Abstract
Background
Focal segmental glomerulosclerosis (FSGS) is a leading cause of steroid-resistant nephrotic syndrome (SRNS) that predominantly affects the podocytes. While mutations in genes causing pediatric SRNS have enhanced our understanding of FSGS, the disease’s etiology remains complex and poorly understood.
Methods
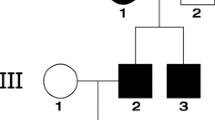
Whole exome sequencing (WES) was performed on a 9-year-old girl with SRNS associated with FSGS (SRNS–FSGS). We analyzed the expression of CRB2, slit diaphragm (SD)-associated proteins, and sphingosine 1-phosphate receptor 1 (S1PR1) in the proband and CRB2 knock-down podocytes.
Results
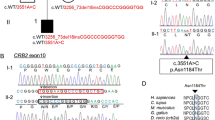
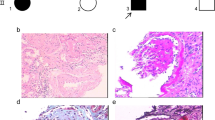
In this study, we identified two novel compound heterozygous mutations in the Crumbs homolog 2 (CRB2) gene (c.2905delinsGCCACCTCGCGCTGGCTG, p.T969Afs*179 and c.3268C > G, p.R1090G) in a family with early-onset SRNS–FSGS. Our findings demonstrate that these CRB2 abnormalities were the underlying cause of SRNS–FSGS. CRB2 defects led to the dysfunction of podocyte SD-related proteins, including podocin, nephrin, and zonula occludens-1 (ZO-1), by reducing the phosphorylation level of S1PR1. Interestingly, the podocytic cytoskeleton remained unaffected, as demonstrated by normal expression and localization of synaptopodin. Our study also revealed a secondary decrease in CRB2 expression in idiopathic FSGS patients, indicating that CRB2 mutations may cause FSGS through a previously unknown mechanism involving SD-related proteins.
Conclusions
Overall, our findings shed new light on the pathogenesis of SRNS–FSGS and revealed that the novel pathogenic mutations in CRB2 contribute to the development of FSGS through a previously unknown mechanism involving SD-related proteins.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information






Similar content being viewed by others
Data availability
All data and materials included in this study are available upon request by contacting with the corresponding author.
References
Chapman FA, Nyimanu D, Maguire JJ, Davenport AP, Newby DE, Dhaun N (2021) The therapeutic potential of apelin in kidney disease. Nat Rev Nephrol 17:840–853. https://doi.org/10.1038/s41581-021-00461-z
Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V (2021) Chronic kidney disease. Lancet 398:786–802. https://doi.org/10.1016/s0140-6736(21)00519-5
Jacobs-Cachá C, Vergara A, García-Carro C, Agraz I, Toapanta-Gaibor N, Ariceta G, Moreso F, Serón D, López-Hellín J, Soler MJ (2021) Challenges in primary focal segmental glomerulosclerosis diagnosis: from the diagnostic algorithm to novel biomarkers. Clin Kidney J 14:482–491. https://doi.org/10.1093/ckj/sfaa110
De Vriese AS, Wetzels JF, Glassock RJ, Sethi S, Fervenza FC (2021) Therapeutic trials in adult FSGS: lessons learned and the road forward. Nat Rev Nephrol 17:619–630. https://doi.org/10.1038/s41581-021-00427-1
Weng PL, Majmundar AJ, Khan K, Lim TY, Shril S, Jin G, Musgrove J, Wang M, Ahram DF, Aggarwal VS, Bier LE, Heinzen EL, Onuchic-Whitford AC, Mann N, Buerger F, Schneider R, Deutsch K, Kitzler TM, Klämbt V, Kolb A, Mao Y, Moufawad El Achkar C, Mitrotti A, Martino J, Beck BB, Altmüller J, Benz MR, Yano S, Mikati MA, Gunduz T, Cope H, Shashi V, Trachtman H, Bodria M, Caridi G, Pisani I, Fiaccadori E, AbuMaziad AS, Martinez-Agosto JA, Yadin O, Zuckerman J, Kim A, John-Kroegel U, Tyndall AV, Parboosingh JS, Innes AM, Bierzynska A, Koziell AB, Muorah M, Saleem MA, Hoefele J, Riedhammer KM, Gharavi AG, Jobanputra V, Pierce-Hoffman E, Seaby EG, O’Donnell-Luria A, Rehm HL, Mane S, D’Agati VD, Pollak MR, Ghiggeri GM, Lifton RP, Goldstein DB, Davis EE, Hildebrandt F, Sanna-Cherchi S (2021) De novo TRIM8 variants impair its protein localization to nuclear bodies and cause developmental delay, epilepsy, and focal segmental glomerulosclerosis. Am J Hum Genet 108:357–367. https://doi.org/10.1016/j.ajhg.2021.01.008
