in 2050: precision medicine. Intensive Care Med 43:1507–1509
Chung KF (2018) Precision medicine in asthma: linking phenotypes to targeted treatments. Curr Opin Pulm Med 24:4–10
Basu RK, Hackbarth R, Gillespie S, Akcan-Arikan A, Brophy P, Bagshaw S, Alobaidi R, Goldstein SL (2021) Clinical phenotypes of acute kidney injury are associated with unique outcomes in critically ill septic children. Pediatr Res 90:1031–1038
Basu RK, Wong HR, Krawczeski CD, Wheeler DS, Manning PB, Chawla LS, Devarajan P, Goldstein SL (2014) Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 64:2753–2762
Stanski N, Menon S, Goldstein SL, Basu RK (2019) Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children. J Crit Care 53:1–7
Gist KM, Selewski DT, Brinton J, Menon S, Goldstein SL, Basu RK (2020) Assessment of the independent and synergistic effects of fluid overload and acute kidney injury on outcomes of critically ill children. Pediatr Crit Care Med 21:170–177
Gist KM, Borasino S, SooHoo M, Soranno DE, Mack E, Hock KM, Rahman A, Brinton JT, Basu RK, Alten JA (2022) Transient and persistent acute kidney injury phenotypes following the Norwood operation: a retrospective study. Cardiol Young 32:564–571
Kaddourah A, Basu RK, Goldstein SL, Sutherland SM; Assessment of Worldwide Acute Kidney Injury, Renal Angina and, Epidemiology (AWARE) Investigators (2019) Oliguria and acute kidney injury in critically ill children: implications for diagnosis and outcomes. Pediatr Crit Care Med 20:332–339
Sutherland SM, Kaddourah A, Gillespie SE, Soranno DE, Woroniecki RP, Basu RK, Zappitelli M; Assessment of Worldwide Acute Kidney Injury, Renal Angina and, Epidemiology (AWARE) Investigators (2021) Cumulative application of creatinine and urine output staging optimizes the kidney disease: improving global outcomes definition and identifies increased mortality risk in hospitalized patients with acute kidney injury. Crit Care Med 49:1912–1922
Penk J, Gist KM, Wald EL, Kitzmiller L, Webb TN, Li Y, Cooper DS, Goldstein SL, Basu RK (2019) Furosemide response predicts acute kidney injury in children after cardiac surgery. J Thorac Cardiovasc Surg 157:2444–2451
Borasino S, Wall KM, Crawford JH, Hock KM, Cleveland DC, Rahman F, Martin KD, Alten JA (2018) Furosemide response predicts acute kidney injury after cardiac surgery in infants and neonates. Pediatr Crit Care Med 19:310–317
Kellum JA, Lameire N; KDIGO AKI Guideline Work Group (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17:204
Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, Bagshaw SM (2018) Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr 172:257–268
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA; ADQI Workgroup (2017) Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13:241–257
LoBasso M, Schneider J, Sanchez-Pinto LN, Del Castillo S, Kim G, Flynn A, Sethna CB (2022) Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr Nephrol 37:659–665
Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Kimmel PL, Seneff MG (2013) Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care 17:R207
Hasson DC, Zhang B, Krallman K, Rose JE, Kempton KM, Steele P, Devarajan P, Goldstein SL, Alder MN (2023) Acute kidney injury biomarker olfactomedin 4 predicts furosemide responsiveness. Pediatr Nephrol. https://doi.org/10.1007/s00467-023-05920-2
Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, Chawla LS, Cruz D, Ince C, Okusa MD; ADQI Workgroup (2014) Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 85:513–521
Acknowledgements
The ADQI 26 workgroup: The following individuals contributed to the formulation and content of this work in accordance with their participation in the 26th Acute Disease Quality Initiative (ADQI XXVI).
Chairs:
Ayse Akcan Arikan, MD, Baylor College of Medicine, Department of Pediatrics, Divisions of Critical Care Medicine and Nephrology, Houston, TX, USA.
Rajit K. Basu, MD, MS, Northwestern University Feinberg School of Medicine, Ann & Robert Lurie Children’s Hospital of Chicago, Department of Pediatrics, Division of Critical Care Medicine, Chicago, IL, USA.
Stuart L. Goldstein, MD, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, Division of Nephrology & Hypertension, Cincinnati, OH, USA.
