Abstract
Learning health systems (LHS) align science, informatics, incentives, and culture for continuous improvement and innovation. In this organizational system, best practices are seamlessly embedded in the delivery process, and new knowledge is captured as an integral byproduct of the care delivery experience aimed to transform clinical practice and improve patient outcomes. The objective of this review is to describe how building better health systems that integrate clinical care, improvement, and research as part of an LHS can improve care within pediatric nephrology. This review will provide real-world examples of how this system can be established in a single center and across multiple centers as learning health networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the constant expansion of electronic health records (EHRs), the amount of clinical data available is staggering. Ideally, the clinician can access the right information for the right patient at the right time, and patients are empowered to partner with clinicians in their care. Edward Wagner and colleagues described an ideal process in the Chronic Care Model with the community and health system working together so that a prepared and proactive practice team has productive interactions with an informed and activated patient [1, 2]. This is particularly relevant in pediatric chronic disease management.
In 2007, the National Academy of Medicine (formerly the Institute of Medicine) first described the learning health system (LHS)—a system in which science, informatics, incentives, and culture are aligned for continuous improvement and innovation, with best practices seamlessly embedded in the delivery process and new knowledge captured as an integral byproduct of the delivery experience [3]. In the report Best Care at Lower Cost: The Path to Continuously Learning Health Care in America [4], the National Academy of Medicine describes a continuous LHS as comprising several key characteristics:
-
Real-time access to knowledge—the system continuously and reliably captures, curates, and delivers the best available evidence to guide, support, tailor, and improve clinical decision-making and care safety and quality.
-
Digital capture of the care experience—the system captures data from the care experience for real-time generation and application of knowledge for care improvement.
-
Engaged, empowered patients—the system is anchored on patient needs and perspectives and promotes the inclusion of patients, families, and other caregivers as vital members of the team.
-
Incentives aligned for value—the system incentivizes continuous improvement, identifies and reduces waste, and rewards high-value care.
-
Full transparency—the system monitors the safety, quality, processes, prices, costs, and outcomes of care, and makes this information available for care improvement and informed choices and decision-making by clinicians, patients, and their families.
-
Leadership-instilled culture of learning—the system is stewarded by leadership committed to a culture of teamwork, collaboration, and adaptability in support of continuous learning as a core aim.
-
Supportive system competencies—the system constantly refines complex care operations and processes through ongoing team training and skill building, systems analysis and information development, and creation of the feedback loops for continuous learning and system improvement.
One key feature of data collection in the LHS framework that distinguishes it from traditional models of data collection for research is the purpose for a robust data collection system. The data collected in an LHS enables learning cycles that occur at various speeds and levels of scale to transform this data into knowledge and apply knowledge to improve clinical performance and outcomes. It can take years and even decades to go through the traditional research system of grant preparation, award, planning, performing the research, data analysis, publication of results, and implementation trials to understand how to spread the new knowledge. The LHS model strives to be nimbler—aiming to identify and address areas of concern in “real-time” through frequent assessment and continuous process improvement.
How might the LHS approach benefit pediatric nephrology? Consider one of the most common conditions the nephrologist cares for: chronic kidney disease (CKD). The Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines for the Evaluation and Management of CKD is a 163-page document that includes a litany of recommendations [5]. During a routine follow-up visit for CKD care, the nephrologist must evaluate cardiovascular health (blood pressure control, last 24-h ABPM, last echocardiogram), bone health (calcium, phosphorous, hyperparathyroidism), acid–base status, appropriate growth and nutrition, and infectious complications. Assessing and responding to any of these problems individually may seem straightforward but treating them all simultaneously creates a complexity of clinical care that the current healthcare system does not support. Indeed, despite many effective therapies, 30% of patients have either uncontrolled casual prehypertension or uncontrolled hypertension [6]. In a multicenter study of pediatric transplant centers, six cardiovascular risk factors recommended by the Kidney Disease Outcomes Quality Initiative were assessed only 57% of the time, and only 10% of patients had all six documented in the patient’s chart [7]. Thus, one of the greatest challenges to improving long-term outcomes for children with kidney disease is not a lack of effective medical therapies, but rather the lack of effective healthcare delivery systems to monitor the patient condition in real time and implement effective therapies reliably in clinical practice.
The objective of this review is to describe how building better health systems that integrate clinical care, improvement, and research as part of an LHS could improve care within pediatric nephrology. This review will describe general strategies and provide examples for putting this framework into practice both within a single practice and across practices through a learning health network (LHN). Since pediatric nephrology is a largely academic pursuit and most full-time positions are part of an academic practice [8], this model for the integration of clinical practice and research applies to every nephrologist.
