Abstract
Genetic forms of focal and segmental glomerulosclerosis (FSGS) often have extra-renal manifestations. This study examined FSGS-associated genes from the Genomics England Renal proteinuria panel for reported and likely ocular features. Thirty-two of the 55 genes (58%) were associated with ocular abnormalities in human disease, and a further 12 (22%) were expressed in the retina or had an eye phenotype in mouse models. The commonest genes affected in congenital nephrotic syndrome (NPHS1, NPHS2, WT1, LAMB2, PAX2 but not PLCE1) may have ocular manifestations . Many genes affected in childhood–adolescent onset FSGS (NPHS1, NPHS2, WT1, LAMB2, SMARCAL1, NUP107 but not TRPC6 or PLCE1) have ocular features. The commonest genes affected in adult-onset FSGS (COL4A3–COL4A5, GLA ) have ocular abnormalities but not the other frequently affected genes (ACTN4, CD2AP, INF2, TRPC6). Common ocular associations of genetic FSGS include cataract, myopia, strabismus, ptosis and retinal atrophy. Mitochondrial forms of FSGS (MELAS, MIDD, Kearn’s Sayre disease) are associated with retinal atrophy and inherited retinal degeneration. Some genetic kidney diseases (CAKUT, ciliopathies, tubulopathies) that result in secondary forms of FSGS also have ocular features. Ocular manifestations suggest a genetic basis for FSGS, often help identify the affected gene, and prompt genetic testing. In general, ocular abnormalities require early evaluation by an ophthalmologist, and sometimes, monitoring or treatment to improve vision or prevent visual loss from complications. In addition, the patient should be examined for other syndromic features and first degree family members assessed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal and segmental glomerulosclerosis (FSGS) is a histopathological diagnosis characterised by sclerosis of less than half the glomerular tuft in fewer than half the glomeruli in a kidney biopsy [1].
FSGS is commonly classified into primary, secondary or genetic forms. Primary autoimmune FSGS typically presents with the nephrotic syndrome (NS), responds to immunosuppression (‘steroid-sensitive’ , SSNS) and recurs after transplantation [2, 3]. The genetic forms of FSGS frequently manifest with lower levels of proteinuria [4], and neither respond to steroid treatment (‘steroid-resistant’ nephrotic syndrome, SRNS) nor recur post transplantation [5]. Secondary FSGS results from hyperfiltration after nephron loss. Each disease type has implications for management and prognosis.
An accurate and timely diagnosis is critical in providing effective treatment, advising other family members of the risk of being affected and in avoiding the complications of steroid treatment. However, identification of individual genetic forms of FSGS is generally not possible with biopsy alone and requires genetic testing [6].
There are however clues to the likelihood of a genetic basis for FSGS [7]. These include a positive family history [8], a young age at onset [9], SRNS [10] and no other obvious cause [11]. The association with extra-renal features, such as a hearing loss, skeletal, cardiac or ocular anomalies, is also important [12].
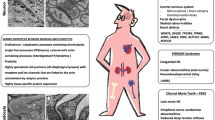
More than 70 genes have been implicated in FSGS, and inheritance is mainly autosomal recessive (AR) in children but often autosomal dominant (AD) in adults, as well as X-linked (XL) or mitochondrial [13]. The commonest genes associated with nephrotic syndrome differ at birth (NPHS1, NPHS2, WT1, LAMB2, PAX2, PLCE1), in childhood or adolescence (NPHS1, NPHS2, WT1, LAMB2, SMARCAL1, NUP107, TRPC6, PLCE1) and in adults (COL4A3-COL4A5, GLA, ACTN4, CD2AP, INF2, TRPC6).
Overall, the commonest genes affected in FSGS are COL4A5 (XL Alport syndrome) and COL4A3 and COL4A4 (usually AD rather than the rare AR Alport syndrome) INF2, TRPC6 and ACTN4 [8, 14, 15]. Most of these genes code for proteins that are found in the glomerular podocyte or adjacent extracellular matrix. There is also overlap with genes that result in other kidney phenotypes including some forms of congenital abnormalities of the kidney and urinary tract (CAKUT), cystic kidney diseases, renal ciliopathies and tubulopathies [13, 16]. These include Dent disease (CLCN5, OCRL), AD tubulointerstitial kidney disease (ADTKD due to UMOD variants), nephronophthisis (TTC21B, NPHP4), Imerslund-Grasbeck syndrome 1 (CUBN) and papillorenal syndrome (PAX2). Finally, some mitochondrial diseases (mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS) [17], maternally inherited diabetes and deafness (MIDD) [18], and Kearns-Sayre syndrome [19]) are associated with FSGS.
Some of these diseases are suspected on the basis of their extra-renal features. Ocular abnormalities are particularly common in genetic kidney disease and while these may not severely affect vision, they are helpful indicators of the genetic nature of the underlying disease [20, 21]. The association between kidney and eye disease is attributable to developmental, structural and physiological similarities between the kidney and the eye [22]. Both the kidney and the eye are ‘paired’ organs that share some transcription factors; and the glomerular filtration barrier resembles the retina [23] with epithelial cells overlying a basement membrane of mainly collagen IV α3α4α5 and a capillary endothelium [23]. Other similarities occur in the microcirculation with a localised renin–angiotensin system in both the glomerular and retinal vasculature [24].
The presence of ophthalmological abnormalities in a person with FSGS suggests a genetic basis and encourages genetic testing. It also facilitates early ophthalmological evaluation and monitoring to prevent vision loss. It may also provide insights into genetic kidney disease pathogenesis.
This review characterises the ocular associations of the individual genes affected in FSGS that may be useful in indicating an underlying genetic disorder and, in some cases, the specific gene affected.
Methods
The genes for FSGS from the Renal Proteinuria panel were down-loaded from the Genomics England Panel App in October 2020 (v2.77, green and amber genes).
Genomics England uses a traffic light system where ‘green’ genes have a high level of evidence for an association with a disease (having been reported in 3 unrelated families or 2 families with further strong evidence) as decided by an expert panel. These represent the genes that should be examined in a diagnostic genetic laboratory. ‘Amber’ genes have borderline levels of evidence and 'red' genes have low levels for a disease association.
Genes associated with FSGS were then searched in Medline (OVID), Embase and the Cochrane Database of Systematic Reviews with the terms (eye* or ocular or retina* or lens or cornea* or vision or ophthalm*) and ‘gene name’ to identify all reports of ocular manifestations. All manuscripts in English likely to include ocular manifestations of FSGS based on their abstracts were read. Full-text articles that did not report FSGS or ocular findings, and those where only the abstract was available or were a conference proceeding were excluded. Additional references from studies were hand- searched. In addition, Online Mendelian Inheritance in Man (OMIM: https://www.omim.org/) was used to identify renal, extra-renal and further ocular features in October 2022.