Kawachi H, Fukusumi Y (2020) New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol 24:193–204. https://doi.org/10.1007/s10157-020-01854-3
Ashraf S, Kudo H, Rao J, Kikuchi A, Widmeier E, Lawson JA, Tan W, Hermle T, Warejko JK, Shril S, Airik M, Jobst-Schwan T, Lovric S, Braun DA, Gee HY, Schapiro D, Majmundar AJ, Sadowski CE, Pabst WL, Daga A, van der Ven AT, Schmidt JM, Low BC, Gupta AB, Tripathi BK, Wong J, Campbell K, Metcalfe K, Schanze D, Niihori T, Kaito H, Nozu K, Tsukaguchi H, Tanaka R, Hamahira K, Kobayashi Y, Takizawa T, Funayama R, Nakayama K, Aoki Y, Kumagai N, Iijima K, Fehrenbach H, Kari JA, El Desoky S, Jalalah S, Bogdanovic R, Stajić N, Zappel H, Rakhmetova A, Wassmer SR, Jungraithmayr T, Strehlau J, Kumar AS, Bagga A, Soliman NA, Mane SM, Kaufman L, Lowy DR, Jairajpuri MA, Lifton RP, Pei Y, Zenker M, Kure S, Hildebrandt F (2018) Mutations in six nephrosis genes delineate a pathogenic pathway amenable to treatment. Nat Commun 9:1960. https://doi.org/10.1038/s41467-018-04193-w
Tan W, Lovric S, Ashraf S, Rao J, Schapiro D, Airik M, Shril S, Gee HY, Baum M, Daouk G, Ferguson MA, Rodig N, Somers MJG, Stein DR, Vivante A, Warejko JK, Widmeier E, Hildebrandt F (2018) Analysis of 24 genes reveals a monogenic cause in 11.1% of cases with steroid-resistant nephrotic syndrome at a single center. Pediatr Nephrol 33:305–314. https://doi.org/10.1007/s00467-017-3801-6
Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F (2015) A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26:1279–1289. https://doi.org/10.1681/asn.2014050489
Sun H, Perez-Gill C, Schlöndorff JS, Subramanian B, Pollak MR (2021) Dysregulated dynein-mediated trafficking of nephrin causes INF2-related podocytopathy. J Am Soc Nephrol 32:307–322. https://doi.org/10.1681/asn.2020081109
Butt L, Unnersjö-Jess D, Höhne M, Hahnfeldt R, Reilly D, Rinschen MM, Plagmann I, Diefenhardt P, Brähler S, Brinkkötter PT, Brismar H, Blom H, Schermer B, Benzing T (2022) Super-resolution imaging of the filtration barrier suggests a role for podocin R229Q in Genetic predisposition to glomerular disease. J Am Soc Nephrol 33:138–154. https://doi.org/10.1681/asn.2020060858
Buvall L, Wallentin H, Sieber J, Andreeva S, Choi HY, Mundel P, Greka A (2017) Synaptopodin is a coincidence detector of tyrosine versus serine/threonine phosphorylation for the modulation of rho protein crosstalk in podocytes. J Am Soc Nephrol 28:837–851. https://doi.org/10.1681/asn.2016040414
Takano T, Bareke E, Takeda N, Aoudjit L, Baldwin C, Pisano P, Matsuda J, El Andalousi J, Muhtadie L, Bernard C, Majewski J, Miyazaki T, Yamamura KI, Gupta IR (2019) Recessive mutation in CD2AP causes focal segmental glomerulosclerosis in humans and mice. Kidney Int 95:57–61. https://doi.org/10.1016/j.kint.2018.08.014
Farmer LK, Rollason R, Whitcomb DJ, Ni L, Goodliff A, Lay AC, Birnbaumer L, Heesom KJ, Xu SZ, Saleem MA, Welsh GI (2019) TRPC6 binds to and activates calpain, independent of its channel activity, and regulates podocyte cytoskeleton, cell adhesion, and motility. J Am Soc Nephrol 30:1910–1924. https://doi.org/10.1681/asn.2018070729
Carrasco-Rando M, Prieto-Sánchez S, Culi J, Tutor AS, Ruiz-Gómez M (2019) A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms. J Cell Biol 218:2294–2308. https://doi.org/10.1083/jcb.201810171
Knust E, Dietrich U, Tepass U, Bremer KA, Weigel D, Vässin H, Campos-Ortega JA (1987) EGF homologous sequences encoded in the genome of Drosophila melanogaster, and their relation to neurogenic genes. EMBO J 6:761–766. https://doi.org/10.1002/j.1460-2075.1987.tb04818.x
Katoh M, Katoh M (2004) Identification and characterization of Crumbs homolog 2 gene at human chromosome 9q33.3. Int J Oncol 24:743–749