Building a learning health system
Focus on what matters most
The goal of the LHS is to achieve better health by focusing on the decisions that matter most to all stake holders including patients and caregivers, clinicians, researchers, and administrators by integrating the activities of clinical care, quality improvement, operations, and research. This is done by building a community with a shared understanding of and commitment to rigorously applying a theory for change and proven methodology (e.g., the chronic care model [1, 2], the model for improvement [9], research methods [10], etc.), by leveraging technology to support clinical operations, and by developing strategies to measure impact and identify research questions. These elements together allow stakeholders within a center or across centers to apply a continuous learning system to change systems of care resulting in better health.
Measuring impact
To track improvement over time, it is necessary to define relevant measures for the patient population. Measures should be centered on improved patient outcomes and determined using evidence-based guidelines and available research. Data used to calculate measures come directly from the interaction between patients/families and the clinician at the point of care, where a large amount of data is recorded both in structured (i.e., discrete; specifically entered to be organized and accessed at another time) and unstructured (i.e., narrative, text; not entered in such a way to be easily retrieved) formats. Data recorded in unstructured formats must be converted into structured data before it can be utilized for reporting. Once key outcomes have been identified and operationally defined, processes that affect this outcome can be identified, measured, and tested under the hypothesis that this process will affect the given clinical outcome [9, 11]. For example, if the outcome of interest is to improve blood pressure control, one hypothesis could be that the clinic team must appropriately measure and classify the patient’s blood pressure, providing the physician with accurate information for medical decision-making. Since systems of care do not exist in isolation and any change can have unintended consequences, balancing measures should also be identified [11]. In this example, documenting how long it takes to check a patient in after changing how blood pressure is measured would be a possible balancing measure to determine how the new intervention affects patient flow.
Figure 1 is an example of a model developed by our team in 2011 to guide outcomes improvement work for patients with a kidney transplant. It defines clinical outcome measures for kidney transplant patients and the specific processes in place that affect these clinical outcomes, which are themselves affected by processes that can be measured and reported. Continuing with the blood pressure example, consider what processes drive this outcome. There are three process measures the team hypothesized would affect this outcome: measurement and documentation—is the blood pressure being measured and documented in the EHR; assessment—are clinicians appropriately reviewing the blood pressure and documenting the classification of the blood pressure during the visit; treatment—is the patient being treated with either diet/exercise, antihypertensive medication, or is their antihypertensive medication adherence being addressed. These processes are all tracked and reported back to the transplant team. The hypothesis is that if each of the described processes is happening reliably, then the number of patients with controlled blood pressure will increase.
Clinical outcome measures with process measures that affect the outcome and the foundational care processes in place that support these processes. In this example, “hypertension” is the clinical outcome of interest. Each clinical outcome measure will have an associated operational definition that details what the goal is. Each outcome will have associated process measures; in this example, for appropriate hypertension management, blood pressure needs to be measured and documented appropriately, classified and assessed, and treated (if necessary). Each of these processes have subprocesses. For example, the clinician attempts to classify and assess the blood pressure (correctly or incorrectly). This figure is simplified to show only the process measures for “hypertension,” but process measures exist for each outcome measure
For each outcome, process, or balancing measure, it is important to identify the appropriate analysis to detect improvement over time. This may be a run chart [12] or any number of statistical process control charts [11, 13]. A run chart is a graphical display of data plotted over some type of order—most often over time—but could also include sequential patients, visits, or procedures [12]. While run charts are used to study variation over time to understand the impact of changes on a given measure, statistical process control charts are used to distinguish between common cause (causes inherent in the system of interest) and special cause (causes not part of the system of interest) variation [11]. The chart should be selected based on the type of data being collected, the frequency of events, and the desired sensitivity to detect change. Once the appropriate chart is selected, the measure should be operationally defined, and a data collection strategy should be specified. Ideally, data capture should be as seamless as possible and embedded in usual clinical care [11]. This may require figuring out how to structure unstructured data. For instance, the EHR can be configured to allow data from biopsies or ambulatory blood pressure monitors to be entered as structured data elements rather than uploaded as scanned documents. Another example is integrating “outside labs” from external laboratories into the institution’s EHR—which is especially relevant for pediatric nephrologists due to the large regional catchment areas served by any given nephrology program. The upfront resources and effort required to develop systems to input data as structured rather than unstructured elements provide long-term benefits of efficiency as these data can then automatically populate clinical notes, letters, and other communications in addition to being extracted for analysis.
