Abstract
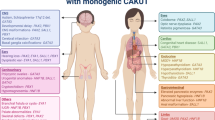
Congenital anomalies of the kidney and urinary tract (CAKUT) are among the most common birth defects worldwide and a major cause of kidney failure in children. Extra-renal manifestations are also common. This study reviewed diseases associated with the Genomics England CAKUT-associated gene panel for ocular anomalies. In addition, each gene was examined for expression in the human retina and an ocular phenotype in mouse models using the Human Protein Atlas and Mouse Genome Informatics databases, respectively. Thirty-four (54%) of the 63 CAKUT-associated genes (55 ‘green’ and 8 ‘amber’) had a reported ocular phenotype. Five of the 6 most common CAKUT-associated genes (PAX2, EYA1, SALL1, GATA3, PBX1) that represent 30% of all diagnoses had ocular features. The ocular abnormalities found with most CAKUT-associated genes and with five of the six commonest were coloboma, microphthalmia, optic disc anomalies, refraction errors (astigmatism, myopia, and hypermetropia), and cataract. Seven of the CAKUT-associated genes studied (11%) had no reported ocular features but were expressed in the human retina or had an ocular phenotype in a mouse model, which suggested further possibly-unrecognised abnormalities. About one third of CAKUT-associated genes (18, 29%) had no ocular associations and were not expressed in the retina, and the corresponding mouse models had no ocular phenotype. Ocular abnormalities in individuals with CAKUT suggest a genetic basis for the disease and sometimes indicate the affected gene. Individuals with CAKUT often have ocular abnormalities and may require an ophthalmic review, monitoring, and treatment to preserve vision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
CAKUT
Genetic kidney disease comprises congenital anomalies of the kidney and urinary tract (CAKUT), cystic kidney diseases and ciliopathies, glomerulopathies such as Alport syndrome, complementopathies, and focal and segmental glomerulosclerosis (FSGS), as well as the tubulopathies.
CAKUT are a diverse group of developmental anomalies and the most common (20–30%) of all birth defects [1]. CAKUT is found in nearly 1% of live births worldwide, and is the cause in almost half the children who develop kidney failure [1].
The kidney phenotypes of CAKUT include agenesis, hypoplasia, dysplasia, cysts, ectopia, fusion, hydronephrosis, urinary tract agenesis, duplication, megaureter, ureteropelvic junction obstruction, vesicoureteral reflux, and posterior urethral valves. Defects may be unilateral or bilateral. Some changes, such as bilateral kidney agenesis, are almost always fatal [2]. Some progress to kidney failure necessitating dialysis or transplantation within the first few years of life or later [3]. Some have an excellent prognosis [3]. At present, many individuals with CAKUT may be diagnosed antenatally with ultrasound but others are found incidentally after imaging, repeated urinary tract infections, or the detection of impaired kidney function.
CAKUT occurs in isolation or together with defects in other organ systems in syndromic disease. Many cases are sporadic [4], with no identifiable cause, and probably result from both genetic and environmental factors, including maternal diabetes or obesity [5]. However, familial CAKUT consistent with a genetic basis still represents at least 10–20% of cases [4].
More than 150 genes are associated with CAKUT and more remain to be identified. Many of these genes are transcription factors that are important in embryonic development. Six are common (PAX2, HNF1B, EYA1, SALL1, GATA3, PBX1) but many of the others are found only in individual families [6]. Inheritance is usually autosomal dominant (AD) with incomplete penetrance and variable expression. This means that clinical features vary even within a family, and one affected family member may have a single kidney, another has vesicoureteric reflux and another affected family member has normal kidneys. CAKUT may be misdiagnosed as FSGS since some genes result in a reduced nephron number. In addition, the diagnosis of CAKUT may be overlooked when it results from copy number variants that are difficult to detect with Whole Exome Sequencing.
Ocular anomalies occur in different forms of CAKUT. This is because of the developmental and structural overlap between the kidney and the eye despite their different functions. The kidney and the eye develop embryologically at about the same time (5th to 12th weeks of gestation) and under the control of some of the same transcription factors, including BMP7, EYA1, FOXC1, PAX, and WNT1 [7]. Surprisingly, the kidney and the eye also share structural features such as the epithelial cell barrier, basement membrane and capillary network in the glomerular filter and in the retinal pigment epithelial cells, Bruch’s membrane and choriocapillaris [7]. In addition, the glomerular and retinal basement membranes both mainly comprise the collagen IV α3α4α5 network. Importantly the kidney and the retina also depend on ciliated cells for their functions.
This review examines monogenic causes of CAKUT for their ocular associations. Identifying ocular features suggests that the disease has a genetic basis, and may indicate the affected gene and the need for treatment or ophthalmic monitoring to prevent complications.