Since some genes were reported in only a few individuals who had not necessarily undergone a complete ophthalmological examination, two further databases were studied to determine whether ophthalmic features were likely. These were the Human Protein Atlas (https://www.proteinatlas.org/) which was examined for mRNA expression in the retina and the Mouse Genome Informatics database (http://www.informatics.jax.org/) which was examined for an ocular phenotype in mouse models. Searches were undertaken August–September 2020 and reviewed October 2022.
In addition to the Renal Proteinuria panel, the genes associated with secondary FSGS (CLCN5, OCRL, CUBN, PAX2) and the common FSGS-associated mitochondrial diseases (MELAS, MIDD, Kearns Sayre disease) were searched.
Results
In all, 4702 records were identified from the databases and a further 179 from hand searching. After duplicates were removed, 3417 abstracts were screened to yield 774 full texts, and after review, 303 records were examined (Fig. 1).
Fifty-five genes from the Genomics England Renal proteinuria panel were studied. Thirty-two (58%) had ocular manifestations reported in human disease (Tables 1, 2 and 3; Figs. 2, 3 and 4).
Ocular features associated with genetic causes of FSGS demonstrating a congenital cataract; b inferior coloboma; c microphthalmia with inferior coloboma in partner eye of (b); d retinal profile on optical coherence tomography in Pierson syndrome presenting in adulthood; e retinal profile in X-linked Alport syndrome demonstrating the resemblance; f cherry red spot macula typical of many metabolic diseases including Fabry disease; and g optical coherence tomography profile of the retina with accumulation at the level of the external limiting membrane
Ocular features associated with XL or AR Alport syndrome demonstrating a perimacular fleck retinopathy; b subtle perimacular fleck retinopathy that must be distinguished from the normal retinal sheen seen in young people; c black and white image of (b) demonstrating the fleck retinopathy more clearly; d black and white image demonstrating the margins of a large macular hole; e maculopathy; f typical profile on optical coherence tomography demonstrating the > 10% temporal retinal atrophy; and g retinal profile on optical coherence tomography demonstrating a lamellar macular hole
Ocular features associated with FSGS due to mitochondrial diseases demonstrating a MELAS with focal atrophy in the right eye and generalised perifoveal thinning; b red-free photograph with more extensive areas of foveal atrophy; c optical coherence tomography scan with areas of atrophy confirming the foveal defects in the outer retina and retinal pigment epithelium; d MIDD demonstrating normal fovea surrounded by extensive atrophy; and e optical coherence tomography demonstrating parafoveal atrophy of the outer retinal layer
Most of the genes commonly associated with congenital nephrotic syndrome have reported ocular manifestations (NPHS1, NPHS2, WT1, LAMB2, PAX2, PLCE1). Many genes associated with childhood–adolescent FSGS also have ocular abnormalities (NPHS1, NPHS2, WT1, LAMB2, SMARCAL1, NUP107, PLCE1 but not TRPC6). The commonest genes associated with adult-onset FSGS (COL4A3, COL4A4, COL4A5) have ocular abnormalities as does GLA (Fabry disease), but not ACTN4, CD2AP, INF2 or TRPC6. LMX1B and MYH9 are infrequent causes of FSGS but ocular features are common.
Mitochondrial diseases that result in FSGS (MELAS, MIDD, Kearn’s Sayre disease) typically result in inherited retinal degeneration and retinal atrophy (Table 2, Fig. 4).
The Renal proteinuria gene list corresponded to some genetic kidney diseases (CAKUT, tubulopathies, ciliopathies) that result in secondary FSGS such as Dent disease (CLCN5, OCRL), nephronophthisis (TTC21B, NPHP4), ADTKD-UMOD and Imerslund-Grasbeck syndrome 1 (CUBN). Of these, Dent disease and nephronophthisis both have ocular features.
Of the 55 genes studied, 51 (93%) had transcripts expressed in the retina, but only 16 (29%) had more than 10 transcripts per million, expression was not examined in other parts of the eye and protein levels were not quantitated. Twenty-seven of these genes (49%) were associated with an ocular phenotype in a mouse model. This meant that a further 12 genes (22%) had > 10 transcripts per million in the retina or an ocular phenotype in a mouse model, suggesting that an uncommon or milder ocular phenotype, even if not recognised to date, might still be found in human disease.
Common ocular abnormalities in genetic forms of FSGS
The ocular manifestations associated with the largest number of FSGS-causing genes in this search were ptosis, myopia, strabismus, cataract, retinal atrophy and inherited retinal degeneration (Table 3).
Some ocular features were found only associated with one gene and were common and highly specific . These included Pierson syndrome (LAMB2 variants resulting in microphthalmia); MELAS and MIDD ( mitochondrial variants with inherited retinal degeneration); papillorenal syndrome (PAX2, with abnormal disc vasculature); and the Wilm’s tumour, Aniridia, genitourinary anomalies and impaired intellectual development (WAGR) syndrome (, WT1, with aniridia); Alport syndrome (COL4A3, COL4A4, COL4A5, with lenticonus and fleck retinopathies, temporal retinal thinning, maculopathy and macular hole); and Fabry disease (GLA, with corneal verticillata, and tortuous vessels) (Figs. 2, 3 and 4).
Inherited retinal degeneration and retinal atrophy occurred with pathogenic variants in LAMB2 and with mitochondrial variants (COQ2, COQ8B, MELAS, MIDD, Kearns–Sayre disease) (Fig. 4). Inherited retinal degeneration is a heterogenous group of diseases characterised by premature loss of photoreceptors (rods, cones, or both ) or the underlying choroid. Clinical features include night blindness, loss of peripheral vision and subsequent loss of central vision. In general, there is no effective treatment but gene therapy has been recently approved for some of these diseases [25].
Pierson syndrome (LAMB2)
LAMB2 mutations cause both the more severe disease, Pierson syndrome, as well as nephrotic syndrome type 5. Pierson syndrome presents with congenital nephrotic syndrome that progresses to kidney failure. It is associated with neurodevelopmental delay, and kidney biopsies typically demonstrate diffuse mesangial sclerosis. Individuals with FSGS due to LAMB2 missense variants have a milder phenotype with a slower progression to kidney failure, no neurodevelopmental abnormalities and fewer and milder ocular features [26, 27].
Anophthalmia and microphthalmia are characteristic of Pierson syndrome [27, 28]. Microcoria may be present from birth and characterised by fixed pupils unresponsive to mydriatics [29]. This is likely attributable to iris stroma hypoplasia which appears as a flat, featureless, transilluminable iris, as well as uveal ectropion [27, 29]. Management may require pupilloplasty to improve vision [30].
Other abnormalities also affect vision. Shallow anterior chambers predispose to glaucoma from angle closure or anterior segment dysgenesis [29]. Retinal detachment, scarring and subretinal fibrosis may result in visual loss [31,32,33].
Nephrotic syndrome type 5 or FSGS resulting from milder LAMB2 variants and presenting in later childhood or adolescence is associated with more subtle ocular abnormalities often with no visual consequences.