When determining the outcomes of interest for an LHS registry, it is important to address priorities of all stakeholders. For instance, in order for an LHS to be successful it must be designed to assist clinicians in accomplishing their purpose of providing the best comprehensive care to patients, rather than detracting from it with a large burden of data entry and extra work. In like manner the interests of patients/families, administrators, and researchers must be addressed when prioritizing what data is worth capturing and working to improve. While patient satisfaction scores may not be as helpful for researchers, they tend to be valuable for administrators and patients. Quality of life, while challenging to measure, is typically one of the most important factors for patients and families. Given the amount of time and expertise that is needed to create meaningful data systems with high-quality, valid data, ensuring that all stakeholders are represented can create “buy-in” at all levels and align the various stakeholders to a common vision of continuous process improvement.
Integrating activities with a structured theory
Improvement work is supported by a foundation of processes described in the Chronic Care Model [1, 2]—care coordination, pre-visit planning, population management, and patient/family self-management support—interventions necessary for improvement work to be successful and sustainable in any context. Care coordination aims to achieve high-quality specialty care and successful transitions by ensuring care is timely, safe, effective, patient-centered, efficient, and equitable [14]. Pre-visit planning enables the patient and clinician to conduct the face-to-face visit more effectively by gathering and organizing information ahead of time so they can devote more attention during the visit to interpreting, discussing, and responding to this information together with the patient [15]. Population management allows a practice or healthcare system to identify patients between visits who are not meeting clinical goals or are at risk for clinical decline and to apply the necessary resources to address this gap in care and prevent adverse clinical outcomes [16]. Self-management is the interaction of health behaviors and related processes that patients and families engage in to care for their chronic condition [17].
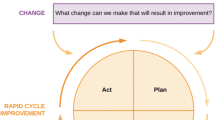
The model for improvement provides a framework for developing, testing, and implementing change using three fundamental questions: (1) What are we trying to accomplish? (2) How will we know that a change is an improvement? (3) What changes can we make that will result in an improvement [9]? Once the team has identified the outcome in need of improvement, a driver diagram should be created to display the theory for improvement by identifying drivers of the outcome and interventions thought to affect the drivers [9]. An example of a driver diagram that guided our work to decrease rejection episodes in our population is displayed in Fig. 2. Often the outcome of interest is the primary outcome measure and process measures are developed for each key driver. Plan-Do-Study-Act (PDSA) cycles [9] or planned experimentation [10] are then used to test interventions at the point of care for their impact on key drivers (process measures) and the clinical outcome (outcome measures). It is essential that a rigorous process is applied to make sure a measurement system is in place prior to initiating interventions, such that the team can answer whether a change is resulting in improvement.
Applying the continuous learning system to change systems of care
Creating an infrastructure to manage, learn from, and present the outcomes and measures in a meaningful way to clinicians, researchers, patients, and families is a foundational step of the LHS. One way to conceptualize this includes an afferent or “sensing” arm that collects patient data from the clinical setting, a data processing unit that analyzes and processes these data, and an efferent or “effector” arm that is the execution and application of the knowledge generated and applied to the right patient, in the right setting, at the right time (Fig. 3). The afferent arm collects the outcome, process, and balancing measures and other relevant clinical data identified by the team.
The foundation of the data processing unit is the patient registry. Once in the registry, applications can process the data and provide several outputs, including (1) pre-visit planning and population management reports that provide clinical decision support and identify gaps in care for individual patients and the population [18], and (2) quality and outcome reports that identify trends in care and outcomes for individual patients or the population over time. These reports both facilitate clinical care and allow a center to understand how their clinical practice and quality improvement efforts are affecting their processes and outcomes. The registry database can also support the research operations of a practice by (1) identifying patients eligible for research studies based on study selection criteria and (2) generating high quality data for observational and comparative effectiveness research. The efferent arm is the application of this new knowledge back to the point of care. This is done by applying the principles and strategies previously described by the chronic care model (i.e., pre-visit planning and population management) and model for improvement. To keep the LHS constantly progressing, the team requires structure and discipline to regularly review the data that is being collected and applying frequent tests of change on the system of care to achieve continuous improvement.
