Abstract
Background
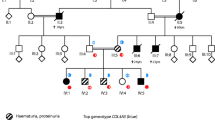
Pathogenic mutations in the non-muscle single-headed myosin, myosin 1E (Myo1e), are a rare cause of pediatric focal segmental glomerulosclerosis (FSGS). These mutations are biallelic, to date only reported as homozygous variants in consanguineous families. Myo1e regulates the actin cytoskeleton dynamics and cell adhesion, which are especially important for podocyte functions.
Methods
DNA and RNA sequencing were used to identify novel MYO1E variants associated with FSGS. We studied the effects of these variants on the localization of Myo1e in kidney sections. We then analyzed the clinical and histological observations of all known pathogenic MYO1E variants.
Results
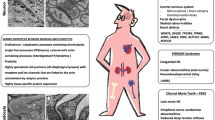
We identified a patient compound heterozygote for two novel variants in MYO1E and a patient homozygous for a deletion of exon 19. Computer modeling predicted these variants to be disruptive. In both patients, Myo1e was mislocalized. As a rule, pathogenic MYO1E variants map to the Myo1e motor and neck domain and are most often associated with steroid-resistant nephrotic syndrome in children 1–11 years of age, leading to kidney failure in 4–10 years in a subset of patients. The ultrastructural features are the podocyte damage and striking diffuse and global Alport-like glomerular basement membrane (GBM) abnormalities.
Conclusions
We hypothesize that MYO1E mutations lead to disruption of the function of podocyte contractile actin cables resulting in abnormalities of the podocytes and the GBM and dysfunction of the glomerular filtration barrier. The characteristic clinicopathological data can help to tentatively differentiate this condition from other genetic podocytopathies and Alport syndrome until genetic testing is done.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information.





Similar content being viewed by others
Data availability
No datasets were generated that would be necessary to interpret or replicate the findings in this article.
References
McConnell RE, Tyska MJ (2010) Leveraging the membrane - cytoskeleton interface with myosin-1. Trends Cell Biol 20:418–426
Krendel M, Kim SV, Willinger T, Wang T, Kashgarian M, Flavell RA, Mooseker MS (2009) Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol 20:86–94
Bi J, Chase SE, Pellenz CD, Kurihara H, Fanning AS, Krendel M (2013) Myosin 1e is a component of the glomerular slit diaphragm complex that regulates actin reorganization during cell-cell contact formation in podocytes. Am J Physiol Renal Physiol 305:F532-544
Chase SE, Encina CV, Stolzenburg LR, Tatum AH, Holzman LB, Krendel M (2012) Podocyte-specific knockout of myosin 1e disrupts glomerular filtration. Am J Physiol Renal Physiol 303:F1099-1106
Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M, PodoNet Consortium (2011) MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365:295–306
Skowron JF, Bement WM, Mooseker MS (1998) Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil Cytoskeleton 41:308–324
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Ramage IJ, Howatson AG, McColl JH, Maxwell H, Murphy AV, Beattie TJ (2002) Glomerular basement membrane thickness in children: a stereologic assessment. Kidney Int 62:895–900
Kollmar M, Durrwang U, Kliche W, Manstein DJ, Kull FJ (2002) Crystal structure of the motor domain of a class-I myosin. EMBO J 21:2517–2525
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
van der Velde KJ, Dhekne HS, Swertz MA, Sirigu S, Ropars V, Vinke PC, Rengaw T, van den Akker PC, Rings EH, Houdusse A, van Ijzendoorn SC (2013) An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum Mutat 34:1597–1605
Sanna-Cherchi S, Burgess KE, Nees SN, Caridi G, Weng PL, Dagnino M, Bodria M, Carrea A, Allegretta MA, Kim HR, Perry BJ, Gigante M, Clark LN, Kisselev S, Cusi D, Gesualdo L, Allegri L, Scolari F, D’Agati V, Shapiro LS, Pecoraro C, Palomero T, Ghiggeri GM, Gharavi AG (2011) Exome sequencing identified MYO1E and NEIL1 as candidate genes for human autosomal recessive steroid-resistant nephrotic syndrome. Kidney Int 80:389–396
Al-Hamed MH, Al-Sabban E, Al-Mojalli H, Al-Harbi N, Faqeih E, Al Shaya H, Alhasan K, Al-Hissi S, Rajab M, Edwards N, Al-Abbad A, Al-Hassoun I, Sayer JA, Meyer BF (2013) A molecular genetic analysis of childhood nephrotic syndrome in a cohort of Saudi Arabian families. J Hum Genet 58:480–489
Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, VegaWarner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Group SS, Hildebrandt F (2015) A single-gene cause in 295% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26:1279–1289
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Laboratory Quality Assurance Committee ACMG (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Gubler M, Levy M, Broyer M, Naizot C, Gonzales G, Perrin D, Habib R (1981) Alport’s syndrome. A report of 58 cases and a review of the literature. Am J Med 70:493–505
Cosgrove D, Liu S (2017) Collagen IV diseases: a focus on the glomerular basement membrane in Alport syndrome. Matrix Biol 57–58:45–54