Papillorenal syndrome (PAX2)
Pathogenic variants in PAX2 commonly result in the papillorenal syndrome which more typically manifests as CAKUT with ocular anomalies. However, certain PAX2 variants are also associated with SRNS, and adult-onset FSGS rather than CAKUT.
Affected individuals may have unilateral or bilateral optic disc anomalies that vary from an optic disc pit to a chorioretinal coloboma [34,35,36,37,38]. The characteristic feature is the emergence of the retinal vessels from the periphery rather than the centre of the optic disc. Reduced visual acuity correlates with the degree of foveal involvement. The anomaly may vary in each eye and in different affected family members. The visual consequences also vary. Refractive error is common [39]. Complications include perifoveal splitting, optic nerve atrophy and bilateral glaucomatous cupping [34, 35]. There is no treatment.
WAGR (WT1)
WT1 mutations result in FSGS (nephrotic syndrome type 4), as well as the WAGR, Denys–Drash and Frasier syndromes. These are very rare diseases that present with proteinuria and nephrotic syndrome in the first years of life, as well as extra-renal features.
Aniridia also occurs in the WAGR syndrome that is associated with hemizygosity for the PAX6 gene and deletions of 11p13 including WT1 [40]. Affected individuals have photophobia and reduced visual acuity [41]. Diagnosis of the syndrome is important because of the risk of Wilm’s tumour.
Alport syndrome (COL4A3, COL4A4, COL4A5)
Pathogenic variants in COL4A3, COL4A5 and COL4A5 result in XL (COL4A5) and AR Alport syndrome (where there are two COL4A3 or COL4A4 variants) which are characterised by kidney failure, sensorineural hearing loss and lenticonus, recurrent corneal erosions and a fleck retinopathy, retinal thinning and macular holes [42]. Proteinuria and FSGS are present in the most boys with XL Alport syndrome and boys and girls with AR disease [43]. AD Alport syndrome with heterozygous COL4A3 or COL4A4 variants typically results in haematuria, and sometimes FSGS but without a hearing loss or ocular abnormalities [43, 44].
Corneal erosions occur unilaterally or bilaterally, causing ocular pain or irritation, lacrimation and photophobia [45]. Posterior polymorphous dystrophy is very rare and demonstrated with slit lamp examination [46]. Lesions typically occur on the posterior corneal surface as clear vesicles surrounded by a thickened Descemet’s membrane [46, 47].
Anterior lenticonus is pathognomonic for Alport syndrome and identified on slit lamp examination since the anterior axial projection of the central lens produces an ‘oil droplet’ appearance of the red reflex [48, 49]. Sometimes, a cataract forms after rupture of the lens capsule [50]. Affected individuals have difficulty focusing and reduced visual acuity [51]. Treatment is lens extraction and replacement [52]. Lenticonus does not recur and posterior lenticonus is less common [53].
A fleck retinopathy is the commonest ocular finding in males and females with XL or AR Alport syndrome [54]. The central retinopathy is characterised by multiple white flecks that spare the fovea and is evident on ophthalmoscopy and retinal imaging [55]. The macular reflex may be absent [49]. A peripheral retinopathy appears as larger coalescing lesions that spare the retinal vessels [49]. Visual acuity is not affected, and no treatment is required [21].
Temporal retinal thinning (> 10% of average nasal thickness) is typical on optical coherence tomography in males with XL and in males and females with AR Alport syndrome. Sometimes multiple lamellar holes coalesce to form a ‘giant’ macular hole with loss of central vision [56, 57]. Surgical repair is generally difficult [58] and the patient is typically left with a permanent visual loss [21].
Boys with XL Alport syndrome may have no ocular manifestations, but those who develop kidney failure at a young age will often have a central and peripheral retinopathy, and both lenticonus and temporal retinal thinning have been reported in childhood. Girls with XL Alport syndrome usually have no ocular manifestations. Boys and girls with AR Alport syndrome may have the retinopathy and temporal retinal thinning.
Fabry disease (GLA)
Fabry disease is an XL disorder caused by pathogenic variants in GLA which encodes α-galactosidase. The subsequent accumulation of glycosphingolipids leads to end-organ damage. FSGS manifests as proteinuria but kidney failure usually occurs in adulthood. Extrarenal features include acroparaesthesiae, hypohydrosis and abdominal pain from childhood, angiokeratomas, cardiac hypertrophy and cerebrovascular disease. Uncommonly, FSGS occurs without extrarenal features.
Ocular abnormalities are more common in hemizygous males than heterozygous females [59, 60]. Lacrimal secretions are sometimes reduced [61] and potentially contribute to complaints of sore dry eyes [62]. Bilateral conjunctival and retinal vessel tortuosities occur in nearly all affected males and many females [60]. Corkscrew arterioles with irregular dilatations, constrictions and microaneurysms are seen, especially in the inferior conjunctivae. The presence of conjunctival tortuosity correlates with increased disease severity, as measured by the Fabry Outcome Survey-Mainz Severity Score Index (FOS-MSSI), and affected individuals have a more rapid decline in kidney function and increase in cardiac size with age [63]. Tortuous vessels also occur on the upper eyelids [64]. Enzyme replacement therapy may prevent progression of conjunctival and upper lid tortuosity [65] and slow the development of kidney and cardiac disease.
Corneal verticillata are subtle, fine, subepithelial streaks running from the centre of the cornea in whorls to the periphery [66,67,68]. They develop early in life, are usually located inferiorly and vary from creamy white to golden brown [68]. A brownish, grey or white subepithelial corneal haze may be seen [61]. These corneal changes progress and become more evident with time [69]. Visual acuity is preserved but there may be reduced night vision and increased glare [62, 70]. These features are highly specific for Fabry disease after pharmacological causes such as amiodarone, chloroquine [71], and chlorpromazine use [68] have been excluded [72]. The verticillata do not correlate with disease progression [63] and may regress with enzyme replacement therapy [73, 74].
A granular anterior capsular lens opacity frequently occurs bilaterally in the inferior quadrants [68, 75]. These are usually wedge-shaped, with bases towards the equator and apices towards the centre of the anterior capsule [68]. Posterior lens anomalies are less common, but a cataract with thin, branching, spoke-like opacities radiating from the centre of the posterior capsule is pathognomonic for Fabry disease [67] and may be the only ocular manifestation [68]. Cataracts continue to develop despite enzyme replacement therapy [65].
Retinal vascular changes are also more frequent in hemizygotes [68]. Arterioles appear narrowed with arteriovenous nicking at the peripheries; and capillary micro- and macro-aneurysms occur throughout the retina [75]. Corkscrew tortuosity, especially of the veins, is also seen at the posterior pole [67]. These changes continue to worsen despite enzyme treatment [65].
Further serious ocular complications include central retinal artery occlusion [68, 76, 77], anterior ischemic optic neuropathy [78, 79] and central retinal vein occlusion [80].