Creating the learning health system—a case study
Our kidney transplant team developed an LHS to monitor the kidney transplant population according to 7 health domains—kidney function, chronic kidney disease, cardiovascular health, rejection, growth, infection, and malignancy—according to pass/fail criteria based on KDIGO and Transplantation Society guidelines [5, 19] (Fig. 4). Using these domains, the EHR was configured to capture necessary data in structured formats to automatically populate a population level outcomes dashboard to follow the outcomes of interest and identify areas in need of improvement (Fig. 5). Figure 5 graphically displays each outcome measure over time (x-axis) and the percentage of the population that has achieved the desired state for this outcome measure (y-axis). A real-world example using this data for improvement is our work on cholesterol screening and treatment according to published guidelines. Different lab monitoring guidelines were created for patients based on dyslipidemia risk. Using the data from the EHR, a regular reporting and feedback structure to the transplant team, and quality improvement methods over a 4-year period, the percent of cholesterol levels checked according to guidelines increased from 84 to 95%, the number of dyslipidemia patients on statin therapy increased from 52 to 88%, and patients with an LDL < 130 mg/dL increased from 65 to 83% [20]. This improvement in the population LDL cholesterol was done without any new medications or therapies; rather, the existing guidelines and recommendations were applied reliably in clinical practice. A similar approach was used to develop a Medication Adherence Promotion System to reduce rejection in our population [21]. This system identifies patients at risk for rejection [22] by systematically assessing for and addressing barriers to immunosuppression medication adherence [23] and has increased the number of patients rejection-free for the prior year from 80 to > 90% [21]. Table 1 summarizes the published measures and improvement of these two initiatives. A similar approach has led to improvement in the percentage of patients with normal hemoglobin, proteinuria, and vitamin D (Fig. 5).
Composite Ideal Outcome for kidney transplant patients. This figure illustrates one way to map the ideal outcome for a transplant patient. This composite outcome is comprised of relevant health domains (shaded boxes) with the measure(s) associated with each domain and future states (dashed line boxes). Abbreviations: CKD, chronic kidney disease; Ca, calcium; CO2, bicarbonate; Hgb, hemoglobin; PO4, phosphorus; UPC, urine protein/creatinine ratio; GFR, glomerular filtration rate; BKV, BK virus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; UTI, urinary tract infection; BMI, body mass index; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol
Example of population level clinical outcomes for kidney transplant patients. Each graph shows a given outcome measure over time (x-axis) and the percentage of the population that achieved the goal for this outcome measure (y-axis). Abbreviations: BMI, body mass index; CM, cytomegalovirus; UTI, urinary tract infection; GFR, glomerular filtration rate; Chol, cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; SBP, systolic blood pressure; Ca, calcium; UPC, urine protein/creatinine ratio; HgB, hemoglobin; PO4, phosphorus; CO2, bicarbonate
In our practice, these clinical outcomes were combined to create a composite “ideal” outcome (Fig. 3) [19]. In this cross-sectional study, patients were assessed at 1-, 3-, 5-, and 10-year transplant anniversaries according to an outcome measure consisting of 15 criteria in the aforementioned health domains. At 1 year, an ideal outcome was achieved for 63% of patients, 37% at 3 years, 40% at 5 years, and 26% at 10 years [19]. The composite ideal outcome can also be applied to the population to track changes over time (Fig. 6). During this 5-year period, our kidney transplant program was actively pursuing improvement projects focused on blood pressure control, reducing acute rejections, and appropriately managing chronic kidney disease. This approach allows for measurement and monitoring of individual and population data over time to measure the health and outcomes at all relevant levels—the individual patient, the population, and the system supporting the work. Future work is planned to continue to incorporate patient-reported outcomes and value of care (quality and cost).
Ideal outcome for a kidney transplant program. This figure illustrates how these measures can be displayed over time for population management. During this 5-year period our kidney transplant program was actively pursuing improvement projects focused on blood pressure control (brown), reducing acute rejection (red), and appropriately managing chronic kidney disease (purple)
Learning health networks in pediatric nephrology
One of the challenges faced by pediatric nephrologists is that kidney disease is uncommon in children, making it difficult to produce generalizable knowledge through research from a single center [24]. The LHS model can also be applied across multiple care centers in an LHN. Nearly all pediatric chronic diseases are considered rare according to the National Institutes of Health definition of a prevalence of fewer than 200,000 affected individuals in the USA [25]. In addition, due to small cohorts and a historical lack of well-defined treatment pathways, there is substantial variation in care practices and clinical outcomes between centers [25]. These limitations can be overcome with LHNs through shared data models and reliance agreements with a single-center institutional review board. Marsolo and colleagues describe a model network architecture that integrates the clinical data from different EHR platforms into an enhanced registry to support care for patients with inflammatory bowel disease in ImproveCareNow [26]. They describe the many facets of creating and maintaining an effective registry, including regulatory and legal considerations, working with multiple EHR vendors, and creating structured data in the EHR so it can be extracted, analyzed, and shared to inform measures, clinical decision support, and automating registry reports [26].
The LHN is aligned with the single-center LHS approach: focusing on the health of patients, measuring impact, integrating activities with a structured theory, and striving for continuous learning to change systems of care. Distinct advantages arise with a multicentered, network approach—specifically they allow for collective action by many individuals and teams to answer questions and solve problems that otherwise could not be answered at the level of a single center. Additionally, single centers that lack the resources to establish an LHS on their own, may benefit from shared resources in an LHN such as a registry, data processing, QI knowledge and training, and access to other centers’ data. To establish clinical and research priorities within a network, it is imperative to engage all stakeholders: patients and families, clinicians, researchers, and administrators. Once established, the network then provides the structure to align the research agenda with clinical questions that underlie patient and clinician uncertainty about what works best for which patients and under what circumstances [25, 27].
