Abstract
Background
There is scarce information on biopsy-verified kidney disease in childhood and its progression to chronic kidney disease stage 5 (CKD 5). This study aims to review biopsy findings in children, and to investigate risk of kidney replacement therapy (KRT).
Methods
We conducted a retrospective long-term follow-up study of children included in the Norwegian Kidney Biopsy Registry (NKBR) and in the Norwegian Renal Registry (NRR) from 1988 to 2021.
Results
In total, 575 children with a median (interquartile range, IQR) age of 10.7 (6.1 to 14.1) years were included, and median follow-up time (IQR) after kidney biopsy was 14.3 (range 8.9 to 21.6) years. The most common biopsy diagnoses were minimal change disease (MCD; n = 92), IgA vasculitis nephritis (IgAVN; n = 76), IgA nephropathy (n = 63), and focal and segmental glomerulosclerosis (FSGS; n = 47). In total, 118 (20.5%) of the biopsied children reached CKD 5, median (IQR) time to KRT 2.3 years (7 months to 8.4 years). Most frequently, nephronophthisis (NPHP; n = 16), FSGS (n = 30), IgA nephropathy (n = 9), and membranoproliferative glomerulonephritis (MPGN; n = 9) led to KRT.
Conclusions
The risk of KRT after a kidney biopsy diagnosis is highly dependent on the diagnosis. None of the children with MCD commenced KRT, while 63.8% with FSGS and 100% with NPHP reached KRT. Combining data from kidney biopsy registries with registries on KRT allows for detailed information concerning the risk for later CKD 5 after biopsy-verified kidney disease in childhood.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney biopsy is the gold standard for diagnosing kidney disease. Kidney biopsy is a safe procedure [1] that supports correct diagnosis and hence the need for prompt or preventive treatment. The core question in preventive, diagnostic, and therapeutic nephrology is how many individuals develop chronic kidney disease stage 5 (CKD 5) ultimately needing kidney replacement therapy (KRT).
Kidney biopsy registries capture most glomerulonephritis in childhood including those who proceed to KRT. In addition, a KRT registry will include congenital anomalies of the kidney and urinary tract (CAKUT) and different hereditary nephropathies including nephronophthisis (NPHP) that are responsible for 34–43% and 19–34% of pediatric KRT, respectively [2,3,4,5].
A more comprehensive overview of kidney disease outcome is achievable by linking biopsy data with data on progression to CKD 5.
The primary aim of the study was to present, based on data from a national kidney biopsy registry with the support from clinical findings and genetics, comprehensive data on biopsy-verified kidney disease in children aged 0–15 years. The secondary aim was to evaluate how many of these children, and which diagnoses, led to progressive kidney disease and need of KRT in the long term.
Subjects and methods
Registries
The Norwegian Kidney Biopsy Registry (NKBR) registers information on pathology data from native kidney biopsies and selected clinical data. Norwegian Renal Registry (NRR) is a national quality registry including patients in all age groups treated with KRT. Inclusion into the NKBR and NRR is based on informed, written consent by the patient and/or their designees. A few regional hospitals perform pediatric kidney biopsies and Oslo University Hospital performs all transplantations in Norway, which gives the registries nearly complete follow-up possibilities. NNR, but not NKBR, does not record refusal of inclusion.
All patients below 16 years of age registered in NKBR from March 1, 1988 to December 31, 2013 were included. We merged data from NKBR with information from the NRR to identify patients who received KRT until December 31, 2020. If the reports in NKBR were insufficient, we searched the journal for supplementary information regarding biopsy data and diagnosis. If the information still was lacking, the patients were excluded. All children with dialysis as the bridge to transplantation were included; the start of KRT was set at the time of starting dialysis or the day of transplantation. To assess for changes in the different diagnoses over time in NKBR, we compared an early period (1990–1994) with a late period (2009–2013).
Classifications
We grouped the kidney diagnosis according to Hou et al., modified to include inherited tubulopathies in hereditary disease [6]. Hereditary kidney disease in a childhood dataset contains various syndromes and hereditary kidney diseases (Table 1). NPHP, polycystic kidney disease, and other ciliopathies are gathered under the term renal ciliopathies [7]. Focal segmental glomerulosclerosis (FSGS) in children frequently has a genetic background, but we chose to use the traditional classification considering FSGS as a primary glomerular disease. The term IgA vasculitis nephritis (IgAVN) was preferred to Henoch Schönlein Purpura nephritis [8]. We used the term mesangioproliferative glomerulonephritis (MesPGN) in mesangioproliferative glomerulonephritis without IgA deposits and no other manifestations consistent with another kidney disease or infection. Calcineurin inhibitor (CNI) toxicity as diagnosis only reflects children with another cause for use of calcineurin inhibitor rather than kidney disease. Biopsy-proven CNI toxicity connected to treatment for primary GN like MCD was thus classified as MCD and not CNI toxicity.
