Abstract
Background
Icodextrin has a lower absorption rate, and icodextrin peritoneal dialysate contributes to more water removal than glucose dialysate in patients with high peritoneal permeability. There are limited data on icodextrin dialysate use in children.
Methods
This study included all pediatric patients who received peritoneal equilibration tests and peritoneal dialysis with icodextrin dialysate at the study center. The factors related to ultrafiltration volume with icodextrin dialysate with long dwell time were statistically analyzed. Then the ultrafiltration volume with icodextrin and medium-concentration glucose dialysate was compared in individual cycles in the same patients.
Results
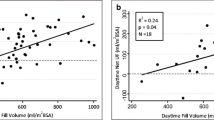
Thirty-six samples were included in the icodextrin group, and nine samples were used to compare the ultrafiltration volume with icodextrin and glucose dialysate. Dwell time, D/P-creatinine, D/D0-glucose, age, height, and weight correlated significantly with the ultrafiltration volume of icodextrin dialysate (p < 0.05). A dwell volume equal to or more than 550 mL/m2 was associated with a significantly higher ultrafiltration volume than a lower dwell volume (p = 0.039). Multiple regression analysis revealed that dwell time (p = 0.038) and height (p < 0.01) correlated with ultrafiltration volume significantly. In addition, the ultrafiltration volume was superior (p < 0.01), and dwell time was longer (p = 0.02), with icodextrin dialysate than with medium-concentration glucose dialysate.
Conclusions
The ultrafiltration volume with icodextrin dialysate decreases in patients with small stature. Providing sufficient dwell time and volume is important for maximal water removal even in children. Ultrafiltration volume is superior with icodextrin than medium-concentration glucose dialysate for long dwell times.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information




Similar content being viewed by others
Data availability
This retrospective study was based on data obtained from electronic medical records at the study center.
Code availability
IBM SPSS statistics was licensed by the study center.
References
Twardowski ZJ, Khanna R, Nolph KD (1986) Osmotic agents and ultrafiltration in peritoneal dialysis. Nephron 42:93–101. https://doi.org/10.1159/000183645
Peers E, Gokal R (1997) Icodextrin: overview of clinical experience. Perit Dial Int 17:22–26
Mistry CD, Gokal R, Peers E (1994) A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int 46:496–503. https://doi.org/10.1038/ki.1994.300
Pannekeet MM, Imholz AL, Struijk DG, Koomen GC, Langedijk MJ, Schouten N, de Waart R, Hiralall J, Krediet RT (1995) The standard peritoneal permeability analysis: a tool for the assessment of peritoneal permeability characteristics in CAPD patients. Kidney Int 48:866–875. https://doi.org/10.1038/ki.1995.363
Olszowska A, Waniewski J, Stachowska-Pietka J, Garcia-Lopez E, Lindholm B, Wańkowicz Z (2019) Long peritoneal dialysis dwells with icodextrin: Kinetics of transperitoneal fluid and polyglucose transport. Front Physiol 10:1326. https://doi.org/10.3389/fphys.2019.01326
Posthuma N, ter Wee PM, Verbrugh HA, Oe PL, Peers E, Sayers J, Donker AJ (1997) Icodextrin instead of glucose during the daytime dwell in CCPD increases ultrafiltration and 24-h dialysate creatinine clearance. Nephrol Dial Transplant 12:550–553. https://doi.org/10.1093/ndt/12.3.550
de Boer AW, Schröder CH, van Vliet R, Willems JL, Monnens LA (2000) Clinical experience with icodextrin in children: ultrafiltration profiles and metabolism. Pediatr Nephrol 15:21–24. https://doi.org/10.1007/s004670000406
Rousso S, Banh TM, Ackerman S, Piva E, Licht C, Harvey EA (2016) Impact of fill volume on ultrafiltration with icodextrin in children on chronic peritoneal dialysis. Pediatr Nephrol 31:1673–1679. https://doi.org/10.1007/s00467-016-3398-1