LHNs differ from other large research networks and patient registries in that the primary objective of the LHS is to improve patient outcomes by direct, rather than indirect impact on clinical care processes. This is done by democratizing the decision-making and prioritization of which areas to direct time, effort, and resources. The distributive nature of learning throughout the individual centers involved in LHNs allows for multiple ways to address a problem or improve an outcome, and this data is shared openly and freely to the rest of the network. This sharing of successes and failures allows the network to progress faster to achieve its goals than would occur using traditional research methods.
LHNs focused on a variety of chronic conditions have proven to improve patient outcomes across inpatient care, outpatient care, and perioperative care [28, 29]. Britto and colleagues describe multiple real-world examples, including improved rate of remission for patients with inflammatory bowel disease (chronic disease management), decreased mortality for infants with single ventricle congenital heart disease (perioperative/inpatient care), decreased catheter-associated urinary tract infection for admitted patients (patient safety), and more [29]. LHNs can also provide individual centers with data analysis and population management services that they might not have the resources or information systems support to create and manage on their own. Furthermore, being part of an LHN allows a program to compare its outcomes to those of peer institutions and potentially garner support from program administration to more fully engage in improvement activities.
The following are several examples of LHNs in pediatric nephrology along with their goals and selected successes.
The Standardizing Care to improve Outcomes in Pediatric End stage renal disease (SCOPE) Collaborative [30] was launched in 2011 using a model to combine rigorous quality improvement methodologies and real-time, transparent reporting of process and outcome measures [31]. This model has led to impressive clinical outcomes improvement, including reduction of peritonitis rates in children on peritoneal dialysis through a standardized follow-up care bundle. In this effort, mean monthly peritonitis rates across 24 centers decreased from 0.63 episodes per patient year before launch of the standardized bundle to 0.42 at 36 months after launch [32]. Additionally, the SCOPE collaborative has reported epidemiologic data to describe exit site and tunnel infection rates for children on peritoneal dialysis [33] and risk factors for early onset peritonitis [34] and fungal peritonitis [35]—data that is critical in the work to improve such outcomes.
The Improving Renal Outcomes Collaborative (IROC) [36, 37] was started in 2016 with the vision to partner with patients who have kidney disease and their families to improve health, longevity, and quality of life. IROC provides member centers with structured quality improvement training and a central registry for patient demographic and medical data that is used to generate pre-visit planning and population management reports. IROC’s initial efforts focused on improving blood pressure measurement, documentation, treatment, and control according to established guidelines. Prior to this project, only 11% of transplant clinic visits from 17 participating centers documented an appropriately measured blood pressure—a number that increased to more than 85% within 20 weeks [38]. As a response to the COVID-19 pandemic, IROC centers were able to rapidly organize and deploy network-wide calls for clinicians and patients/families to learn how practices were being impacted and sharing of best practices learned by centers that were earliest and most significantly impacted [39]. IROC also established a COVID-19 testing registry that was supported by the existing patient registry to report the largest cohort of COVID-19 testing and outcomes for pediatric kidney transplant patients [39].
The Nephrotoxic Injury Negated by Just-In-Time Action (NINJA) program, originally created at a single institution and found to decrease acute kidney injury (AKI) by identifying patients at high risk for AKI [40], has since spread to form a multicenter learning network. This collaborative recently reported that across 9 pediatric centers, AKI rates for inpatients decreased from 1.7 to 1.3 episodes per 1000 patient days (23.8% reduction) and AKI rates per nephrotoxic medication exposure decreased from 23.6 to 15.0% (36.7% reduction) [41]. Through this work, nephrotoxic AKI was selected as one of 12 preventable hospital-acquired conditions by the Children’s Hospitals’ Solutions for Patient Safety—perhaps the largest LHN in pediatrics consisting of 145 Children's Hospitals collaborating to eliminate serious harm to patients [42].
The Pediatric Glomerular Disease Learning Network (GLEAN) was recently created. This group has previously described using EHR data to rapidly and accurately identify children with glomerular disease using a computable phenotype [43]. This development is an important step in identifying the cohort of interest and will not only enhance and accelerate comparative effectiveness and health outcomes research for patients with glomerular disease but also can be applied to structured outcomes improvement and can be applied to other cohorts of patients with rare diseases [43].