Statistics
We applied SPSS Statistics 26 for statistical analysis and used descriptive statistics to describe the different diagnoses and the respective long-term outcomes. The chi-square test was used for comparison between observed results and p < 0.05 was considered statistically significant. Interquartile range (IQR) defined the difference in spread between the 25th and 75th percentiles in age at biopsy and over all follow-up time. We used Kaplan–Meier plot for survival curves. The population below 16 years in Norway between 1990 to 1994 and 2009 to 2013 was found in Statistics Norway (07,459: Population, by year and contents. Statbank Norway (ssb.no).
Ethical approval
The Regional Ethics Committee of Eastern Norway (2014/2319/REK) approved the study including approval to review medical files and if necessary, to clarify the diagnosis.
Results
We identified 586 children with a kidney biopsy of which 575 children were included, 313 (54.4%) boys. Median (IQR) age at biopsy was 10.7 (6.1 to 14.1) years, and the median (IQR) follow-up time after kidney biopsy was 14.2 (8.9 to 21.6) years.
Primary glomerular disease was the predominant category in the kidney biopsies (n = 289, 50.3%), followed by secondary systemic glomerular disease (n = 142, 24.7%). See Table 1 for a complete overview of the distribution of the different groups.
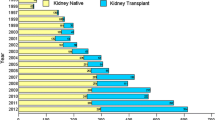
Minimal change disease (MCD) was the most common histological diagnosis (n = 92, 16.0%), followed by IgAVN (n = 76, 13.2%), IgA nephropathy (n = 63, 11%), and FSGS (n = 47, 8.2%). See Fig. 1 for a complete report. Shown in Fig. 2 are survival curves for some of the kidney diseases.
Number of kidney biopsy diagnoses in children in the time period 1988 to 2014 and number that reached KRT within 2021. ATN acute tubular necrosis, CNS congenital nephrotic syndrome, CKD 5 chronic kidney disease stage 5, FSGS focal segmental glomerulosclerosis, GN glomerulonephritis, HUS hemolytic uremic syndrome, MCD minimal change disease, MesPGN mesangioproliferative glomerulonephritis, MPGN membranoproliferative glomerulonephritis, NPHP nephronophthisis, PIGN post-infectious glomerulonephritis, TBMD thin basement membrane disease, TIN tubulointerstitial nephritis
In total, 118 children with a biopsy-confirmed diagnosis reached KRT (20.5%), 59 boys (50%), with a median (IQR) time to KRT of 2.3 (7 months to 8.4) years (Fig. 1). The risk for commencing KRT was greatest for hereditary kidney disease (44.4%) and primary glomerular disease (27.6%) (Table 1). Of the specific diagnoses, all children with NPHP reached KRT (100%) followed by FSGS (63.8%) (Table 2). Similar proportions of children with IgA nephropathy and IgAVN reached KRT: IgA nephropathy 14.5% KRT (median age 20.9 years, range 12.5–33.1 years) and IgAVN 7.9% KRT (median age at KRT 22.2 years, range 8.8–45.9 years), p = 0.21. Table 2 shows details for common biopsy diagnoses leading to KRT.
There were only 8 patients that had not commenced kidney transplantation in NRR, of which 4 had died while on dialysis, with the remaining 4 still waiting for transplantation.
The number of kidney biopsies were 1.6/100,000 per year in children below 16 years from 1990 to 1994 and 2.9/100,000 per year between 2009 and 2013, but there were no significant differences in distribution across time (primary kidney disease p = 0.68, secondary systemic glomerular disease p = 0.08, and hereditary kidney disease p = 0.4). IgAVN was the only diagnosis that increased in frequency across time. For comparison of the four most common diagnosis between 1990 to 1994 and 2009 to 2013, see Table 3. There was no major increase in number of patients reaching KRT between 1990 to 1994 and 2009 to 2013, 18 and 21 patients, respectively. Due to small numbers, it was not possible to ascertain temporal changes for time to KRT in individual diagnoses.