Search strategy for ocular features in CAKUT
Sixty-three genes associated with CAKUT from the ‘green’ (n = 55) or ‘amber’ lists (n = 8) of the Genomics England CAKUT panel (https://panelapp.genomicsengland.co.uk/) were searched for ocular manifestations in the Online Mendelian Inheritance in Man (OMIM, https://www.omim.org/), PubMed and Google Scholar (https://scholar.google.com/) databases between July 2022 and March 2023. Genomics England uses a traffic light system where green genes have a high level of evidence for a disease association (reported in 3 unrelated families or 2 families with additional strong evidence) as decided by an expert panel. These panels represent the genes often examined in a diagnostic genetic laboratory. Amber and red genes have borderline and low levels of evidence for disease association.
The 63 genes were also examined for mRNA expression in the retina in The Human Protein Atlas (https://www.proteinatlas.org), and for an ocular phenotype in mouse models in the Mouse Genome Informatics (MGI) database (http://www.informatics.jax.org/).
Ocular features associated with CAKUT
About half of the 63 CAKUT-associated genes in the Genomics England panels (34, 54%) have reported ocular abnormalities (Supplementary Table 1). About one third of CAKUT genes (18, 29%) have no reported ocular associations, are not expressed in the human retina and have no ocular features in a mouse model. Seven further genes (7, 11%) have no reported ocular phenotype in human disease but are expressed in the human retina or the mouse models have ocular features. Thus, further ocular abnormalities may yet be described with these other genes.
Five of the 6 most common CAKUT genes that represent 30% of all cases (PAX2, EYA1, SALL1, GATA3, PBX1) have an ocular phenotype (Table 1). There are no reported ocular features for the HNF1B gene which is affected in HNF1B-nephropathy, formerly known as renal cysts and diabetes syndrome.
The most common ocular abnormalities associated with CAKUT-associated genes are coloboma, optic disc (‘morning glory’) anomalies, microphthalmia, refraction errors (astigmatism, myopia, and hypermetropia), and cataracts (Table 2, Fig. 1).
Common ocular abnormalities associated with CAKUT: a iris coloboma that resembles a ‘keyhole’ inferiorly; b large chorioretinal coloboma; c inferior iris coloboma with microphthalmia (diameter between arrows 7 mm); d subtle papilloretinal syndrome with vessels emerging from the periphery of the disc (arrow); e optic disc hypoplasia, arrows indicate area of missing disc; f optic atrophy; g optic disc pit; h optic disc coloboma; i inherited retinal dystrophy; j macular coloboma with excavated lesion and surrounding pigmented margin; and k optical coherence tomography scan of the fundus in j confirming macular coloboma with excavation, absent retina and choroid, and thinned residual sclera, but more distant normal retina and disc.
Ocular coloboma (CHD7, KMT2D, PAX2, PLVAP, SALL1, STRA6, TFAP2A, BMP4, CENPF, SALL4)
Ocular coloboma affect one in 2000 live births [39], and most have a genetic basis [40].
They derive from incomplete closure of the choroidal fissure during embryonic development at about week 7.
Coloboma in CAKUT affect the iris (anterior segment), or choroid, retina and optic nerve (posterior segment). They affect the anterior and posterior segments equally often [39], and both segments are affected in about one quarter of patients. The extent of the defect depends on the stage at which the fissure fails to close, with a late failure-to-close resulting in only an iris defect [41]. Coloboma are usually unilateral but bilateral in one third of cases [39]. Ocular coloboma are also associated with developmental delay [39], and other neurological, skeletal, and craniofacial, as well as kidney anomalies [41].
Coloboma are often associated with microphthalmia, and are present from birth. The visual prognosis depends mainly on the involvement of the macula and optic nerve. Isolated coloboma of the iris do not affect vision and rarely require surgical treatment, for cosmetic reasons [42]. Chorioretinal coloboma are usually asymptomatic, require a formal ophthalmic examination for their demonstration, but may result in a visual field defect [41]. Unilateral cases may develop strabismus. Bilateral cases often present in infancy with poor vision and nystagmus.
At diagnosis, the extent of the coloboma and associated abnormalities such as microphthalmia, amblyopia, squint, and refractive errors should be identified. Direct and indirect ophthalmoscopy, accurate refraction, assessment for microphthalmia, and visual fields should be performed. Evaluation may be difficult in an infant. Individuals with a coloboma, especially of the posterior segment, require monitoring by an ophthalmologist.
In general, coloboma cannot be corrected, and their management focuses on monitoring for and treating complications such as cataract, glaucoma, retinal detachment and subretinal neovasculariation [42,43,44,45,46]. More than half of children with a coloboma develop another ocular abnormality including amblyopia or strabismus over a 9-year period [39]. Amblyopia may be helped with part-time occlusion, strabismus can be treated surgically and spectacles may help. Chorioretinal coloboma are associated with retinal detachments in up to 40% of affected individuals [41]. These may be overlooked because of already-limited vision. Subretinal neovascularisation at the coloboma edge may be managed with antiVEGF treatment.
The patient with CAKUT and a coloboma must also be assessed for other syndromic features, and first-degree relatives examined for coloboma, other ocular features and for CAKUT.