Nail-patella syndrome (LMX1B)
Pathogenic variants in LMX1B result in the Nail-patella syndrome which is associated with haematuria, and sometimes kidney failure as well as cataract, hearing loss, limb and pelvic skeletal abnormalities [81, 82]. Interestingly, however, many individuals with Nail-patella syndrome have only kidney manifestations [83,84,85,86], and, in particular, no ocular features [87].
Ocular abnormalities in mitochondrial causes of FSGS
MELAS and MIDD
These diseases result from various pathogenic variants in mitochondrial DNA and are not detected with whole exome sequencing (Table 3).
The m.3242A > G variant is responsible for many individuals with MELAS [88]. The characteristic features have an onset prior to age 40, typically with stroke, seizures, lactic acidosis and ragged red fibres on muscle biopsy [89]. FSGS presents in adulthood and varies from non-nephrotic range proteinuria [17, 90] to SRNS progressing to kidney failure requiring haemodialysis or transplantation [91, 92]. The most common ocular manifestation is a progressive, bilateral macular dystrophy at the level of the retinal pigment epithelium [93, 94]. Lesions first become obvious in adulthood [91, 95] and are graded on ophthalmoscopy and fundus autofluorescence findings. On fundoscopy, mild pigmentary abnormalities initially occur in the central fundus. With disease progression, isolated or multifocal whitish-yellow or hyperpigmented subretinal deposits are seen at the posterior pole [95]. Discontinuous areas of pronounced chorioretinal atrophy develop circumferentially around the fovea and coalesce over time [95,96,97]. Finally, the central fovea is also affected by extensive chorioretinal atrophy [95]. Visual features include blind spots, impaired night vision and photophobia [94]. Vision deteriorates over time but is relatively preserved if the fovea is spared [95]. Proteinuria precedes the macular dystrophy by years [91].
The bilateral vitelliform macular lesions may develop areas of retinal pigment atrophy over a decade [95, 98]. There is no specific treatment for mitochondrial disease but exercise, reduced alcohol intake, smoking cessation and vitamin supplementation are often used although without much evidence for efficacy [99, 100].
MIDD is a mitochondrial disorder characterised by hearing loss and type 2 diabetes in adulthood. There may be additional features such as cardiomyopathy, myopathy, FSGS or other kidney disorders and neuropsychiatric features [101]. MIDD accounts for up to 1% of all new cases of diabetes [102]. It results from a pathogenic variant that impairs oxidative phosphorylation and ATP production.
The ocular features include ptosis and inherited retinal degeneration which occur in almost all the patients [103].
Kearns–Sayre syndrome
This is another mitochondrial disease with variable features including FSGS or renal tubulopathy, short stature, microcephaly, myopathy, cardiomyopathy, cardiac conduction defect, hearing loss and cerebellar ataxia. The typical ocular abnormalities include progressive external ophthalmoplegia, ptosis and inherited retinal degeneration.
Discussion
Thirty-two of the 55 genes from the Genomics England Renal proteinuria panel were associated with an ocular phenotype in human disease. A further 12 genes were expressed in the retina or the corresponding mouse models had ocular features, suggesting that additional ocular manifestations might still be identified. Thus, at least 44 of the 55 genes (80%) currently recognised to be affected in genetic forms of FSGS may have ocular abnormalities.
The commonest reported ocular associations of genetic FSGS genes are cataracts, myopia, strabismus, ptosis, retinal atrophy and inherited retinal degeneration, but these features are often found infrequently. In contrast, some ocular abnormalities are associated with only one gene, but the diseases themselves are relatively common causes of genetic FSGS. These abnormalities include the optic disc anomalies with papillorenal syndrome, the fleck retinopathy in Alport syndrome and the corneal verticillata and vascular tortuosity with Fabry disease. In the mitochondrial diseases, inherited retinal dystrophy and retinal atrophy are often present but the kidney manifestations including FSGS, tubulopathies and cysts are more variable.
In general, it is not possible to deduce how often ocular abnormalities occur in individual cases of genetic forms of FSGS. The likelihood of ocular abnormalities depends on the age of the individual, the gene affected and the variant type, that is, whether it is a nonsense or missense change. The ocular features a may be different in other affected family members. In addition, the demonstration of an ocular abnormality will depend on a thorough ophthalmic examination. Mild or early manifestations may be obvious only with a formal ophthalmic review or investigations such as optical coherence tomography (OCT) or peripheral retinal imaging.
Structural ocular abnormalities such as coloboma, optic disc anomaly or microphthalmia are typically present from birth, and treatment is not usually possible. Strabismus becomes obvious early. The ocular features of Fabry disease may not be present at the time of kidney disease diagnosis, but develop over time, and treatment slows progression [65, 69]. With pathogenic variants in the mitochondrial genome, the kidney disease often precedes the onset of atrophy or inherited retinal degeneration [91].
Many of the genetic kidney diseases previously considered to be paediatric are now also diagnosed for the first time in adults but the ocular manifestations may be less pronounced than those seen in disease with a childhood onset. Thus, Pierson syndrome may manifest with anophthalmia or microphthalmia in neonates but with renal-limited FSGS in adolescents or adults. Nevertheless, it is important for paediatric nephrologists to understand how ocular manifestations in genetic kidney disease differ with increasing age because they may be able to make the diagnosis in an older family member.
The presence of ocular abnormalities may also indicate more severe kidney disease. In Pierson syndrome, severe ocular phenotypes are associated with earlier onset kidney failure [26,27,28]. In Alport syndrome, the more severe genetic variants are associated more often with lenticonus, more severe central retinopathy and temporal atrophy, and earlier onset kidney failure [21, 104, 105]. In Fabry disease, the presence of retinal and conjunctival vessel tortuosity correlates with a more rapid decline in kidney and cardiac function [63].
Ocular phenotypes are still not described for some of the FSGS-associated genes, even where there is retinal expression or a mouse ocular phenotype. However, many of these diseases are very rare, patients may not have undergone formal ophthalmological review; the reporting laboratory may not have had access to the clinical examination findings; the association with the ocular abnormality may not have been recognised; and sometimes, conversely, the reported ocular features are coincidental.
This study has tested for ocular abnormalities as indicators of extrarenal disease that suggest a genetic basis for FSGS, but other organ systems, such as the hearing, heart, skeleton and brain, are commonly affected too. These abnormalities may be more obvious than the ocular features. Nevertheless, a basic ophthalmic examination is inexpensive and non-invasive, and some ocular abnormalities affect vision and must be treated or monitored for complications.
Some ocular features such as coloboma and optic atrophy are obvious to the renal physician and their association with FSGS suggests a genetic cause. In such cases, it is worthwhile seeking a family history of kidney disease and undertaking genetic testing. An early assessment by an interested ophthalmologist where ocular involvement is suspected is important. Even where the abnormality is present at birth and does not typically progress, complications such as strabismus, cataract, glaucoma and retinal detachment can occur. The ophthalmologist is best placed to assess how often the ophthalmic features should be monitored and any treatment required, such as surgery for strabismus or retinal detachment. The patient must be assessed for other syndromic features and first degree relatives also examined.