Paniagua R, Ventura MD, Avila-Díaz M, Hinojosa-Heredia H, Méndez-Durán A, Cueto-Manzano A, Cisneros A, Ramos A, Madonia-Juseino C, Belio-Caro F, García-Contreras F, Trinidad-Ramos P, Vázquez R, Ilabaca B, Alcántara G, Amato D (2010) NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant 25:551–557. https://doi.org/10.1093/ndt/gfp395
Geary DF, Harvey EA, MacMillan JH, Goodman Y, Scott M, Balfe JW (1992) The peritoneal equilibration test in children. Kidney Int 42:102–105. https://doi.org/10.1038/ki.1992.267
Fischbach M, Zaloszyc A, Schaefer B, Schmitt CP (2017) Should sodium removal in peritoneal dialysis be estimated from the ultrafiltration volume? Pediatr Nephrol 32:419–424. https://doi.org/10.1007/s00467-016-3378-5
Fischbach M, Terzic J, Menouer S, Haraldsson B (2000) Optimal volume prescription for children on peritoneal dialysis. Perit Dial Int 20:603–606
de Boer AW, van Schaijk TC, Willems HL, de Haan AF, Monnens LA, Schröder CH (1997) Follow-up study of peritoneal fluid kinetics in infants and children on peritoneal dialysis. Perit Dial Int 19:572–577
Dart A, Feber J, Wong H, Filler G (2005) Icodextrin re-absorption varies with age in children on automated peritoneal dialysis. Pediatr Nephrol 20:683–685. https://doi.org/10.1007/s00467-004-1783-7
Smit W, Parikova A, Struijk DG, Krediet RT (2005) The difference in causes of early and late ultrafiltration failure in peritoneal dialysis. Perit Dial Int 25(Suppl 3):S41–S45
Ha IS, Yap HK, Munarriz RL, Zambrano PH, Flynn JT, Bilge I, Szczepanska M, Lai WM, Antonio ZL, Gulati A, Hooman N, van Hoeck K, Higuita LM, Verrina E, Klaus G, Fischbach M, Riyami MA, Sahpazova E, Sander A, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network Registry (2015) Risk factors for loss of residual renal function in children treated with chronic peritoneal dialysis. Kidney Int 88:605–613. https://doi.org/10.1038/ki.2015.108
Chang TI, Ryu DR, Yoo TH, Kim HJ, Kang EW, Kim H, Chang JH, Kim DK, Moon SJ, Yoon SY, Han SH, Yonsei Associate Network Chronic Kidney Disease Trial (YACHT) investigators (2016) Effect of icodextrin solution on the preservation of residual renal function in peritoneal dialysis patients: a randomized controlled study. Med (Baltimore) 95:e2991. https://doi.org/10.1097/MD.0000000000002991
Acknowledgements
The authors thank Dr. Yoshihiko Morikawa and Dr. Emi Kawaguchi Morikawa for their assistance with the statistical analyses and Mr. James Robert Valera for his assistance with editing the manuscript.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception, data interpretation, and drafting of the manuscript, including reviewing and editing. Material preparation, methodology, data collection, and statistical analysis were performed by Naoaki Mikami and Riku Hamada. The first draft of the manuscript was written by Naoaki Mikami, and all the authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript. The corresponding author, Riku Hamada, had final responsibility for the decision to submit this manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was obtained from the Institutional Research Ethics Board of Tokyo Metropolitan Children’s Medical Center [H30b-173].
Consent to participate and consent for publication
No written informed consent was obtained from the patients or caregivers. Consent to participate was implied in the opt-out clause on the center’s website.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mikami, N., Hamada, R., Harada, R. et al. Factors related to ultrafiltration volume with icodextrin dialysate use in children. Pediatr Nephrol 38, 1267–1273 (2023). https://doi.org/10.1007/s00467-022-05720-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05720-0