Optic nerve dysplasia (PAX2)
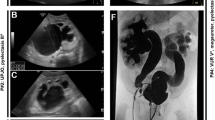
Optic nerve dysplasia also described as ‘optic nerve coloboma’ or a ‘morning glory disc anomaly’ is typical of papillorenal syndrome due to PAX2 pathogenic variants. It occurs in the majority of cases [47], is often bilateral and is characterised by the emergence of the retinal vessels from the periphery rather than the centre of the optic disc.
Microphthalmia (CHD7, DHCR7, FRAS1, GLI3, PAX2, SALl1, STRA6, TFAP2A, BMP4, CENPF, SALL4)
This is a rare developmental disorder affecting one in 5000 individuals [48] where one or both eyes are abnormally small with an axial diameter less than 2SD below the mean for age. Microphthalmia may be associated with microcornea, aniridia, cataract, and retinal degeneration [49]. It usually affects vision, and treatment may be required for the more severe forms [49].
Cataracts (CHD7, DHCR7, EYA1, GPC3, JAG1, KMT2D, LRP4, PAX2, RET, SALL1, TFAP2A, CENPF, HS2ST1, SALL4)
Many of these cataracts are present at birth. Again, these may be unilateral or bilateral, are detected on examination for a red reflex and those more than 3 mm in diameter affect vision. Visually-significant cataracts should be removed surgically within weeks of birth.
Inherited retinal degeneration (GATA3, NPHP3, PAX2, BMP4)
Inherited retinal degeneration is a diverse group of diseases characterised by photoreceptor cell death and progressive loss of vision, and encompassing the retinal dystrophies, including retinitis pigmentosa. It usually reflects ciliary dysfunction which is more commonly associated with the renal ciliopathies and cystic kidney disease. The diagnosis is usually made on the basis of clinical history, fundus examination, and electroretinography. In retinitis pigmentosa, the fundus characteristically demonstrates the triad of 'bone spicules', pale optic disc, and arteriolar attenuation bilaterally.
Hypertelorism (WNT5A, CTU2, LRP4, ZMYM2, H2ST1)
Hypertelorism is an abnormally-increased distance between the orbits which may be corrected surgically between the ages of 5 and 8.
Posterior embryotoxon (NOTCH2, JAG1, HS2ST1) and Axenfeld anomaly (JAG1)
Posterior embryotoxon and Axenfeld anomaly is rare, occurring in one in 200,000 live births. It is usually diagnosed in childhood, where there is a membrane extending from the cornea onto the iris surface in both eyes. It may be associated with iris atrophy and later, glaucoma.
Peter’s anomaly (CENPF, EYA1, NIPBL) is where a thinned and clouded cornea attaches to the iris and results in blurred vision. It is often associated with cataracts and glaucoma.
Refractive errors (CHD7, NIPBL, BMP4), such as myopia and hypermetropia, correspond to short- and long-sightedness, are common, and result from defects in the shape of the eyeball, cornea, or lens.
Neuro-ophthalmic disorders (ANOS1, CHRNA3, DHCR7, EYA1, FAM58A, JAG1, KDM6A, KMT2D, NIPBL, NPHP3, PBX1, SALL1, SALL4) include a poor light reflex, nystagmus, and strabismus.
Duane anomaly (FAM58A) is a congenital anomaly where the horizontal eye movement is limited in abduction, adduction, or both.
Some forms of CAKUT and their ocular associations
Papillorenal (renal-coloboma) syndrome (PAX2)
Papillorenal syndrome is associated with malformations of the kidneys and eyes. More than 250 affected individuals have been reported but many are unrecognised so that population frequencies are likely to be underestimates. A pathogenic variant in PAX2 is identified in nearly 50% of cases [50]. Kidney anomalies occur in 90% of cases, with hypodysplasia in 65%, multicystic dysplasia in 10%, and vesicoureteric reflux in 14% [47, 51]. The principal ocular manifestation is a dysplastic optic nerve, which is usually bilateral [47]. This appears as an excavated optic disc, with the retinal vessels emerging at the periphery rather than centrally (Fig. 1). This is often described as an ‘optic nerve coloboma’ or ‘morning glory disc anomaly’ because of its resemblance to the flower. Detection requires a careful dilated fundus examination. Visual acuity is reduced in at least one eye in 75% of cases but may be normal. Further visual loss occurs with retinal detachment [46]. This risk necessitates close monitoring by an ophthalmologist. The ocular phenotype may differ even among family members with the same variant.
Branchiootorenal syndrome 1 with or without cataracts (EYA1)
Branchiootorenal syndrome 1 is an AD-inherited disease characterised by branchial cysts or fistulae, structural defects and an abnormal shape of the outer, middle or inner ear, preauricular pits, and hearing loss [52]. It affects about one in 40,000 of the population. Kidney abnormalities including agenesis, hypoplastic and cystic kidneys, pelviureteric obstruction, bifid ureters and kidney failure occur in about two-thirds of affected individuals. The ocular associations include congenital anterior segment anomalies including cataract. However, these features are not common [14], and sometimes the kidneys are normal.