The strengths of this study were the examination of the Genomics England Renal proteinuria panel which is widely used in testing for the genetic cause of FSGS, and the systematic approach to identifying ocular abnormalities from OMIM and the literature as well as examining retinal expression and the effect in mouse models. Genes associated with FSGS will continue to be identified, and some of these will have ocular features. However, the aim of this study was not to identify all the FSGS genes with an ocular association, but rather to determine whether ocular features were common enough to identify a genetic cause, and the gene itself, whether they indicated more severe disease and whether they affected vision.
The study’s major limitations were that the Renal proteinuria panel did not include some genes that might be considered pathogenic. In addition,some reports of genetic FSGS were rare; the ophthalmic examinations often absent or incomplete; and sometimes a gene was not only associated with FSGS but also with CAKUT, a tubulopathy or cystic kidney disease [8, 106]. Finally, there was little data on how often the ocular manifestations were present in affected children and adults and the age by which features had developed if not apparent at birth.
In conclusion, ocular abnormalities are common in genetic forms of FSGS, suggest their genetic nature and often a specific diagnosis, and may predict renal disease severity. Importantly, some genetic forms of FSGS are associated with ocular features that must be monitored and treated to avoid complications and to maintain vision.
Data Availability
All data used in this study is available in the manuscript or the Supplementary file.
References
Fogo AB (2015) Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 11:76–87
Rydel JJ, Korbet SM, Borok RZ, Schwartz MM (1995) Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am J Kidney Dis 25:534–542
Chun MJ, Korbet SM, Schwartz MM, Lewis EJ (2004) Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol 15:2169–2177
Praga M, Morales E, Herrero JC, Pérez Campos A, Domínguez-Gil B, Alegre R, Vara J, Martínez MA (1999) Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis 33:52–58
De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC (2018) Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 29:759–774
Ahn W, Bomback AS (2020) Approach to diagnosis and management of primary glomerular diseases due to podocytopathies in adults: core curriculum 2020. Am J Kidney Dis 75:955–964
Savige J, Rana K, Tonna S, Buzza M, Dagher H, Wang YY (2003) Thin basement membrane nephropathy. Kidney Int 64:1169–1178
Gast C, Pengelly RJ, Lyon M, Bunyan DJ, Seaby EG, Graham N, Venkat-Raman G, Ennis S (2016) Collagen (COL4A) mutations are the most frequent mutations underlying adult focal segmental glomerulosclerosis. Nephrol Dial Transplant 31:961–970
Bierzynska A, McCarthy HJ, Soderquest K, Sen ES, Colby E, Ding WY, Nabhan MM, Kerecuk L, Hegde S, Hughes D, Marks S, Feather S, Jones C, Webb NJ, Ognjanovic M, Christian M, Gilbert RD, Sinha MD, Lord GM, Simpson M, Koziell AB, Welsh GI, Saleem MA (2017) Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int 91:937–947
Gribouval O, Boyer O, Hummel A, Dantal J, Martinez F, Sberro-Soussan R, Etienne I, Chauveau D, Delahousse M, Lionet A, Allard J, Pouteil Noble C, Tete MJ, Heidet L, Antignac C, Servais A (2018) Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int 94:1013–1022
Braunisch MC, Riedhammer KM, Herr PM, Draut S, Gunthner R, Wagner M, Weidenbusch M, Lungu A, Alhaddad B, Renders L, Strom TM, Heemann U, Meitinger T, Schmaderer C, Hoefele J (2021) Identification of disease-causing variants by comprehensive genetic testing with exome sequencing in adults with suspicion of hereditary FSGS. Eur J Hum Genet 29:262–270
Connaughton DM, Kennedy C, Shril S, Mann N, Murray SL, Williams PA, Conlon E, Nakayama M, van der Ven AT, Ityel H, Kause F, Kolvenbach CM, Dai R, Vivante A, Braun DA, Schneider R, Kitzler TM, Moloney B, Moran CP, Smyth JS, Kennedy A, Benson K, Stapleton C, Denton M, Magee C, O’Seaghdha CM, Plant WD, Griffin MD, Awan A, Sweeney C, Mane SM, Lifton RP, Griffin B, Leavey S, Casserly L, de Freitas DG, Holian J, Dorman A, Doyle B, Lavin PJ, Little MA, Conlon PJ, Hildebrandt F (2019) Monogenic causes of chronic kidney disease in adults. Kidney Int 95:914–928
Knoers N, Antignac C, Bergmann C, Dahan K, Giglio S, Heidet L, Lipska-Zietkiewicz BS, Noris M, Remuzzi G, Vargas-Poussou R, Schaefer F (2022) Genetic testing in the diagnosis of chronic kidney disease: recommendations for clinical practice. Nephrol Dial Transplant 37:239–254
Yao T, Udwan K, John R, Rana A, Haghighi A, Xu L, Hack S, Reich HN, Hladunewich MA, Cattran DC, Paterson AD, Pei Y, Barua M (2019) Integration of genetic testing and pathology for the diagnosis of adults with FSGS. Clin J Am Soc Nephrol 14:213–223
Rood IM, Deegens JKJ, Wetzels JFM (2012) Genetic causes of focal segmental glomerulosclerosis: implications for clinical practice. Nephrol Dial Transplant 27:882–890
Sambharia M, Rastogi P, Thomas CP (2022) Monogenic focal segmental glomerulosclerosis: a conceptual framework for identification and management of a heterogeneous disease. Am J Med Genet C Semin Med Genet 190:377–398
Hotta O, Inoue CN, Miyabayashi S, Furuta T, Takeuchi A, Taguma Y (2001) Clinical and pathologic features of focal segmental glomerulosclerosis with mitochondrial tRNALeu(UUR) gene mutation. Kidney Int 59:1236–1243
Cao XY, Wei RB, Wang YD, Zhang XG, Tang L, Chen XM (2013) Focal segmental glomerulosclerosis associated with maternally inherited diabetes and deafness: clinical pathological analysis. Indian J Pathol Microbiol 56:272–275
Narumi K, Mishima E, Akiyama Y, Matsuhashi T, Nakamichi T, Kisu K, Nishiyama S, Ikenouchi H, Kikuchi A, Izumi R, Miyazaki M, Abe T, Sato H, Ito S (2018) Focal segmental glomerulosclerosis associated with chronic progressive external ophthalmoplegia and mitochondrial DNA A3243G Mutation. Nephron 138:243–248
