Abstract
Background
Various definitions used to describe cisplatin nephrotoxicity potentially lead to differences in determination of risk factors. This study evaluated incidence of kidney injury according to commonly used and alternative definitions in two cohorts of children who received cisplatin.
Methods
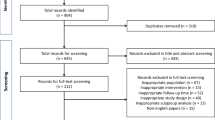
This retrospective cohort study included children from Vancouver, Canada (one center), and Mexico City, Mexico (two centers), treated with cisplatin for a variety of solid tumors. Serum creatinine–based definitions (KDIGO and Pediatric RIFLE (pRIFLE)), electrolyte abnormalities consisted of hypokalemia, hypophosphatemia and hypomagnesemia (based on NCI-CTCAE v5), and an alternative definition (Alt-AKI) were used to describe nephrotoxicity. Incidence with different definitions, definitional overlap, and inter-definition reliability was analyzed.
Results
In total, 173 children (100 from Vancouver, 73 from Mexico) were included. In the combined cohort, Alt-AKI criteria detected more patients with cisplatin nephrotoxicity compared to pRIFLE and KDIGO criteria (82.7 vs. 63.6 vs. 44.5%, respectively). Nephrotoxicity and all electrolyte abnormalities were significantly more common in Vancouver cohort than in Mexico City cohort except when using KDIGO definition. The most common electrolyte abnormalities were hypomagnesemia (88.9%, Vancouver) and hypophosphatemia (24.2%, Mexico City). The KDIGO definition provided highest overlap of cases in Vancouver (100%), Mexico (98.6%), and the combined cohort (99.4%). Moderate overall agreement was found among Alt-AKI, KDIGO, and pRIFLE definitions (κ = 0.18, 95% CI 0.1–0.27) in which KDIGO and pRIFLE showed moderate agreement (κ = 0.48, 95% CI 0.36–0.60).
Conclusions
Compared to pRIFLE and KDIGO criteria, Alt-AKI criteria detected more patients with cisplatin nephrotoxicity. pRIFLE is more sensitive to detect not only actual kidney injury but also patients at risk of cisplatin nephrotoxicity, while KDIGO seems more useful to detect clinically significant kidney injury.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ruggiero A, Rizzo D, Trombatore G, Maurizi P, Riccardi R (2016) The ability of mannitol to decrease cisplatin-induced nephrotoxicity in children: real or not? Cancer Chemother Pharmacol 77:19–26
O’Leary M, Krailo M, Anderson JR, Reaman GH, Children’s Oncology Group (2008) Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol 35:484–493
Barton CD, Pizer B, Jones C, Oni L, Pirmohamed M, Hawcutt DB (2018) Identifying cisplatin-induced kidney damage in paediatric oncology patients. Pediatr Nephrol 33:1467–1474
Wensing KU, Ciarimboli G (2013) Saving ears and kidneys from cisplatin. Anticancer Res 33:4183–4188
Khrunin AV, Moisseev A, Gorbunova V, Limborska S (2010) Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J 10:54–61
Jones DP, Spunt SL, Green D, Springate JE, Children’s Oncology Group (2008) Renal late effects in patients treated for cancer in childhood: a report from the Children’s Oncology Group. Pediatr Blood Cancer 51:724–731
Knijnenburg SL, Mulder RL, Schouten-Van Meeteren AY, Bokenkamp A, Blufpand H, van Dulmen-den Broeder E, Veening MA, Kremer LC, Jaspers MW (2013) Early and late renal adverse effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev:CD008944
Skinner R (2018) Late renal toxicity of treatment for childhood malignancy: risk factors, long-term outcomes, and surveillance. Pediatr Nephrol 33:215–225
Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, Chakaravarthi R, Holden AL, Goldstein SL (2015) Phenotype standardization for drug-induced kidney disease. Kidney Int 88:226–234
Lajer H, Daugaard G (1999) Cisplatin and hypomagnesemia. Cancer Treat Rev 25:47–58
Oronsky B, Caroen S, Oronsky A, Dobalian VE, Oronsky N, Lybeck M, Reid TR, Carter CA (2017) Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol 80:895–907
Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI (2011) An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol 41:803–821
Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, Sleijfer DT (2000) Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 355:1075–1076
Kooijmans EC, Bokenkamp A, Tjahjadi NS, Tettero JM, van Dulmen-den Broeder E, van der Pal HJ, Veening MA (2019) Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev 3:CD008944
McMahon KR, Harel-Sterling M, Pizzi M, Huynh L, Hessey E, Zappitelli M (2018) Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: a pilot study. Pediatr Nephrol 33:2311–2320
McMahon KR, Rassekh SR, Schultz KR, Blydt-Hansen T, Cuvelier GDE, Mammen C, Pinsk M, Carleton BC, Tsuyuki RT, Ross CJD, Palijan A, Huynh L, Yordanova M, Crepeau-Hubert F, Wang S, Boyko D, Zappitelli M, Applying Biomarkers to Minimize Long-term Effects of Childhood/Adolescent Cancer Treatment Research Study Group (2020) Epidemiologic characteristics of acute kidney injury during cisplatin infusions in children treated for cancer. JAMA Netw Open 3:e203639
Jimenez-Triana CA, Castelan-Martinez OD, Rivas-Ruiz R, Jimenez-Mendez R, Medina A, Clark P, Rassekh R, Castaneda-Hernandez G, Carleton B, Medeiros M, Canadian Pharmacogenomics Network for Drug Safety Consortium (2015) Cisplatin nephrotoxicity and longitudinal growth in children with solid tumors: a retrospective cohort study. Medicine (Baltimore) 94:e1413
Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD (2009) Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: relevance of age and dose as risk factors. Eur J Cancer 45:3213–3219
Carleton B, Poole R, Smith M, Leeder J, Ghannadan R, Ross C, Phillips M, Hayden M (2009) Adverse drug reaction active surveillance: developing a national network in Canada’s children’s hospitals. Pharmacoepidemiol Drug Saf 18:713–721
Tanoshima R, Khan A, Biala AK, Trueman JN, Drogemoller BI, Wright GEB, Hasbullah JS, Groeneweg GSS, Ross CJD, Carleton BC, Canadian Pharmacogenomics Network for Drug Safety Consortium (2019) Analyses of adverse drug reactions-nationwide active surveillance network: Canadian pharmacogenomics network for drug safety database. J Clin Pharmacol 59:356–363
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
KDIGO AKI Working Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
National Institutes of Health, National Cancer Institute (2017) Common Terminology Criteria for Adverse Events (CTCAE) v5.0. U.S. Department of Health and Human Services
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, Goldstein SL (2015) AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10:554–561
Nahum E, Kadmon G, Kaplan E, Weissbach A, Hijazi H, Haskin O, Mozer-Glassberg Y (2019) Prevalence of acute kidney injury after liver transplantation in children: comparison of the pRIFLE, AKIN, and KDIGO criteria using corrected serum creatinine. J Crit Care 50:275–279
(2013) Chapter 1: definition and classification of CKD. Kidney Int Suppl 3:19–62
Zazuli Z, Kos R, Veltman JD, Uyterlinde W, Longo C, Baas P, Masereeuw R, Vijverberg SJH, Maitland-van der Zee AH (2020) Comparison of myelotoxicity and nephrotoxicity between daily low-dose cisplatin with concurrent radiation and cyclic high-dose cisplatin in non-small cell lung cancer patients. Front Pharmacol 11:975
Moke DJ, Luo C, Millstein J, Knight KR, Rassekh SR, Brooks B, Ross CJD, Wright M, Mena V, Rushing T, Esbenshade AJ, Carleton BC, Orgel E (2021) Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study. Lancet Child Adolesc Health 5:274–283
Tzvetkov MV, Behrens G, O’Brien VP, Hohloch K, Brockmoller J, Benohr P (2011) Pharmacogenetic analyses of cisplatin-induced nephrotoxicity indicate a renoprotective effect of ERCC1 polymorphisms. Pharmacogenomics 12:1417–1427
Windsor RE, Strauss SJ, Kallis C, Wood NE, Whelan JS (2012) Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: a pilot study. Cancer 118:1856–1867
Powrozek T, Mlak R, Krawczyk P, Homa I, Ciesielka M, Koziol P, Prendecka M, Milanowski J, Malecka-Massalska T (2016) The relationship between polymorphisms of genes regulating DNA repair or cell division and the toxicity of platinum and vinorelbine chemotherapy in advanced NSCLC patients. Clin Transl Oncol 18:125–131