Townes-Brocks syndrome 1 (SALL1)
Townes-Brocks syndrome 1 is an AD-inherited disease characterised by kidney, limb and ear anomalies as well as an imperforate anus, rectal atresia, polydactyly, and a triphalangeal thumb [50]. Other features include preauricular tags, overfolded helices, cardiac anomalies, hypospadias, and impaired kidney function. Coloboma and the Duane anomaly are rare. The features may overlap with branchiootorenal syndrome since the EYA1 and SALL1 genes are involved in the same biochemical pathways.
Hypoparathyroidism, sensorineural deafness and renal dysplasia (HDR syndrome) (GATA3)
GATA3 is a zinc-finger transcription factor that binds to the enhancer elements (A/T)GATA (A/G) of all four T cell antigen receptors [53], and is required for the embryonic development of the parathyroids, hearing, and kidneys. Hypoparathyroidism in HDR syndrome ranges from asymptomatic disease to features including myalgia, sensory problems, and tetany from hypocalcaemia. Parathyroid hormone levels vary from low to high. Hearing loss is obvious early. Kidney problems include developmental abnormalities such as dysplasia and hypoplasia but also cystic kidneys, vesicoureteric reflux, proteinuria, renal tubular acidosis, nephrocalcinosis, and kidney failure. Penetrance varies in different family members. Other manifestations include pyloric stenosis and female genital tract malformations [54]. Hearing loss ranges from mild to severe and is usually bilateral. The prognosis depends on disease severity. Ophthalmic features include band keratopathy and inherited retinal degeneration [55].
CAKUT with or without hearing loss, abnormal ears or developmental delay (PBX1)
PBX1 is a transcription factor that regulates morphologic patterning, organogenesis, and haematopoiesis in the embryo. It does this through modulation of the HOX protein [34]. This syndrome is sometimes referred to as CAKUTHED (Hearing loss, abnormal ears, and developmental delay). The disease usually presents in childhood. Ocular features include strabismus, corneal clouding, iris abnormalities, and glaucoma [38].
Other forms of CAKUT with interesting ocular manifestations include the following.
CHARGE syndrome (CHD7)
CHARGE syndrome (Coloboma of the eye, Heart defects, nasal choanae, growth Retardation, and Genital and urinary tract abnormalities and Ear anomalies with deafness) affects one in 10,000 births [56], and results from a pathogenic variant in CHD7 in two-thirds of cases [57]. However, these are almost all de novo so that there is often no family history [58]. Inheritance is otherwise AD and there is phenotypic variation even between affected family members. The kidney manifestations include unilateral agenesis, fusion, ectopia, and malrotation, duplex collecting systems and ureteral agenesis. The other classical features are choanal atresia, developmental delay, hypoplasia or aplasia of the semicircular canals, and cardiac defects in 80% of cases [59].
Coloboma are characteristic and seen in nearly all affected individuals [60]. They are typically chorioretinal, but may also affect the iris or optic nerve [61]. They are usually bilateral. Other reported ocular anomalies include microphthalmia and anophthalmia, microcornea, cataract, ectopia lentis, and persistent foetal vasculature [62]. There is an association with high myopia, but overall, visual acuity ranges from near normal to an absence of light perception [62]. Patients require management by an ophthalmologist, because of the risk of retinal detachment [63], and to ensure refractive errors are corrected.
Alagille syndrome (JAG1, NOTCH2)
Alagille syndrome results from a pathogenic variant in JAG1 in 97% of cases and NOTCH2 in fewer than 1% [64]. The population frequency is one in 70,000 but this is probably an underestimate [64]. Inheritance is AD. With a JAG1 variant, kidney anomalies occur in about 40% [65], with dysplasia being the most common, and others including vesicoureteral reflex and ureteropelvic junction obstruction [65]. Posterior embryotoxon, an anteriorly displaced and thickened Schwalbe’s line, is a cardinal feature of Alagille syndrome, occurring in 95% of cases [66]. It does not affect vision but is helpful diagnostically. Optic disc drusen are also common and usually occur bilaterally [67]. Other common ocular anomalies include those of the optic disc (76%), diffuse fundus hypopigmentation (57%), and a speckled retinal pigment epithelium (33%) [66]. The Axenfeld anomaly occurs in 13% [68].
Rubinstein-Taybi syndrome (CREBBP, EP300)
Rubinstein-Taybi syndrome is characterised by short stature, microcephaly, intellectual disability, dysmorphic facial features, broad thumbs and big toes and cryptorchidism. It affects about one in 100,000 births and has AD inheritance. Pathogenic variants in CREBBP account for 60% of cases, EP300 for 10% and the other genes are not known [69, 70]. Kidney malformations occur in half the cases, including kidney agenesis, duplication, hypoplasia, hydronephrosis, and vesicoureteric reflux.
More than half the affected individuals have ocular abnormalities [71]. These include strabismus (in 70%); refractive error (60%) including high myopia, in 25%; and coloboma affecting the iris, retina, choroid, or optic nerve in 10% [71,72,73]. Congenital and juvenile glaucoma, and congenital cataract occur [72]. Inherited retinal degeneration may be present with an abnormal retinal pigment epithelium, absent foveal reflex, and electroretinogram findings suggesting cone or cone-rod dysfunction [72], as well as peripheral retinal avascularity [74].