Savige J, Ratnaike S, Colville D (2011) Retinal abnormalities characteristic of inherited renal disease. J Am Soc Nephrol 22:1403–1415
Savige J, Sheth S, Leys A, Nicholson A, Mack HG, Colville D (2015) Ocular features in Alport syndrome: pathogenesis and clinical significance. Clin J Am Soc Nephrol 10:703–709
Farrah TE, Dhillon B, Keane PA, Webb DJ, Dhaun N (2020) The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int 98:323–342
Booij JC, Baas DC, Beisekeeva J, Gorgels TGMF, Bergen AAB (2010) The dynamic nature of Bruch’s membrane. Prog Retin Eye Res 29:1–18
Wilkinson-Berka JL, Agrotis A, Deliyanti D (2012) The retinal renin–angiotensin system: roles of angiotensin II and aldosterone. Peptides 36:142–150
Nash BM, Wright DC, Grigg JR, Bennetts B, Jamieson RV (2015) Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl Pediatr 4:139–163
Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nürnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Bröking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nürnberg P, Zenker M, Hildebrandt F (2006) Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70:1008–1012
Wuhl E, Kogan J, Zurowska A, Matejas V, Vandevoorde RG, Aigner T, Wendler O, Lesniewska I, Bouvier R, Reis A, Weis J, Cochat P, Zenker M (2007) Neurodevelopmental deficits in Pierson (microcoria-congenital nephrosis) syndrome. Am J Med Genet Part A 143:311–319
Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, Dötsch J, Reis A, Müntefering H, Neumann LM (2004) Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet Part A 130A:138–145
Bredrup C, Matejas V, Barrow M, Blahova K, Bockenhauer D, Fowler DJ, Gregson RM, Maruniak-Chudek I, Medeira A, Mendonca EL, Kagan M, Koenig J, Krastel H, Kroes HY, Saggar A, Sawyer T, Schittkowski M, Swietlinski J, Thompson D, VanDeVoorde RG, Wittebol-Post D, Woodruff G, Zurowska A, Hennekam RC, Zenker M, Russell-Eggitt I (2008) Ophthalmological aspects of Pierson syndrome. Am J Ophthalmol 146:602–611
Arima M, Tsukamoto S, Akiyama R, Nishiyama K, Kohno RI, Tachibana T, Hayashida A, Murayama M, Hisatomi T, Nozu K, Iijima K, Ohga S, Sonoda KH (2018) Ocular findings in a case of Pierson syndrome with a novel mutation in laminin β2 gene. J AAPOS 22:401-403.e401
Matejas V, Al-Gazali L, Amirlak I, Zenker M (2006) A syndrome comprising childhood-onset glomerular kidney disease and ocular abnormalities with progressive loss of vision is caused by mutated LAMB2. Nephrol Dial Transplant 21:3283–3286
Mohney BG, Pulido JS, Lindor NM, Hogan MC, Consugar MB, Peters J, Pankratz VS, Nasr SH, Smith SJ, Gloor J, Kubly V, Spencer D, Nielson R, Puffenberger EG, Strauss KA, Morton DH, Eldahdah L, Harris PC (2011) A novel mutation of LAMB2 in a multigenerational mennonite family reveals a new phenotypic variant of Pierson syndrome. Ophthalmology 118:1137–1144
Lehnhardt A, Lama A, Amann K, Matejas V, Zenker M, Kemper MJ (2012) Pierson syndrome in an adolescent girl with nephrotic range proteinuria but a normal GFR. Pediatr Nephrol 27:865–868
Iwafuchi Y, Morioka T, Morita T, Yanagihara T, Oyama Y, Morisada N, Iijima K, Narita I (2016) Diverse renal phenotypes observed in a single family with a genetic mutation in paired box protein 2. Case Rep Nephrol Dial 6:61–69
Rachwani Anil R, Rocha-de-Lossada C, Ayala CH, Contreras ME (2019) A new mutation in the PAX2 gene in a Papillorenal Syndrome patient. Am J Ophthalmol Case Rep 16:100563
Vivante A, Chacham OS, Shril S, Schreiber R, Mane SM, Pode-Shakked B, Soliman NA, Koneth I, Schiffer M, Anikster Y, Hildebrandt F (2019) Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol 34:1607–1613
Rossanti R, Morisada N, Nozu K, Kamei K, Horinouchi T, Yamamura T, Minamikawa S, Fujimura J, Nagano C, Sakakibara N, Ninchoji T, Kaito H, Ito S, Tanaka R, Iijima K (2020) Clinical and genetic variability of PAX2-related disorder in the Japanese population. J Hum Genet 65:541–549
Saida K, Kamei K, Morisada N, Ogura M, Ogata K, Matsuoka K, Nozu K, Iijima K, Ito S (2020) A novel truncating PAX2 mutation in a boy with renal coloboma syndrome with focal segmental glomerulosclerosis causing rapid progression to end-stage kidney disease. CEN Case Rep 9:19–23
Olsen TW, Summers CG, Knobloch WH (1996) Predicting visual acuity in children with colobomas involving the optic nerve. J Pediatr Ophthalmol Strabismus 33:47–51
Bremond-Gignac D, Crolla JA, Copin H, Guichet A, Bonneau D, Taine L, Lacombe D, Baumann C, Benzacken B, Verloes A (2005) Combination of WAGR and Potocki-Shaffer contiguous deletion syndromes in a patient with an 11p11.2-p14 deletion. Eur J Hum Genet 13:409–413
Lee H, Khan R, O’Keefe M (2008) Aniridia: current pathology and management. Acta Ophthalmol 86:708–715
Alport AC (1927) Hereditary familial congenital haemorrhagic nephritis. Br Med J 1:504–506
Malone AF, Phelan PJ, Hall G, Cetincelik U, Homstad A, Alonso AS, Jiang R, Lindsey TB, Wu G, Sparks MA, Smith SR, Webb NJ, Kalra PA, Adeyemo AA, Shaw AS, Conlon PJ, Jennette JC, Howell DN, Winn MP, Gbadegesin RA (2014) Rare hereditary COL4A3/COL4A4 variants may be mistaken for familial focal segmental glomerulosclerosis. Kidney Int 86:1253–1259
Pierides A, Voskarides K, Athanasiou Y, Ioannou K, Damianou L, Arsali M, Zavros M, Pierides M, Vargemezis V, Patsias C, Zouvani I, Elia A, Kyriacou K, Deltas C (2009) Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant 24:2721–2729
Burke JP, Talbot JF, Clearkin LG (1991) Recurrent corneal epithelial erosions in Alport’s syndrome. Acta Ophthalmol 69:555–557
Teekhasaenee C, Nimmanit S, Wutthiphan S, Vareesangthip K, Laohapand T, Malasitr P, Ritch R (1991) Posterior polymorphous dystrophy and Alport syndrome. Ophthalmology 98:1207–1215
Bower KS, Edwards JD, Wagner ME, Ward TP, Hidayat A (2009) Novel corneal phenotype in a patient with alport syndrome. Cornea 28:599–606