Paniagua AE, Segurado A, Dolón JF, Esteve-Rudd J, Velasco A, Williams DS, Lillo C (2021) Key role for CRB2 in the maintenance of apicobasal polarity in retinal pigment epithelial cells. Front Cell Dev Biol 9:701853. https://doi.org/10.3389/fcell.2021.701853
Thompson BJ, Pichaud F, Röper K (2013) Sticking together the Crumbs - an unexpected function for an old friend. Nat Rev Mol Cell Biol 14:307–314. https://doi.org/10.1038/nrm3568
Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A (2009) A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol 334:1–9. https://doi.org/10.1016/j.ydbio.2009.04.017
Ebarasi L, Ashraf S, Bierzynska A, Gee HY, McCarthy HJ, Lovric S, Sadowski CE, Pabst W, Vega-Warner V, Fang H, Koziell A, Simpson MA, Dursun I, Serdaroglu E, Levy S, Saleem MA, Hildebrandt F, Majumdar A (2015) Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am J Hum Genet 96:153–161. https://doi.org/10.1016/j.ajhg.2014.11.014
Slavotinek A, Kaylor J, Pierce H, Cahr M, DeWard SJ, Schneidman-Duhovny D, Alsadah A, Salem F, Schmajuk G, Mehta L (2015) CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am J Hum Genet 96:162–169. https://doi.org/10.1016/j.ajhg.2014.11.013
Lu J, Guo YN, Dong LQ (2021) Crumbs homolog 2 mutation in two siblings with steroid-resistant nephrotic syndrome: two case reports. World J Clin Cases 9:3056–3062. https://doi.org/10.12998/wjcc.v9.i13.3056
Möller-Kerutt A, Rodriguez-Gatica JE, Wacker K, Bhatia R, Siebrasse JP, Boon N, Van Marck V, Boor P, Kubitscheck U, Wijnholds J, Pavenstädt H, Weide T (2021) Crumbs2 is an essential slit diaphragm protein of the renal filtration barrier. J Am Soc Nephrol 32:1053–1070. https://doi.org/10.1681/asn.2020040501
Tanoue A, Katayama K, Ito Y, Joh K, Toda M, Yasuma T, D’Alessandro-Gabazza CN, Kawachi H, Yan K, Ito M, Gabazza EC, Tryggvason K, Dohi K (2021) Podocyte-specific Crb2 knockout mice develop focal segmental glomerulosclerosis. Sci Rep 11:20556. https://doi.org/10.1038/s41598-021-00159-z
Udagawa T, Jo T, Yanagihara T, Shimizu A, Mitsui J, Tsuji S, Morishita S, Onai R, Miura K, Kanda S, Kajiho Y, Tsurumi H, Oka A, Hattori M, Harita Y (2017) Altered expression of Crb2 in podocytes expands a variation of CRB2 mutations in steroid-resistant nephrotic syndrome. Pediatr Nephrol 32:801–809. https://doi.org/10.1007/s00467-016-3549-4
Chen H, Wang J, Zhang C, Ding P, Tian S, Chen J, Ji G, Wu T (2022) Sphingosine 1-phosphate receptor, a new therapeutic direction in different diseases. Biomed Pharmacother 153:113341. https://doi.org/10.1016/j.biopha.2022.113341
Wang H, Huang H, Ding SF (2018) Sphingosine-1-phosphate promotes the proliferation and attenuates apoptosis of Endothelial progenitor cells via S1PR1/S1PR3/PI3K/Akt pathway. Cell Biol Int 42:1492–1502. https://doi.org/10.1002/cbin.10991
Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108:689–701. https://doi.org/10.1172/jci12450
Lin L, Wang Q, Qian K, Cao Z, Xiao J, Wang X, Li X, Yu Z (2018) bFGF protects against oxygen glucose deprivation/reoxygenation-induced endothelial monolayer permeability via S1PR1-dependent mechanisms. Mol Neurobiol 55:3131–3142. https://doi.org/10.1007/s12035-017-0544-0
Li Q, Chen B, Zeng C, Fan A, Yuan Y, Guo X, Huang X, Huang Q (2015) Differential activation of receptors and signal pathways upon stimulation by different doses of sphingosine-1-phosphate in endothelial cells. Exp Physiol 100:95–107. https://doi.org/10.1113/expphysiol.2014.082149
Yanagida K, Liu CH, Faraco G, Galvani S, Smith HK, Burg N, Anrather J, Sanchez T, Iadecola C, Hla T (2017) Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc Natl Acad Sci U S A 114:4531–4536. https://doi.org/10.1073/pnas.1618659114