Discussion
This study found that about half the genes associated with CAKUT have ocular features and that more may be expected based on the retinal expression data and the phenotypes of mouse models. There is, however, little information on the proportion of individuals with each form of CAKUT who have ocular abnormalities.
Coloboma are one of the ocular features associated with the most CAKUT genes. They are typically present from birth and do not progress during life, but complications such as amblyopia, strabismus, retinal detachment, cataract and subretinal neovascularisation may occur. Coloboma are also sometimes seen in other genetic kidney diseases such as the ciliopathies, focal and segmental glomerulosclerosis, and tubulopathies, as well as genetic diseases that do not affect the kidney, and sometimes without an obvious cause.
Many ocular abnormalities, such as coloboma, microphthalmia or strabismus, are obvious to a renal physician and should prompt a formal ophthalmological review. However, the diagnosis of CAKUT itself indicates that screening for syndromic features including an ophthalmological examination is warranted. The input from an interested ophthalmologist is important in assessment of an ocular phenotype, and in deciding on the need for active treatment or ongoing monitoring.
The demonstration of a coloboma or other ocular abnormality in a person with a structural kidney disease suggests a genetic cause, and in some cases a specific gene. However, more than 150 genes have been identified in CAKUT and the genetic detection rate is less than 20% so that testing for a variant is only advocated where a defect in a specific gene is suspected, such as for PAX2 with optic disc dysplasia [75].
Most genes causing CAKUT demonstrate AD inheritance and first-degree family members should also be examined. However, clinical features are often incompletely penetrant and even affected family members may have no phenotype. The variable penetrance means that it is often not possible to accurately predict the kidney and visual consequences for a future child.
This study’s strengths were the use of the Genomics England CAKUT panel, and of curated retinal expression and mouse model databases to identify the ocular associations. The study’s major limitations were that individual forms of CAKUT diseases are rare, data is limited on how often ocular features occur with different forms of CAKUT, affected individuals have not necessarily undergone an ophthalmic examination, and reported ocular features may have been coincidental. The list of CAKUT genes is not exhaustive but nevertheless represents those considered by many laboratories in their search for pathogenic variants and is representative of the genes causing CAKUT and their ocular phenotypes.
Thus, clinicians should consider the possibility of ocular disease in patients with CAKUT, since these can be helpful diagnostically and may require further ophthalmic management. The ocular abnormalities in CAKUT generally do not progress over time, but complications may occur, and monitoring and treatment may be necessary.
Data Availability
All relevant data is included in the manuscript or in the Supplementary Information.
References
Loane M, Dolk H, Kelly A, Teljeur C, Greenlees R, Densem J, EUROCAT Working Group (2011) Paper 4: EUROCAT statistical monitoring: identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91(Suppl 1):S31–S43
Wuhl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, Zurriaga O, Hoitsma A, Niaudet P, Palsson R, Ravani P, Jager KJ, Schaefer F (2013) Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol 8:67–74
Riddle S, Habli M, Tabbah S, Lim FY, Minges M, Kingma P, Polzin W (2020) Contemporary outcomes of patients with isolated bilateral renal agenesis with and without fetal intervention. Fetal Diagn Ther 47:675–681
Capone VP, Morello W, Taroni F, Montini G (2017) Genetics of congenital anomalies of the kidney and urinary tract: the current state of play. Int J Mol Sci 18:796
Macumber I, Schwartz S, Leca N (2017) Maternal obesity is associated with congenital anomalies of the kidney and urinary tract in offspring. Pediatr Nephrol 32:635–642
Kagan M, Pleniceanu O, Vivante A (2022) The genetic basis of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 37:2231–2243
Izzedine H, Bodaghi B, Launay-Vacher V, Deray G (2003) Eye and kidney: from clinical findings to genetic explanations. J Am Soc Nephrol 14:516–529
Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J (2004) Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med 140:510–517