Mettier SR Jr (1961) Ocular defects associated with familial renal disease and deafness: case reports and review of literature. Arch Ophthalmol 65:386–391
Govan JA (1983) Ocular manifestations of Alport’s syndrome: a hereditary disorder of basement membranes? Br J Ophthalmol 67:493–503
Wilson ME, Trivedi RH, Biber JM, Golub R (2006) Anterior capsule rupture and subsequent cataract formation in Alport syndrome. J AAPOS 10:182–183
Streeten BW, Robinson MR, Wallace R, Jones DB (1987) Lens capsule abnormalities in Alport’s syndrome. Arch Ophthalmol 105:1693–1697
Kato T, Watanabe Y, Nakayasu K, Kanai A, Yajima Y (1998) The ultrastructure of the lens capsule abnormalities in Alport’s syndrome. Jpn J Ophthalmol 42:401–405
Nielsen CE (1978) Lenticonus anterior and Alport’s syndrome. Acta Ophthalmol 56:518–530
Colville D, Savige J, Morfis M, Ellis J, Kerr P, Agar J, Fasset R (1997) Ocular manifestations of autosomal recessive Alport syndrome. Ophthalmic Genet 18:119–128
Polak BC, Hogewind BL (1977) Macular lesions in Alport’s disease. Am J Ophthalmol 84:532–535
Mete UO, Karaaslan C, Ozbilgin MK, Polat S, Tap O, Kaya M (1996) Alport’s syndrome with bilateral macular hole. Acta Ophthalmol Scand 74:77–80
Rahman W, Banerjee S (2007) Giant macular hole in Alport syndrome. Can J Ophthalmol 42:314–315
Raimundo M, Fonseca C, Silva R, Figueira J (2019) Bilateral giant macular holes: a rare manifestation of Alport syndrome. Eur J Ophthalmol 29:NP13–NP16
Galanos J, Nicholls K, Grigg L, Kiers L, Crawford A, Becker G (2002) Clinical features of Fabry’s disease in Australian patients. Intern Med J 32:575–584
Nguyen TT, Gin T, Nicholls K, Low M, Galanos J, Crawford A (2005) Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin Exper Ophthalmol 33:164–168
Orssaud C, Dufier J, Germain D (2003) Ocular manifestations in Fabry disease: a survey of 32 hemizygous male patients. Ophthalmic Genet 24:129–139
Davey PG (2014) Fabry disease: a survey of visual and ocular symptoms. Clin Ophthalmol 8:1555–1560
Sodi A, Ioannidis AS, Mehta A, Davey C, Beck M, Pitz S (2007) Ocular manifestations of Fabry's disease: data from the Fabry outcome survey. Br J Ophthalmol 91:210–4
Michaud L (2013) Vascular tortuosities of the upper eyelid: a new clinical finding in Fabry patient screening. J Ophthalmol 2013:207573
Michaud L (2019) Longitudinal study on ocular manifestations in a cohort of patients with Fabry disease. PLoS One 14:e0213329
Pompen AWM, Ruiter M, Wyers HJG (1947) Angiokeratoma corporis diffusum (universale) Fabry, as a sign of an unknown internal disease; two autopsy reports. Acta Med Scand 128:234–255
Spaeth GL, Frost P (1965) Fabry’s disease Its ocular manifestations. Arch Ophthalmol 74:760–769
Sher NA, Letson RD, Desnick RJ (1979) The ocular manifestations in Fabry’s disease. Arch Ophthalmol 97:671–676
Sivley MD, Benjamin WJ (2020) Fabry keratopathy: manifestations and changes over time. Br J Ophthalmol 104:1148–1155
Koh S, Haruna M, Asonuma S, Maeda N, Hamano T, Sakai N, Hara C, Maruyama K, Nishida K (2019) Quantitative evaluation of visual function in patients with cornea verticillata associated with Fabry disease. Acta Opthalmol 97:e1098–e1104
Mäntyjärvi M, Tuppurainen K, Ikäheimo K (1998) Ocular side effects of amiodarone. Surv Ophthalmol 42:360–366
Pitz S, Kalkum G, Arash L, Karabul N, Sodi A, Larroque S, Beck M, Gal A (2015) Ocular signs correlate well with disease severity and genotype in Fabry disease. PLoS One 10:e0120814
Fledelius HC, Sandfeld L, Rasmussen AK, Madsen CV, Feldt-Rasmussen U (2015) Ophthalmic experience over 10 years in an observational nationwide Danish cohort of Fabry patients with access to enzyme replacement. Acta Opthalmol 93:258–264
Koh S, Sakai N, Maeda N, Nishida K (2018) Prominent regression of corneal deposits in Fabry disease 16 years after initiation of enzyme replacement therapy. Acta Opthalmol 96:e255–e256
Karr WJ Jr (1959) Fabry’s disease (angiokeratoma corporis diffusum universale). An unusual syndrome with multisystem involvement and unique skin manifestations. Am J Med 27:829–835
Sher NA, Reiff W, Letson RD, Desnick RJ (1978) Central retinal artery occlusion complicating Fabry’s disease. Arch Ophthalmol 96:815–817
Tuppurainen K, Collan Y, Rantanen T, Hollmen A (1981) Fabry’s disease and cornea verticillata. A report of 3 cases. Acta Ophthalmol 59:674–682
Abe H, Sakai T, Sawaguchi S, Hasegawa S, Takagi M, Yoshizawa T, Usui T, Horikawa Y (1992) Ischemic optic neuropathy in a female carrier with Fabry’s disease. Ophthalmologica 205:83–88
Kumagai K, Mitamura Y, Mizunoya S, Fujimoto N, Yamamoto S (2008) A case of anterior ischemic optic neuropathy associated with Fabry’s disease. Jpn J Ophthalmol 52:421–423
Oto S, Kart H, Kadayifcilar S, Ozdemir N, Aydin P (1998) Retinal vein occlusion in a woman with heterozygous Fabry’s disease. Eur J Ophthalmol 8:265–267
Sweeney E, Fryer A, Mountford R, Green A, McIntosh I (2003) Nail patella syndrome: a review of the phenotype aided by developmental biology. J Med Genet 40:153–162
Bongers EM, Huysmans FT, Levtchenko E, de Rooy JW, Blickman JG, Admiraal RJ, Huygen PL, Cruysberg JR, Toolens PA, Prins JB, Krabbe PF, Borm GF, Schoots J, van Bokhoven H, van Remortele AM, Hoefsloot LH, van Kampen A, Knoers NV (2005) Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur J Hum Genet 13:935–946
Boyer O, Woerner S, Yang F, Oakeley EJ, Linghu B, Gribouval O, Tete MJ, Duca JS, Klickstein L, Damask AJ, Szustakowski JD, Heibel F, Matignon M, Baudouin V, Chantrel F, Champigneulle J, Martin L, Nitschke P, Gubler MC, Johnson KJ, Chibout SD, Antignac C (2013) LMX1B mutations cause hereditary FSGS without extrarenal involvement. J Am Soc Nephrol 24:1216–1222
Andeen NK, Schleit J, Blosser CD, Dorschner MO, Hisama FM, Smith KD (2018) LMX1B-Associated nephropathy with type III collagen deposition in the glomerular and tubular basement membranes. Am J Kidney Dis 72:296–301