Challenges for the learning health system/network
One of the most pressing issues at the network level is how to handle the vast amounts of data that exist across various institutions and that are being uploaded to multiple patient registries. At present, none of the LHNs or large patient registries in pediatric nephrology utilize automated electronic data transfer—where the data that is being collected and tracked is automatically retrieved from an individual center EHR and uploaded to the patient registry of interest. This leaves much of the work for data upload to be manually entered at the center level, which is a major limitation to greater engagement with these networks. To address this, some networks have developed processes to allow for bulk upload of EHR data or other third-party data into central registries; however, manual manipulation and formatting of data elements is still required. An additional data challenge is that many institutions participate in more than one of these networks and/or patient registries which leads to entering or uploading similar data on the same patients to multiple different registries, each with their own formatting and data entry requirements. There is a need for implementation of a common data model that organizes the relevant data into standard structures that can be shared across networks and registries to ease the administrative burden of participation in multiple networks/registries. An additional hurdle is the majority of EHR content is unstructured and locked into proprietary systems which is problematic when trying to collect and analyze this data across different systems [44]. While the technology exists to overcome these barriers, one of the greatest challenges is that this also requires legal and regulatory agreements between networks, hospitals, and organizations to allow handling of protected health information. Alignment and streamlining of network priorities will be required for this to occur.
An additional consideration is the role of the pediatric nephrologist’s participation in one or many of such networks. The currency of academia is grant funding and publications. With many academic positions on either a “research,” “clinical,” or “educational” track there can be a lack of time to fully engage with the structured quality improvement methods that are needed to successfully improve the local health care system while also fulfilling academic requirements. Participation in these networks can be encouraged by formal recognition of the time and effort needed for successful work to be completed and by including this work as a pathway in the reappointment, promotion, and tenure process at the institutional level. Benefits of participating in an LHN include opportunities to address many academic requirements through leading or participating in multicenter clinical studies or leading a multicenter QI effort.
Finally, to develop robust LHSs and LHNs, they must be funded appropriately. Providing the highest quality and most efficient care is the explicitly stated mission of nearly all academic institutions and payors, including government entities, yet when funding priorities are established, the development of LHSs and LHNs is often considered “additional” work that must be funded through extramural grant support rather than through operational budgets. Staff are often expected to engage in LHS and LHN work without the aforementioned formal recognition or protected time to do so. However, if the vision of better health and patient experience at lower cost is to be achieved through LHSs, institutions must support quality and outcomes improvement work with necessary recourses as part of their primary missions rather than just a means to academic productivity or recognition. For instance, the pediatric nephrologist cannot be expected to add “additional work” to existing commitments; rather, institutions must provide dedicated effort, staff, and other resources to develop LHSs that ultimately improve the efficiency and quality of care. The emphasis needs to shift from participation as “additional” work, to acknowledging that the work of LHSs and LHNs is “central” to the business and mission of all healthcare organizations and payors. Payors—both private and public—stand to gain the greatest financial benefit from improved health of populations. As such, they should be willing to invest the financial capital to establish robust LHSs and LHNs.
Summary and future directions
As LHSs continue to emerge and prove successful at improving patient outcomes, this creates an opportunity for a new field of expertise. Engaging patients and colleagues, implementing quality improvement, and performing research through learning networks requires a unique skillset. To address the need for future experts in the field, thought leaders in the field have developed a set of 33 core competencies for LHS researchers that encompass 7 competency domains [45], including (1) systems science, (2) research questions and standards of scientific evidence, (3) research methods, (4) informatics, (5) ethics of research and implementation in health systems, (6) improvement and implementation science, and (7) engagement, leadership, and research management. Distinguishing features of this field from traditional clinical research are the real-world milieu of LHS research, the embeddedness of the researcher within the health system, and engagement of all stakeholders [45]. The PEDSNet Scholars Program was created to prepare and equip researchers with these needed skills [46]. Founded in 2018, this program is a grant awarded to junior faculty under the Agency for Healthcare Research and Quality – Patient-Centered Outcomes Research Institute (AHRQ-PCORI) Institutional Mentored Career Development Program (K12).
Participating in an LHN provides a number of benefits for physicians—including allowing for peer-to-peer comparison and benchmarking on best results, opportunities to formally test what works and what does not and quickly spread the result within the network, and access to coaching from peers and individuals with expertise in quality improvement methods. Participation in these networks can be used to fulfill Maintenance of Certification requirements for pediatricians [47]. Additionally, there are opportunities for patient-clinician collaboration on projects that is not fostered by the traditional clinic interaction [48, 49]. For example, in IROC the Community Engagement Workgroup (comprising patients with a kidney transplant and their parents) developed a list of questions about acute rejection that were answered by the physicians within the network. The answers were collated and used to create two versions of educational materials—an illustrated comic book for children and a comprehensive packet for adults. These resources have since been translated into Spanish and made available to all participating centers within the network. The Community Engagement Workgroup has their own leadership and meeting structure and can set priorities consistent with the wants and needs of its members.