None of the biopsied children with MCD, post-infectious glomerulonephritis (PIGN) or tubulointerstitial nephritis (TIN) progressed to CKD 5. The median follow-up time (IQR) for patients not reaching CKD 5 was 16.2 (11.8 to 22.8) years.
By the end of 2020, 27 (4.7%) of the included patients had died, 12 of whom were in KRT (cardiac arrest and malignancy, both n = 3; infection, n = 2; stroke, gastrointestinal bleeding, accident, and refusal of further KRT, all n = 1). Due to lack of follow-up data in NKBR, we do not know the cause of death for patients not treated with KRT.
Discussion
In this retrospective study on kidney biopsy findings in children and the kidney long-term outcome, MCD, IgAVN, IgA nephropathy, and FSGS accounted for nearly 50% of the biopsy diagnoses. Several other studies have also found that these four diagnoses are highly prevalent in childhood kidney biopsy [9,10,11,12,13,14]. MCD was the most common single diagnosis with no children progressing to CKD 5, whereas NPHP was as expected the most common single diagnosis associated with KRT. Our data confirm findings from other studies that MCD is a leading histology finding in kidney biopsies in children [9, 10, 15,16,17]. Generally, children presenting with a typical clinical picture of MCD do not need a kidney biopsy [18, 19]. The risk of later KRT in MCD is negligible with symptom-based treatment, and hence, the main challenge with this “benign” disease is the disease burden and the therapeutic challenges to avoid relapses. The high appearance of MCD reflects the incidence of the disease, the tendency of frequent relapses, steroid resistance, and the problems with nephrotoxic drugs used in the treatment. Interestingly, idiopathic FSGS and MCD may represent two histological pictures of the same podocytopathy [20, 21]. FSGS is a heterogeneous disease with many underlying causes, including more than 50 genetic mutations [21]. Some of our children with FSGS subsequently were diagnosed with various genetic mutations associated with podocyte integrity and function, predisposing for a more rapid deterioration in kidney function likely due to lower response to immunosuppressive therapy [21, 22]. Unfortunately, genetic information in the patient charts was not complete, and was thus not implemented in the present analysis. Nearly 60% of our children with FSGS reached KRT, similar to other studies [2, 23].
IgA nephropathy was the second most common primary glomerulonephritis (Fig. 1). Due to the timing, this study did not implement the Oxford classification for IgA nephropathy launched in 2009 [24]. Our analysis supports the reports from several studies that boys more often than girls develop IgA nephropathy and progress to CKD 5 [25,26,27,28].
Interestingly, some see IgA nephropathy and IgAVN as different expressions of the same disease [29,30,31]. Clinical course and extrarenal manifestations are necessary to differentiate IgA nephropathy and IgAVN. In 2018, Suzuki et al. suggested a shared etiology between IgA nephropathy and IgAVN when they detected glomerular galactose-deficient IgA1 in both IgA nephropathy and IgAVN, but not in other kidney diseases [32]. High serum galactose-deficient IgA1 levels are also associated with poor prognosis in IgA nephropathy [33, 34]. Based on clinical manifestations before biopsy, IgAVN represented half of the secondary systemic glomerular diseases detected in our study (Fig. 1). There was an increase in the number of kidney biopsies performed in the IgAVN group between 1990 to 1994 and 2009 to 2013. We interpret this as an increased awareness of the crucial role of biopsies in IgAVN in order to evaluate the severity of the kidney disease and implement treatment that might reduce the risk of CKD [35]. Our study shows that despite small numbers, there are similarities between IgA nephropathy and IgAVN with respect to occurrence, numbers reaching KRT and age at KRT in children with a biopsy-verified diagnosis (Table 2). The similarity in outcomes of IgA nephropathy and IgAVN in our study may be skewed by the different indications for biopsy in the two diseases: diagnosis in the former and severity and appropriateness in the latter. The actual incidence for KRT in both IgA nephropathy and IgAVN is highly uncertain as the prevalence of both diseases are uncertain and vary across the world [25, 36,37,38,39,40].
Clinical phenotype or genetic analyses may render a kidney biopsy redundant in many cases of kidney disease, limiting the number of such children included in kidney biopsy registries [2,3,4,5]. Collecting clinical, genetic, and pathology data in the same registry may improve our understanding of chronic kidney disease in children. Additionally, reviewing histology results in the context of genetic information may be beneficial when educating patients and their parents on disease course and management, and may indeed improve patient care [41].