Rashid Alobaidi1, David J. Askenazi2, Sean M. Bagshaw1, Matthew Barhight3, Erin Barreto4, Benan Bayrakci5, O.N. Ray Bignall II6, Erica Bjornstad2, Patrick Brophy7, Jennifer Charlton8, Rahul Chanchlani9, Andrea L. Conroy10, Akash Deep11, Prasad Devarajan12, Kristin Dolan13, Dana Fuhrman14, Katja M. Gist12, Stephen M. Gorga15, Jason H. Greenberg16, Denise Hasson12, Emma Heydari1, Arpana Iyengar17, Jennifer Jetton18, Catherine Krawczeski6, Leslie Meigs19, Shina Menon20, Catherine Morgan1, Jolyn Morgan12, Theresa Mottes3, Tara Neumayr21, Zaccaria Ricci22, David T. Selewski23, Danielle Soranno10, Natalja Stanski12, Michelle Starr10, Scott M. Sutherland24, Jordan Symons20, Marcelo Tavares25, Molly Vega26, Michael Zappitelli27, Claudio Ronco28, Ravindra L. Mehta29, John Kellum30, and Marlies Ostermann31.
1Alberta Health Sciences University, Edmonton, Alberta, Canada.
2University of Alabama at Birmingham, Children’s Hospital of Alabama, Birmingham, AL, USA.
3Northwestern University Feinberg School of Medicine, Ann & Robert Lurie Children’s Hospital of Chicago, Chicago, IL, USA.
4Hacettepe University, Ankara, Türkiye
5Mayo Clinic, Rochester, MN, USA.
6Nationwide Children’s Hospital, Ohio State University, Columbus, OH, USA.
7Golisano Children’s Hospital, Rochester University Medical Center, Rochester, NY, USA.
8University of Virginia, Charlottesville, VA, USA.
9McMaster University, McMaster Children’s Hospital, Hamilton, ON, Canada.
10Riley Children’s Hospital, Indiana University School of Medicine, Indianapolis, IN, USA.
11King’s College London, King’s College Hospital NHS Foundation Trust, London, UK
12Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
13Mercy Children’s Hospital, University of Missouri-Kansas City School of Medicine, Kansas City, MO, USA.
14Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
15C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI, USA.
16Yale University Medical Center, New Haven, CT, USA.
17St. John’s National Academy of Health Sciences, Bangalore, India.
18Stead Family Children’s Hospital, University of Iowa, Iowa City, IA, USA.
19University of St. Thomas, Houston, TX USA.
20Seattle Children’s Hospital, University of Washington, Seattle, WA, USA.
21Washington University School of Medicine, St. Louis, MO, USA.
22University of Florence, Florence, Italy.
23Medical University of South Carolina, Charleston, SC, USA.
24Lucille Packard Children’s Hospital, Stanford University, Palo Alto, CA, USA.
25Santa Casa dela Belo Horizonte, Belo Horizonte, Brazil.
26Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA.
27Hospital for Sick Children, Toronto, Ontario, Canada.
28San Bartolo Hospital, Universiti di Padova, Vicenza, Italy.
29University of California San Diego School of Medicine, La Jolla, CA, USA.
30University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
31Guy’s and St. Thomas’ NHS Foundation Trust, London, UK.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dana Y. Fuhrman and Natalja L. Stanski contributed equally to the writing of this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
467_2023_6133_MOESM1_ESM.jpg
Supplementary Figure 1. The Renal Angina Index. Derived and validated in critically ill children, the renal angina index is calculated 12 hours after intensive care unit admission from demographic and clinical data. A score of 8 or higher defines “renal angina” and has been demonstrated to predict severe acute kidney injury 72 hours later. (JPG 360 KB)
467_2023_6133_MOESM2_ESM.jp2
Supplementary Figure 2. Priorities for pediatric acute kidney injury phenotyping. Acute kidney injury phenotyping efforts in children should focus on identifying those most likely to suffer meaningful outcomes of interest (i.e. prognostic enrichment) and identifying those with shared underlying biology that may direct therapy (i.e. predictive enrichment). Identifying these unique subsets of patients has the ability to inform care and improve outcomes, including via targeted clinical trial enrollment and the delivery of patient-specific biology-based therapies. (JP2 88 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fuhrman, D.Y., Stanski, N.L., Krawczeski, C.D. et al. A proposed framework for advancing acute kidney injury risk stratification and diagnosis in children: a report from the 26th Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol 39, 929–939 (2024). https://doi.org/10.1007/s00467-023-06133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06133-3