In summary, the LHS and multicenter LHNs attempt to align, organize, and empower what have traditionally been disparate (and potentially adversarial) components of the health care system into an organization focused on improving patient health and clinical outcomes. This is done through a reorganization of the older, hierarchical model of healthcare delivery into a more distributed leadership and accountability model. While tech companies have been quick to adapt to this more distributed model (e.g., Wikipedia, AirBnB, Uber, etc.) there is work to be done in healthcare. Reshaping the culture of medicine will be important to achieve this. In the ideal LHS, clinicians and health systems, public health and social services, businesses and community organizations, and individuals and families are not only seamlessly linked but are active contributors to and beneficiaries of the learning culture [50]. This is done by harnessing and relying on the inherent motivations of all stakeholders toward the shared goal of improving health and clinical outcomes.
References
Wagner E (1998) Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract 1:2–4
Wagner EH, Austin BT, Von Korff M (1996) Organizing care for patients with chronic illness. Milbank Q 74:511–544
Olsen LA, Aisner D, McGinnis JM (2007) Institute of medicine roundtable on evidence-based medicine. The Learning Healthcare System: Workshop Summary. National Academies Press (US), Washington, DC
Smith M, Saunders R, Stuckhardt L, McGinnis JM (2012) Institute of medicine best care at lower cost: the path to continuously learning health care in America. National Academies Press (US), Washington DC
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:1–150
Barletta G-M, Pierce C, Mitsnefes M, Samuels J, Warady BA, Furth S, Flynn J (2018) Is blood pressure improving in children with chronic kidney disease? Hypertension 71:444–450
Hooper DK, Williams JC, Carle AC, Amaral S, Chand DH, Ferris ME, Patel HP, Licht C, Barletta GM, Zitterman V, Mitsnefes M, Patel UD (2013) The quality of cardiovascular disease care for adolescents with kidney disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 28:939–949
Primack WA, Meyers KE, Kirkwood SJ, Ruch-Ross HS, Radabaugh CL, Greenbaum LA (2015) The US pediatric nephrology workforce: a report commissioned by the American Academy of Pediatrics. Am J Kidney Dis 66:33–39
Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP (2009) The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco
Moen RD, Nolan TW, Provost LP (2012) Quality improvement through planned experimentation. McGraw-Hill, New York
Provost LP, Murray SK (2011) The health care data guide: learning from data for improvement. Jossey-Bass, San Francisco
Perla RJ, Provost LP, Murray SK (2011) The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 20:46–51
Benneyan JC, Lloyd RC, Plsek PE (2003) Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 12:458–464
MacColl Institute for Healthcare Innovation (2011) Reducing care fragmentation: a toolkit for coordinating care. http://www.improvingchroniccare.org/. Accessed 25 Aug 2020
Sinsky CA, Sinsky TA, Rajcevich E (2015) Putting pre-visit planning into practice. Fam Pract Manag 22:34–38
Gupta R, Skootsky SA, Kahn KL, Chen L, Abtin F, Kee S, Nicholas SB, Vangala S, Wilson J (2021) A system-wide population health value approach to reduce hospitalization among chronic kidney disease patients: an observational study. J Gen Intern Med 36:1613–1621
Modi AC, Pai AL, Hommel KA, Hood KK, Cortina S, Hilliard ME, Guilfoyle SM, Gray WN, Drotar D (2012) Pediatric self-management: a framework for research, practice, and policy. Pediatrics 129:e473–e485
Patwardhan MB, Kawamoto K, Lobach D, Patel UD, Matchar DB (2009) Recommendations for a clinical decision support for the management of individuals with chronic kidney disease. Clin J Am Soc Nephrol 4:273–283
Taylor VA, Kirby CL, Nehus EJ, Goebel J, Hooper DK (2019) Composite health outcomes in pediatric and young adult kidney transplant recipients. J Pediatr 204:196–202
Hooper DK, Kirby CL, Margolis PA, Goebel J (2013) Reliable individualized monitoring improves cholesterol control in kidney transplant recipients. Pediatrics 131:e1271-1279