The main strengths of this national study are the long follow-up time regarding progression to KRT after childhood kidney biopsy, and the fact that we could link data from two national registries both with very good attendance based on the authors’ personal experiences. It was a strength that clinicians caring for the patients retrieved clinical data at their respective hospitals. Unfortunately, the retrospective study design has several limitations including information bias, missing data, and a potential selection bias of patients selected for biopsy. Limited sample size is a general challenge in pediatric kidney disease research, and a limitation in our study was the low incidence of CKD 5.
Conclusion
Histopathology in 50% of childhood kidney biopsies showed primary glomerulonephritis; nearly one third of these were MCD. Approximately 20% of the biopsied children had kidney diseases that progressed to CKD 5 during follow-up. NPHP was the most common diagnosis in patients later in need of KRT (100%) followed by FSGS (approximately 60%). Linking national biopsy registry data with national KRT registry data complements our understanding of the outcomes in kidney disease in childhood.
References
Tøndel C, Vikse BE, Bostad L, Svarstad E (2012) Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol 10:1591–1597
Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA (2007) Contributions of the transplant registry: the 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 11:366–373
Vivante A, Hildebrandt F (2016) Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12:133–146
Harambat J, van Stralen KJ, Kim JJ, Tizard EJ (2012) Epidemiology of chronic kidney disease in children. Pediatr Nephrol 27:363–373
Ingelfinger JR, Kalantar-Zadeh K, Schaefer F (2016) World Kidney Day 2016: averting the legacy of kidney disease — focus on childhood. Pediatr Nephrol 31:343–348
Hou JH, Zhu HX, Zhou ML, Le WB, Zeng CH, Liang SS et al (2018) Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis 4:10–19
Arts HH, Knoers NV (2013) Current insights into renal ciliopathies: what can genetics teach us? Pediatr Nephrol 28:863–874
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F et al (2013) 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65:1–11
Hadidi R, Hadidi M, alDabbas M (2014) Spectrum of biopsy-proven kidney disease in children at a Jordanian Hospital. Saudi J Kidney Dis Transpl 25:680–683
Lanewala A, Mubarak M, Akhter F, Aziz S, Bhatti S, Kazi JI (2009) Pattern of pediatric renal disease observed in native renal biopsies in Pakistan. J Nephrol 22:739–746
Arapović A, Vukojević K, Filipović N, Glavina Durdov M, Ljubanović-Galešić D, Saraga-Babić M et al (2020) Epidemiology of 10-year paediatric renal biopsies in the region of southern Croatia. BMC Nephrol 21:65
Coppo R, Gianoglio B, Porcellini MG, Maringhini S (1998) Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant 13:293–297
Fidan K, Isik Gonul I, Buyukkaragoz B, Isiyel E, Arinsoy T, Soylemezoglu O (2016) Changing trends in pediatric renal biopsies: analysis of pediatric renal biopsies in national nephrology registry data. Ren Fail 38:1228–1233
Lee SA, Kim MS, Kim SC, Lee D-Y (2017) Clinical and pathological findings of renal biopsy in children: outcomes from a single center over 27 years. Child Kidney Dis 21:8–14
Choi IJ, Jeong HJ, Han DS, Lee JS, Choi KH, Kang SW et al (2001) An analysis of 4,514 cases of renal biopsy in Korea. Yonsei Med J 42:247–254
Bakr A, Eid R, Sarhan A, Hammad A, El-Refaey AM, El-Mougy A et al (2014) Fifteen years of kidney biopsies in children: a single center in Egypt. Saudi J Kidney Dis Transpl 25:1321–1327
Souilmi F, Houssaini T, Alaoui H, Harmouch T, Atmani S, Hida M (2015) Indications and results of renal biopsy in children: a single-center experience from Morocco. Saudi J Kidney Dis Transpl 26:810–815
Lombel RM, Gipson DS, Hodson EM (2013) Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 28:415–426
Pasini A, Benetti E, Conti G, Ghio L, Lepore M, Massella L et al (2017) The Italian Society for Pediatric Nephrology (SINePe) consensus document on the management of nephrotic syndrome in children: part I — diagnosis and treatment of the first episode and the first relapse. Ital J Pediatr 43:41
Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF (2016) Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol 12:768–776
Kopp JB, Anders H-J, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F et al (2020) Podocytopathies. Nat Rev Dis Primers 6:1–67
Deegens JK, Wetzels JF (2011) Immunosuppressive treatment of focal segmental glomerulosclerosis: lessons from a randomized controlled trial. Kidney Int 80:798–801
Zagury A, Oliveira AL, Montalvão JA, Novaes RH, Sá VM, Moraes CA et al (2013) Steroid-resistant idiopathic nephrotic syndrome in children: long-term follow-up and risk factors for end-stage renal disease. J Bras Nefrol 35:191–199
Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J et al (2009) The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 76:546–556
Wyatt RJ, Kritchevsky SB, Woodford SY, Miller PM, Roy S, Holland NH et al (1995) IgA nephropathy: long-term prognosis for pediatric patients. J Pediatr 127:913–919
Suzuki Y, Monteiro RC, Coppo R, Suzuki H (2021) The phenotypic difference of IgA nephropathy and its race/gender-dependent molecular mechanisms. Kidney360 2:1339–1348
Hogg RJ, Silva FG, Wyatt RJ, Reisch JS, Argyle JC, Savino DA (1994) Prognostic indicators in children with IgA nephropathy—report of the Southwest Pediatric Nephrology Study Group. Pediatr Nephrol 8:15–20
Neugarten J, Acharya A, Silbiger SR (2000) Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 11:319–329
Pillebout E (2021) IgA vasculitis and IgA nephropathy: same disease? J Clin Med 10:2310
Song Y, Huang X, Yu G, Qiao J, Cheng J, Wu J et al (2021) Pathogenesis of IgA vasculitis: an up-to-date review. Front Immunol 12:771619
Meadow SR, Scott DG (1985) Berger disease: Henoch-Schönlein syndrome without the rash. J Pediatr 106:27–32
Suzuki H, Yasutake J, Makita Y, Tanbo Y, Yamasaki K, Sofue T et al (2018) IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 93:700–705
Zhao N, Hou P, Lv J, Moldoveanu Z, Li Y, Kiryluk K et al (2012) The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int 82:790–796
Wyatt RJ, Julian BA (2013) IgA nephropathy. N Engl J Med 368:2402–2414
Davin JC, Coppo R (2014) Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol 10:563–573
Ronkainen J, Ala-Houhala M, Autio-Harmainen H, Jahnukainen T, Koskimies O, Merenmies J et al (2006) Long-term outcome 19 years after childhood IgA nephritis: a retrospective cohort study. Pediatr Nephrol 21:1266–1273
Ronkainen J, Nuutinen M, Koskimies O (2002) The adult kidney 24 years after childhood Henoch-Schönlein purpura: a retrospective cohort study. Lancet 360:666–670
Samuel JP, Bell CS, Molony DA, Braun MC (2011) Long-term outcome of renal transplantation patients with Henoch-Schonlein purpura. Clin J Am Soc Nephrol 6:2034–2040
Soylemezoglu O, Ozkaya O, Ozen S, Bakkaloglu A, Dusunsel R, Peru H et al (2009) Henoch-Schönlein nephritis: a nationwide study. Nephron Clin Pract 112:c199–c204
Goldstein AR, White RH, Akuse R, Chantler C (1992) Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet 339:280–282
Murray SL, Dorman A, Benson KA, Connaughton DM, Stapleton CP, Fennelly NK et al (2020) Utility of genomic testing after renal biopsy. Am J Nephrol 51:43–53
Acknowledgements
We would like to acknowledge MD S. Aase, MD J. Aakre, and MD S. Simonsen who also contributed to this article.
Funding
Open access funding provided by University of Oslo (including Oslo University Hospital)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ann Christin Gjerstad, Rannveig Skrunes, Camilla Tøndel, Henrik Døllner, Clara Hammarstrøm, and Anna Kristina Bjerre declare no conflicts of interest. Anders Åsberg’s institution(s) received payment for lectures to Sandoz and Astellas Pharma. Sabine Leh received lecture fees from Sanofi Genzyme. Claus Klingenberg received a consulting fee from Chiesi for being a member of the Nordic Neonatal Meeting Board and received no fees but has participated in the IMNUT study in Oslo, Norway, and in the Sensyn study in the United Kingdom.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gjerstad, A.C., Skrunes, R., Tøndel, C. et al. Kidney biopsy diagnosis in childhood in the Norwegian Kidney Biopsy Registry and the long-term risk of kidney replacement therapy: a 25-year follow-up. Pediatr Nephrol 38, 1249–1256 (2023). https://doi.org/10.1007/s00467-022-05706-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05706-y