Cangiotti AM, Sessa A, Meroni M, Montironi R, Ragaiolo M, Mambelli V, Cinti S (1996) Evolution of glomerular basement membrane lesions in a male patient with Alport syndrome: ultrastructural and morphometric study. Nephrol Dial Transplant 11:1829–1834
Savige J, Storey H, Il Cheong H, Gyung Kang H, Park E, Hilbert P, Persikov A, Torres-Fernandez C, Ars E, Torra R, Hertz JM, Thomassen M, Shagam L, Wang D, Wang Y, Flinter F, Nagel M (2016) X-Linked and autosomal recessive Alport syndrome: pathogenic variant features and further genotype-phenotype correlations. PLoS One 11:e0161802
Moxey-Mims MM, Young G, Silverman A, Selby DM, White JG, Kher KK (1999) End-stage renal disease in two pediatric patients with Fechtner syndrome. Pediatr Nephrol 13:782–786
Kopp JB (2010) Glomerular pathology in autosomal dominant MYH9 spectrum disorders: what are the clues telling us about disease mechanism? Kidney Int 78:130–133
Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R (1996) Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122:3537–3547
Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S (2008) Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol 173:927–937
Francis A, Burke J, Francis L, McTaggart S, Mallett A (2016) Polypoid change of the glomerular basement membrane in a child with steroid resistant nephrotic syndrome and ARHGAP24 mutation: a case report. Open Urol Nephrol J 9:88–93
Ohtsubo H, Morisada N, Kaito H, Nagatani K, Nakanishi K, Iijima K (2012) Alport-like glomerular basement membrane changes with renal-coloboma syndrome. Pediatr Nephrol 27:1189–1192
Ito S, Hataya H, Ikeda M, Takata A, Kikuchi H, Hata J, Morikawa Y, Kawamura S, Honda M (2003) Alport syndrome-like basement membrane changes in Frasier syndrome: an electron microscopy study. Am J Kidney Dis 41:1110–1115
Barger SR, Reilly NS, Shutova MS, Li Q, Maiuri P, Heddleston JM, Mooseker MS, Flavell RA, Svitkina T, Oakes PW, Krendel M, Gauthier NC (2019) Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat Commun 10:1249
Heim JB, Squirewell EJ, Neu A, Zocher G, Sominidi-Damodaran S, Wyles SP, Nikolova E, Behrendt N, Saunte DM, Lock-Andersen J, Gaonkar KS, Yan H, Sarkaria JN, Krendel M, van Deursen J, Sprangers R, Stehle T, Bottcher RT, Lee JH, Ordog T, Meves A (2017) Myosin-1E interacts with FAK proline-rich region 1 to induce fibronectin-type matrix. Proc Natl Acad Sci U S A 114:3933–3938
Gupta P, Gauthier NC, Cheng-Han Y, Zuanning Y, Pontes B, Ohmstede M, Martin R, Knolker HJ, Dobereiner HG, Krendel M, Sheetz M (2013) Myosin 1E localizes to actin polymerization sites in lamellipodia, affecting actin dynamics and adhesion formation. Biol Open 2:1288–1299
Mao J, Wang D, Mataleena P, He B, Niu D, Katayama K, Xu X, Ojala JR, Wang W, Shu Q, Du L, Liu A, Pikkarainen T, Patrakka J, Tryggvason K (2013) Myo1e impairment results in actin reorganization, podocyte dysfunction, and proteinuria in zebrafish and cultured podocytes. PLoS One 8:e72750
Suleiman HY, Roth R, Jain S, Heuser JE, Shaw AS, Miner JH (2017) Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy. JCI Insight 2:e94137
Lennon R, Stuart HM, Bierzynska A, Randles MJ, Kerr B, Hillman KA, Batra G, Campbell J, Storey H, Flinter FA, Koziell A, Welsh GI, Saleem MA, Webb NJ, Woolf AS (2015) Coinheritance of COL4A5 and MYO1E mutations accentuate the severity of kidney disease. Pediatr Nephrol 30:1459–1465
Acknowledgements
We are grateful to Bjørn Westre, Department of Pathology, Ålesund Hospital, for contributing the first kidney biopsy of patient 2. We are indebted to the late Torunn Fiskerstrand from the Department of Medical Genetics, Haukeland University Hospital, Bergen. Without her, patient 2 would never have been correctly diagnosed. We thank Marie Claire Gubler, Laboratoire d’Anatomie Pathologique, Hopital Necker – Enfants Malades, Paris, for conducting the immunofluorescence investigation of a skin biopsy from patient 2 for collagen IV α chains. We thank Dmitry Lyalin, PhD, co-director of Cytogenetics/Molecular Genetics Laboratory, AHWFBMC, and Alicia Byrne, PhD, Clinical Genome Resource, Broad Institute of MIT and Harvard, for their advice and expert help with variant classification.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH under Award R01DK083345 to M.K. and by a pilot research award from the AHWFBMC Department of Pathology to A.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Approved by the Atrium Health Wake Forest Baptist Medical Center Institutional Review Board, ID IRB00063833.
Consent to participate
Not required according to IRB; however, informed consent obtained from the patient’s parents or the patient himself (patient 2).
Consent for publication
Not required according to IRB. Consent for publication is given by patient 2.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Krendel, M., Leh, S., Garone, M.E. et al. Focal segmental glomerulosclerosis and proteinuria associated with Myo1E mutations: novel variants and histological phenotype analysis. Pediatr Nephrol 38, 439–449 (2023). https://doi.org/10.1007/s00467-022-05634-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05634-x