Kaplan BS, Gordon I, Pincott J, Barratt TM (1989) Familial hypoplastic glomerulocystic kidney disease: a definite entity with dominant inheritance. Am J Med Genet 34:569–573
Nakayama M, Nozu K, Goto Y, Kamei K, Ito S, Sato H, Emi M, Nakanishi K, Tsuchiya S, Iijima K (2010) HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 25:1073–1079
Rizzoni G, Loirat C, Levy M, Milanesi C, Zachello G, Mathieu H (1982) Familial hypoplastic glomerulocystic kidney. A new entity? Clin Nephrol 18:263–268
Eccles MR, Schimmenti LA (1999) Renal-coloboma syndrome: a multi-system developmental disorder caused by PAX2 mutations. Clin Genet 56:1–9
Schimmenti LA (2011) Renal coloboma syndrome. Eur J Hum Genet 19:1207–1212
Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M (2000) Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet 9:363–366
Carmi R, Binshtock M, Abeliovich D, Bar-Ziv J (1983) The branchio-oto-renal (BOR) syndrome: report of bilateral renal agenesis in three sibs. Am J Med Genet 14:625–627
Chitayat D, Hodgkinson KA, Chen MF, Haber GD, Nakishima S, Sando I (1992) Branchio-oto-renal syndrome: further delineation of an underdiagnosed syndrome. Am J Med Genet 43:970–975
Fraser FC, Ling D, Clogg D, Nogrady B (1978) Genetic aspects of the BOR syndrome–branchial fistulas, ear pits, hearing loss, and renal anomalies. Am J Med Genet 2:241–252
Fraser FC, Ayme S, Halal F, Sproule J (1983) Autosomal dominant duplication of the renal collecting system, hearing loss, and external ear anomalies: a new syndrome? Am J Med Genet 14:473–478
Legius E, Fryns JP, Van den Berghe H (1990) Dominant branchial cleft syndrome with characteristics of both branchio-oto-renal and branchio-oculo-facial syndrome. Clin Genet 37:347–350
Melnick M, Bixler D, Silk K, Yune H, Nance WE (1975) Autosomal dominant branchiootorenal dysplasia. Birth Defects Orig Artic Ser 11:121–128
Melnick M, Bixler D, Nance WE, Silk K, Yune H (1976) Familial branchio-oto-renal dysplasia: a new addition to the branchial arch syndromes. Clin Genet 9:25–34
Blanck C, Kohlhase J, Engels S, Burfeind P, Engel W, Bottani A, Patel MS, Kroes HY, Cobben JM (2000) Three novel SALL1 mutations extend the mutational spectrum in Townes-Brocks syndrome. J Med Genet 37:303–307
Botzenhart EM, Green A, Ilyina H, Konig R, Lowry RB, Lo IF, Shohat M, Burke L, McGaughran J, Chafai R, Pierquin G, Michaelis RC, Whiteford ML, Simola KO, Rosler B, Kohlhase J (2005) SALL1 mutation analysis in Townes-Brocks syndrome: twelve novel mutations and expansion of the phenotype. Hum Mutat 26:282
Botzenhart EM, Bartalini G, Blair E, Brady AF, Elmslie F, Chong KL, Christy K, Torres-Martinez W, Danesino C, Deardorff MA, Fryns JP, Marlin S, Garcia-Minaur S, Hellenbroich Y, Hay BN, Penttinen M, Shashi V, Terhal P, Van Maldergem L, Whiteford ML, Zackai E, Kohlhase J (2007) Townes-Brocks syndrome: twenty novel SALL1 mutations in sporadic and familial cases and refinement of the SALL1 hot spot region. Hum Mutat 28:204–205
Ferraz FG, Nunes L, Ferraz ME, Sousa JP, Santos M, Carvalho C, Maroteaux P (1989) Townes-Brocks syndrome. Report of a case and review of the literature. Ann Genet 32:120–123
Johnson JP, Poskanzer LS, Sherman S (1996) Three-generation family with resemblance to Townes-Brocks syndrome and Goldenhar/oculoauriculovertebral spectrum. Am J Med Genet 61:134–139
Kohlhase J, Taschner PE, Burfeind P, Pasche B, Newman B, Blanck C, Breuning MH, ten Kate LP, Maaswinkel-Mooy P, Mitulla B, Seidel J, Kirkpatrick SJ, Pauli RM, Wargowski DS, Devriendt K, Proesmans W, Gabrielli O, Coppa GV, Wesby-van Swaay E, Trembath RC, Schinzel AA, Reardon W, Seemanova E, Engel W (1999) Molecular analysis of SALL1 mutations in Townes-Brocks syndrome. Am J Hum Genet 64:435–445
Kurnit DM, Steele MW, Pinsky L, Dibbins A (1978) Autosomal dominant transmission of a syndrome of anal, ear, renal, and radial congenital malformations. J Pediatr 93:270–273
Rossmiller DR, Pasic TR (1994) Hearing loss in Townes-Brocks syndrome. Otolaryngol Head Neck Surg 111:175–180
Barakat AJ, Raygada M, Rennert OM (2018) Barakat syndrome revisited. Am J Med Genet A 176:1341–1348
Bilous RW, Murty G, Parkinson DB, Thakker RV, Coulthard MG, Burn J, Mathias D, Kendall-Taylor P (1992) Brief report: autosomal dominant familial hypoparathyroidism, sensorineural deafness, and renal dysplasia. N Engl J Med 327:1069–1074
Ferraris S, Del Monaco AG, Garelli E, Carando A, De Vito B, Pappi P, Lala R, Ponzone A (2009) HDR syndrome: a novel “de novo” mutation in GATA3 gene. Am J Med Genet A 149A:770–775