Iwata K, Aizawa K, Kamitsu S, Jingami S, Fukunaga E, Yoshida M, Yoshimura M, Hamada A, Saito H (2012) Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin Exp Nephrol 16:843–851
Zhang L, Gao G, Li X, Ren S, Li A, Xu J, Zhang J, Zhou C (2012) Association between single nucleotide polymorphisms (SNPs) and toxicity of advanced non-small-cell lung cancer patients treated with chemotherapy. PLoS One 7:e48350
Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A (2009) Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86:396–402
Zazuli Z, Otten LS, Drogemoller BI, Medeiros M, Monzon JG, Wright GEB, Kollmannsberger CK, Bedard PL, Chen Z, Gelmon KA, McGoldrick N, Kitchlu A, Vijverberg SJH, Masereeuw R, Ross CJD, Liu G, Carleton BC, Maitland-van der Zee AH (2019) Outcome definition influences the relationship between genetic polymorphisms of ERCC1, ERCC2, SLC22A2 and cisplatin nephrotoxicity in adult testicular cancer patients. Genes (Basel) 10:364
Yanagisawa R, Kubota N, Hidaka E, Sakashita K, Tanaka M, Nakazawa Y, Nakamura T (2018) Cisplatin-induced nephrotoxicity in patients with advanced neuroblastoma. Pediatr Blood Cancer 65:e27253
Zazuli Z, Vijverberg S, Slob E, Liu G, Carleton B, Veltman J, Baas P, Masereeuw R, Maitland-van der Zee AH (2018) Genetic variations and cisplatin nephrotoxicity: a systematic review. Front Pharmacol 9:1111
(2020) ALFA Allele Frequency - rs316019. National Center for Biotechnology Information, National Library of Medicine
Griffin BR, Faubel S, Edelstein CL (2019) Biomarkers of drug-induced kidney toxicity. Ther Drug Monit 41:213–226
Zazuli Z, Duin N, Jansen K, Vijverberg SJH, Maitland-van der Zee AH, Masereeuw R (2020) The impact of genetic polymorphisms in organic cation transporters on renal drug disposition. Int J Mol Sci 21:6627
Alaini A, Malhotra D, Rondon-Berrios H, Argyropoulos CP, Khitan ZJ, Raj DSC, Rohrscheib M, Shapiro JI, Tzamaloukas AH (2017) Establishing the presence or absence of chronic kidney disease: uses and limitations of formulas estimating the glomerular filtration rate. World J Methodol 7:73–92
Solanki MH, Chatterjee PK, Gupta M, Xue X, Plagov A, Metz MH, Mintz R, Singhal PC, Metz CN (2014) Magnesium protects against cisplatin-induced acute kidney injury by regulating platinum accumulation. Am J Physiol Renal Physiol 307:F369-384
Motwani SS, Curhan GC (2020) Cisplatin-associated nephrotoxic effects in children. JAMA Netw Open 3:e203612
Cool WP, Grimer RJ, Carter SR, Tillman RM, Davies AM (1998) Longitudinal growth following treatment for osteosarcoma. Sarcoma 2:115–119
Acknowledgements
We gratefully acknowledge the participation of all patients and families who took part in this study. We also acknowledge the contributions of the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) Consortium and Mexican Cooperative Oncology Network (MexiCON).
Funding
This research was funded by Indonesia Endowment Fund for Education (LPDP) Ministry of Finance, the Republic of Indonesia (as a part of ZZ’s Ph.D. project, grant no. 20161022049506). The APC was funded by Indonesia Endowment Fund for Education (LPDP). The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
Conceptualization: ZZ, SV, AHM, and BC; methodology: ZZ, SV, AHM, and BC; validation: ZZ and COH; formal analysis: ZZ and COH; investigation: ZZ and COH; resources: BC and MM; data curation: SRR, MM, RR, and BC; writing—original draft preparation: ZZ; writing—review and editing: ZZ, COH, SV, RM, SRR, MM, RR, AHM, and BC; visualization: ZZ; supervision: SV, RM, AHM, and BC; project administration: ZZ and COH; funding acquisition: ZZ, BC, and MM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zazuli, Z., Op ’t Hoog, C.J.P., Vijverberg, S.J.H. et al. Cisplatin-induced nephrotoxicity in childhood cancer: comparison between two countries. Pediatr Nephrol 38, 593–604 (2023). https://doi.org/10.1007/s00467-022-05632-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05632-z