Yang T, Wang X, Zhou Y, Yu Q, Heng C, Yang H, Yuan Z, Miao Y, Chai Y, Wu Z, Sun L, Huang X, Liu B, Jiang Z, Zhang L (2021) SEW2871 attenuates ANIT-induced hepatotoxicity by protecting liver barrier function via sphingosine 1-phosphate receptor-1-mediated AMPK signaling pathway. Cell Biol Toxicol 37:595–609. https://doi.org/10.1007/s10565-020-09567-9
Yang T, Wang X, Yuan Z, Miao Y, Wu Z, Chai Y, Yu Q, Wang H, Sun L, Huang X, Zhang L, Jiang Z (2020) Sphingosine 1-phosphate receptor-1 specific agonist SEW2871 ameliorates ANIT-induced dysregulation of bile acid homeostasis in mice plasma and liver. Toxicol Lett 331:242–253. https://doi.org/10.1016/j.toxlet.2020.06.018
Dong J, Wang H, Zhao J, Sun J, Zhang T, Zuo L, Zhu W, Gong J, Li Y, Gu L, Li J (2015) SEW2871 protects from experimental colitis through reduced epithelial cell apoptosis and improved barrier function in interleukin-10 gene-deficient mice. Immunol Res 61:303–311. https://doi.org/10.1007/s12026-015-8625-5
Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, Okusa MD (2011) Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int 79:1090–1098. https://doi.org/10.1038/ki.2010.544
Su K, Zeng P, Liang W, Luo Z, Wang Y, Lv X, Han Q, Yan M, Chen C (2017) FTY720 Attenuates angiotensin II-Induced podocyte damage via inhibiting inflammatory cytokines. Mediators Inflamm 2017:3701385. https://doi.org/10.1155/2017/3701385
Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T (2001) Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell 8:693–704. https://doi.org/10.1016/s1097-2765(01)00324-0
Garris CS, Wu L, Acharya S, Arac A, Blaho VA, Huang Y, Moon BS, Axtell RC, Ho PP, Steinberg GK, Lewis DB, Sobel RA, Han DK, Steinman L, Snyder MP, Hla T, Han MH (2013) Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat Immunol 14:1166–1172. https://doi.org/10.1038/ni.2730
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Ni L, Saleem M, Mathieson PW (2012) Podocyte culture: tricks of the trade. Nephrology (Carlton) 17:525–531. https://doi.org/10.1111/j.1440-1797.2012.01619.x
Martin CE, Jones N (2018) Nephrin signaling in the podocyte: an updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol (Lausanne) 9:302. https://doi.org/10.3389/fendo.2018.00302
Shono A, Tsukaguchi H, Yaoita E, Nameta M, Kurihara H, Qin XS, Yamamoto T, Doi T (2007) Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephrol 18:2525–2533. https://doi.org/10.1681/asn.2006101084
D’Agati VD, Kaskel FJ, Falk RJ (2011) Focal segmental glomerulosclerosis. N Engl J Med 365:2398–2411. https://doi.org/10.1056/NEJMra1106556
Acknowledgements
We thank Dr. Yupeng Cun. from the Children’s Hospital of Chongqing Medical University for helpful discussions; the Children’s Hospital of Nanjing Medical University for expert assistance with the cell laboratory.
Author information
Authors and Affiliations
Contributions
Performing the experiments, formal analysis, and writing the manuscript: Qing Yang. Amending the manuscript and designing the experiments: Mo Wang. Collecting clinical data: Tang Dan, Xiaomei Song, and Chun Gan. Formal analysis and amending of the manuscript: Wei Jiang. Technically guiding the experiments: Aihua Zhang, Yaxi Chen, and Mi Bai.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Children’s Hospital of Chongqing Medical University. Information was only collected after written consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
467_2023_6087_MOESM2_ESM.pdf
Supplementary file2 (PDF 67 KB) Supplemental Figure 1 Schematic diagram of the workflow for screening pathogenic mutations
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Tang, D., Gan, C. et al. Novel variants in CRB2 targeting the malfunction of slit diaphragm related to focal segmental glomerulosclerosis. Pediatr Nephrol 39, 149–165 (2024). https://doi.org/10.1007/s00467-023-06087-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06087-6