Hasegawa T, Hasegawa Y, Aso T, Koto S, Nagai T, Tsuchiya Y, Kim KC, Ohashi H, Wakui K, Fukushima Y (1997) HDR syndrome (hypoparathyroidism, sensorineural deafness, renal dysplasia) associated with del(10)(p13). Am J Med Genet 73:416–418
Heidet L, Moriniere V, Henry C, De Tomasi L, Reilly ML, Humbert C, Alibeu O, Fourrage C, Bole-Feysot C, Nitschke P, Tores F, Bras M, Jeanpierre M, Pietrement C, Gaillard D, Gonzales M, Novo R, Schaefer E, Roume J, Martinovic J, Malan V, Salomon R, Saunier S, Antignac C, Jeanpierre C (2017) Targeted exome sequencing identifies PBX1 as involved in monogenic congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol 28:2901–2914
Le Tanno P, Breton J, Bidart M, Satre V, Harbuz R, Ray PF, Bosson C, Dieterich K, Jaillard S, Odent S, Poke G, Beddow R, Digilio MC, Novelli A, Bernardini L, Pisanti MA, Mackenroth L, Hackmann K, Vogel I, Christensen R, Fokstuen S, Bena F, Amblard F, Devillard F, Vieville G, Apostolou A, Jouk PS, Guebre-Egziabher F, Sartelet H, Coutton C (2017) PBX1 haploinsufficiency leads to syndromic congenital anomalies of the kidney and urinary tract (CAKUT) in humans. J Med Genet 54:502–510
Slavotinek A, Risolino M, Losa M, Cho MT, Monaghan KG, Schneidman-Duhovny D, Parisotto S, Herkert JC, Stegmann APA, Miller K, Shur N, Chui J, Muller E, DeBrosse S, Szot JO, Chapman G, Pachter NS, Winlaw DS, Mendelsohn BA, Dalton J, Sarafoglou K, Karachunski PI, Lewis JM, Pedro H, Dunwoodie SL, Selleri L, Shieh J (2017) De novo, deleterious sequence variants that alter the transcriptional activity of the homeoprotein PBX1 are associated with intellectual disability and pleiotropic developmental defects. Hum Mol Genet 26:4849–4860
Murphy MJ, Polok BK, Schorderet DF, Cleary ML (2010) Essential role for Pbx1 in corneal morphogenesis. Invest Ophthalmol Vis Sci 51:795–803
Safgren SL, Olson RJ, Pinto EVF, Bothun ED, Hanna C, Klee EW, Schimmenti LA (2022) De novo PBX1 variant in a patient with glaucoma, kidney anomalies, and developmental delay: an expansion of the CAKUTHED phenotype. Am J Med Genet A 188:919–925
Nakamura KM, Diehl NN, Mohney BG (2011) Incidence, ocular findings, and systemic associations of ocular coloboma: a population-based study. Arch Ophthalmol 129:69–74
Gregory-Evans CY, Williams MJ, Halford S, Gregory-Evans K (2004) Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet 41:881–891
Al AS, Gregory-Evans CY, Gregory-Evans K (2019) An update on the genetics of ocular coloboma. Hum Genet 138:865–880
Onwochei BC, Simon JW, Bateman JB, Couture KC, Mir E (2000) Ocular colobomata. Surv Ophthalmol 45:175–194
Hussain RM, Abbey AM, Shah AR, Drenser KA, Trese MT, Capone A Jr (2017) Chorioretinal coloboma complications: retinal detachment and choroidal neovascular membrane. J Ophthalmic Vis Res 12:3–10
Bavbek T, Ogut MS, Kazokoglu H (1993) Congenital lens coloboma and associated pathologies. Doc Ophthalmol 83:313–322
Bron AJ, Burgess SE, Awdry PN, Oliver D, Arden G (1989) Papillo-renal syndrome. An inherited association of optic disc dysplasia and renal disease. Report and review of the literature. Ophthalmic Paediatr Genet 10:185–198
Jesberg DO, Schepens CL (1961) Retinal detachment associated with coloboma of the choroid. Arch Ophthalmol 65:163–173
Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Moriniere V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L (2012) Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat 33:457–466
Ragge NK, Subak-Sharpe ID, Collin JR (2007) A practical guide to the management of anophthalmia and microphthalmia. Eye (Lond) 21:1290–1300
Verma AS, Fitzpatrick DR (2007) Anophthalmia and microphthalmia. Orphanet J Rare Dis 2:47
Bower MA, Schimmenti LA, Eccles MR (1993) PAX2-related disorder (updated 2018). In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A (eds) GeneReviews®. University of Washington, Seattle
Fletcher J, Hu M, Berman Y, Collins F, Grigg J, McIver M, Juppner H, Alexander SI (2005) Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J Am Soc Nephrol 16:2754–2761
Melnick M, Hodes ME, Nance WE, Yune H, Sweeney A (1978) Branchio-oto-renal dysplasia and branchio-oto dysplasia: two distinct autosomal dominant disorders. Clin Genet 13:425–442
Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K (2000) GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406:419–422
Shim YS, Choi W, Hwang IT, Yang S (2015) Hypoparathyroidism, sensorineural deafness, and renal dysgenesis syndrome with a GATA3 mutation. Ann Pediatr Endocrinol Metab 20:59–63