Pinto EVF, Pichurin PN, Fervenza FC, Nasr SH, Mills K, Schmitz CT, Klee EW, Herrmann SM (2020) Nail-patella-like renal disease masquerading as Fabry disease on kidney biopsy: a case report. BMC Nephrol 21:341
Trimarchi H (2020) Focal segmental glomerulosclerosis and scheduled pretransplant plasmapheresis: a timely diagnosis of Nail-Patella syndrome avoided more futile immunosuppression. Case Rep Nephrol 2020:8879555
Nakata T, Ishida R, Mihara Y, Fujii A, Inoue Y, Kusaba T, Isojima T, Harita Y, Kanda C, Kitanaka S, Tamagaki K (2017) Steroid-resistant nephrotic syndrome as the initial presentation of nail-patella syndrome: a case of a de novo LMX1B mutation. BMC Nephrol 18:100
Sproule DM, Kaufmann P (2008) Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes: basic concepts, clinical phenotype, and therapeutic management of MELAS syndrome. Ann N Y Acad Sci 1142:133–158
Hirano M, Ricci E, Koenigsberger MR, Defendini R, Pavlakis SG, DeVivo DC, DiMauro S, Rowland LP (1992) MELAS: an original case and clinical criteria for diagnosis. Neuromuscul Disord 2:125–135
Doleris LM, Hill GS, Chedin P, Nochy D, Bellanne-Chantelot C, Hanslik T, Bedrossian J, Caillat-Zucman S, Cahen-Varsaux J, Bariety J (2000) Focal segmental glomerulosclerosis associated with mitochondrial cytopathy. Kidney Int 58:1851–1858
Guery B, Choukroun G, Noel LH, Clavel P, Rotig A, Lebon S, Rustin P, Bellane-Chantelot C, Mougenot B, Grunfeld JP, Chauveau D (2003) The spectrum of systemic involvement in adults presenting with renal lesion and mitochondrial tRNA(Leu) gene mutation. J Am Soc Nephrol 14:2099–2108
Lowik MM, Hol FA, Steenbergen EJ, Wetzels JF, van den Heuvel LP (2005) Mitochondrial tRNALeu(UUR) mutation in a patient with steroid-resistant nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant 20:336–341
Harrison TJ, Boles RG, Johnson DR, LeBlond C, Wong LJC (1997) Macular pattern retinal dystrophy, adult-onset diabetes, and deafness: a family study of A3243G mitochondrial heteroplasmy. Am J Ophthalmol 124:217–221
Smith PR, Bain SC, Good PA, Hattersley AT, Barnett AH, Gibson JM, Dodson PM (1999) Pigmentary retinal dystrophy and the syndrome of maternally inherited diabetes and deafness caused by the mitochondrial DNA 3243 tRNA(Leu) A to G mutation. Ophthalmology 106:1101–1108
de Laat P, Smeitink JAM, Janssen MCH, Keunen JEE, Boon CJF (2013) Mitochondrial retinal dystrophy associated with the m.3243A>G mutation. Ophthalmology 120:2684–2696
Michaelides M, Jenkins SA, Bamiou DE, Sweeney MG, Davis MB, Luxon L, Bird AC, Rath PP (2008) Macular dystrophy associated with the A3243G mitochondrial DNA mutation: distinct retinal and associated features, disease variability, and characterization of asymptomatic family members. Arch Ophthalmol 126:320–328
Rath PP, Jenkins S, Michaelides M, Smith A, Sweeney MG, Davis MB, Fitzke FW, Bird AC (2008) Characterisation of the macular dystrophy in patients with the A3243G mitochondrial DNA point mutation with fundus autofluorescence. Br J Ophthalmol 92:623–629
Sivaprasad S, Kung BT, Robson AG, Black G, Webster AR, Bird A, Egan C (2008) A new phenotype of macular dystrophy associated with a mitochondrial A3243G mutation. Clin Exper Ophthalmol 36:92–93
Fraser JA, Biousse V, Newman NJ (2010) The neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol 55:299–334
Parikh S, Goldstein A, Koenig MK, Scaglia F, Enns GM, Saneto R, Anselm I, Cohen BH, Falk MJ, Greene C, Gropman AL, Haas R, Hirano M, Morgan P, Sims K, Tarnopolsky M, Van Hove JLK, Wolfe L, DiMauro S (2015) Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med 17:689–701
Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC (1992) Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet 1:11–15
Murphy R, Turnbull DM, Walker M, Hattersley AT (2008) Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet Med 25:383–399
Guillausseau PJ, Massin P, Dubois-LaForgue D, Timsit J, Virally M, Gin H, Bertin E, Blickle JF, Bouhanick B, Cahen J, Caillat-Zucman S, Charpentier G, Chedin P, Derrien C, Ducluzeau PH, Grimaldi A, Guerci B, Kaloustian E, Murat A, Olivier F, Paques M, Paquis-Flucklinger V, Porokhov B, Samuel-Lajeunesse J, Vialettes B (2001) Maternally inherited diabetes and deafness: a multicenter study. Ann Intern Med 134:721–728
Tan R, Colville D, Wang YY, Rigby L, Savige J (2010) Alport retinopathy results from “severe” COL4A5 mutations and predicts early renal failure. Clin J Am Soc Nephrol 5:34–38
Chen Y, Colville D, Ierino F, Symons A, Savige J (2018) Temporal retinal thinning and the diagnosis of Alport syndrome and Thin basement membrane nephropathy. Ophthalmic Genet 39:208–214
Gulati A, Sevillano AM, Praga M, Gutierrez E, Alba I, Dahl NK, Besse W, Choi J, Somlo S (2020) Collagen IV gene mutations in adults with bilateral renal cysts and CKD. Kidney Int Rep 5:103–108
Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE (2019) Mouse genome database (MGD) 2019. Nucleic Acids Res 47:D801-d806
Acknowledgements
We would like to thank the patients who have given permission to use photographs of their ophthalmic features. We would also like to thank Genomics Englands, the Human Protein Atlas, Mouse Genome Informatics [107], as well as OMIM, Medline, Embase, and the Cochrane Database of Systematic Reviews for access to their databases.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
VZ, TH, and DW were medical students who undertook this research project during a COVID lockdown. HGM has received research funding from Retinal Australia and Honoraria from Novartis. The other authors have no financial or non-financial conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 338 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, V., Huang, T., Wang, D. et al. Ocular manifestations of the genetic causes of focal and segmental glomerulosclerosis. Pediatr Nephrol 39, 655–679 (2024). https://doi.org/10.1007/s00467-023-06073-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06073-y