Kim C, Cheong HI, Kim JH, Yu YS, Kwon JW (2011) Presumed atypical HDR syndrome associated with Band Keratopathy and pigmentary retinopathy. J Pediatr Ophthalmol Strabismus 48:e1-3
Blake KD, Davenport SL, Hall BD, Hefner MA, Pagon RA, Williams MS, Lin AE, Graham JM Jr (1998) CHARGE association: an update and review for the primary pediatrician. Clin Pediatr (Phila) 37:159–173
Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, Hofstra RM, van Ravenswaaij-Arts CM, Hoefsloot LH (2012) Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum Mutat 33:1149–1160
Sanlaville D, Etchevers HC, Gonzales M, Martinovic J, Clement-Ziza M, Delezoide AL, Aubry MC, Pelet A, Chemouny S, Cruaud C, Audollent S, Esculpavit C, Goudefroye G, Ozilou C, Fredouille C, Joye N, Morichon-Delvallez N, Dumez Y, Weissenbach J, Munnich A, Amiel J, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T (2006) Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. J Med Genet 43:211–217
Blake KD, Prasad C (2006) CHARGE syndrome. Orphanet J Rare Dis 1:34
Russell-Eggitt IM, Blake KD, Taylor DS, Wyse RK (1990) The eye in the CHARGE association. Br J Ophthalmol 74:421–426
Metlay LA, Smythe PS, Miller ME (1987) Familial CHARGE syndrome: clinical report with autopsy findings. Am J Med Genet 26:577–581
Nishina S, Kosaki R, Yagihashi T, Azuma N, Okamoto N, Hatsukawa Y, Kurosawa K, Yamane T, Mizuno S, Tsuzuki K, Kosaki K (2012) Ophthalmic features of CHARGE syndrome with CHD7 mutations. Am J Med Genet A 158A:514–518
McMain K, Blake K, Smith I, Johnson J, Wood E, Tremblay F, Robitaille J (2008) Ocular features of CHARGE syndrome. J AAPOS 12:460–465
Turnpenny PD, Ellard S (2012) Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet 20:251–257
Kamath BM, Podkameni G, Hutchinson AL, Leonard LD, Gerfen J, Krantz ID, Piccoli DA, Spinner NB, Loomes KM, Meyers K (2012) Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 158A:85–89
Hingorani M, Nischal KK, Davies A, Bentley C, Vivian A, Baker AJ, Mieli-Vergani G, Bird AC, Aclimandos WA (1999) Ocular abnormalities in Alagille syndrome. Ophthalmology 106:330–337
Nischal KK, Hingorani M, Bentley CR, Vivian AJ, Bird AC, Baker AJ, Mowat AP, Mieli-Vergani G, Aclimandos WA (1997) Ocular ultrasound in Alagille syndrome: a new sign. Ophthalmology 104:79–85
Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA (1999) Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology 29:822–829
Bartsch O, Schmidt S, Richter M, Morlot S, Seemanova E, Wiebe G, Rasi S (2005) DNA sequencing of CREBBP demonstrates mutations in 56% of patients with Rubinstein-Taybi syndrome (RSTS) and in another patient with incomplete RSTS. Hum Genet 117:485–493
Negri G, Milani D, Colapietro P, Forzano F, Della Monica M, Rusconi D, Consonni L, Caffi LG, Finelli P, Scarano G, Magnani C, Selicorni A, Spena S, Larizza L, Gervasini C (2015) Clinical and molecular characterization of Rubinstein-Taybi syndrome patients carrying distinct novel mutations of the EP300 gene. Clin Genet 87:148–154
Milani D, Manzoni FM, Pezzani L, Ajmone P, Gervasini C, Menni F, Esposito S (2015) Rubinstein-Taybi syndrome: clinical features, genetic basis, diagnosis, and management. Ital J Pediatr 41:4
van Genderen MM, Kinds GF, Riemslag FC, Hennekam RC (2000) Ocular features in Rubinstein-Taybi syndrome: investigation of 24 patients and review of the literature. Br J Ophthalmol 84:1177–1184
Rubinstein JH (1990) Broad thumb-hallux (Rubinstein-Taybi) syndrome 1957–1988. Am J Med Genet Suppl 6:3–16
Jacobs DJ, Sein J, Berrocal AM, Grajewski AL, Hodapp E (2012) Fluorescein angiography findings in a case of Rubinstein-Taybi syndrome. Clin Ophthalmol 6:1369–1371
Knoers N, Antignac C, Bergmann C, Dahan K, Giglio S, Heidet L, Lipska-Zietkiewicz BS, Noris M, Remuzzi G, Vargas-Poussou R, Schaefer F (2022) Genetic testing in the diagnosis of chronic kidney disease: recommendations for clinical practice. Nephrol Dial Transplant 37:239–254
Acknowledgements
We would like to thank the many patients who have participated in our studies and their referring clinicians. We would also like to particularly acknowledge the use of the Genomics England CAKUT Panel, and the OMIM, the Human Protein Atlas and the Mouse Genome Informatics databases.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Virth, J., Mack, H.G., Colville, D. et al. Ocular manifestations of congenital anomalies of the kidney and urinary tract (CAKUT). Pediatr Nephrol 39, 357–369 (2024). https://doi.org/10.1007/s00467-023-06068-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06068-9