Hooper DK, Varnell CD Jr, Rich KL, Carle A, Huber J, Mostajabi F, Dahale D, Pai ALH, Goebel JW, Modi AC (2021) A medication adherence promotion system to reduce late kidney allograft rejection: a quality improvement study. Am J Kidney Dis 79:335–346
Varnell CD, Rich KL, Zhang B, Carle AC, Pai ALH, Modi AC, Hooper DK (2021) Predicting acute rejection in children, adolescents, and young adults with a kidney transplant by assessing barriers to taking medication. Pediatr Nephrol 36:2453–2461
Varnell CD, Rich KL, Nichols M, Dahale D, Goebel JW, Pai ALH, Hooper DK, Modi AC (2017) Assessing barriers to adherence in routine clinical care for pediatric kidney transplant patients. Pediatr Transplant 21:e13027
Forrest CB, Margolis P, Seid M, Colletti RB (2014) PEDSnet: how a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood) 33:1171–1177
Lannon CM, Peterson LE (2013) Pediatric collaborative networks for quality improvement and research. Acad Pediatr 13:S69–S74
Marsolo K, Margolis PA, Forrest CB, Colletti RB, Hutton JJ (2015) A digital architecture for a network-based learning health system: integrating chronic care management, quality improvement, and research. EGEMS (Wash DC) 3:1168
Clancy CM, Margolis PA, Miller M (2013) Collaborative networks for both improvement and research. Pediatrics 131(Suppl 4):S210–S214
Mathis MR, Dubovoy TZ, Caldwell MD, Engoren MC (2020) Making sense of big data to improve perioperative care: learning health systems and the multicenter perioperative outcomes group. J Cardiothorac Vasc Anesth 34:582–585
Britto MT, Fuller SC, Kaplan HC, Kotagal U, Lannon C, Margolis PA, Muething SE, Schoettker PJ, Seid M (2018) Using a network organisational architecture to support the development of Learning Healthcare Systems. BMJ Qual Saf 27:937–946
SCOPE Dialysis Collaborative: partnering with families to improve dialysis care for children and adolescents. https://www.childrenshospitals.org/Programs-and-Services/Quality-Improvement-and-Measurement/Collaboratives/SCOPE. Accessed 14 Sept 2020
Neu AM, Miller MR, Stuart J, Lawlor J, Richardson T, Martz K, Rosenberg C, Newland J, McAfee N, Begin B, Warady BA, SCOPE Collaborative Participants (2014) Design of the standardizing care to improve outcomes in pediatric end stage renal disease collaborative. Pediatr Nephrol 29:1477–1484
Neu AM, Richardson T, Lawlor J, Stuart J, Newland J, McAfee N, Warady BA, Participants SC (2016) Implementation of standardized follow-up care significantly reduces peritonitis in children on chronic peritoneal dialysis. Kidney Int 89:1346–1354
Swartz SJ, Neu A, Skversky Mason A, Richardson T, Rodean J, Lawlor J, Warady B, Somers MJG (2018) Exit site and tunnel infections in children on chronic peritoneal dialysis: findings from the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol 33:1029–1035
Keswani M, Redpath Mahon AC, Richardson T, Rodean J, Couloures O, Martin A, Blaszak RT, Warady BA, Neu A, SCOPE Investigators (2019) Risk factors for early onset peritonitis: the SCOPE collaborative. Pediatr Nephrol 34:1387–1394
Munshi R, Sethna CB, Richardson T, Rodean J, Al-Akash S, Gupta S, Neu AM, Warady BA (2018) Fungal peritonitis in the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol 33:873–880
Hooper DK, Misurac J, Blydt-Hansen T, Chua AN (2020) Multicenter data to improve health for pediatric renal transplant recipients in North America: Complementary approaches of NAPRTCS and IROC. Pediatr Transplant 25:e13891
Improving Renal Outcomes Collaborative. http://www.irocnow.org. Accessed 14 Sep 2020
Seifert ME, Dahale DS, Kamel M, Winterberg PD, Barletta G-M, Belsha CW, Chaudhuri A, Flynn JT, Garro R, George RP, Goebel JW, Kershaw DB, Matossian D, Misurac J, Nailescu C, Nguyen CR, Pearl M, Pollack A, Pruette CS, Singer P, VanSickle JS, Verghese P, Warady BA, Warmin A, Weng PL, Wickman L, Wilson AC, Hooper DK (2020) The improving renal outcomes collaborative: blood pressure measurement in transplant recipients. Pediatrics 146:e20192833
Varnell C Jr, Harshman LA, Smith L, Liu C, Chen S, Al-Akash S, Barletta G-M, Belsha C, Brakeman P, Chaudhuri A, Fadakar P, Garro R, Gluck C, Goebel J, Kershaw D, Matossian D, Nailescu C, Patel HP, Pruette C, Ranabothu S, Rodig N, Smith J, Sebestyen VanSickle J, Weng P, Danziger-Isakov L, Hooper DK, Seifert M (2021) COVID-19 in pediatric kidney transplantation: the Improving Renal Outcomes Collaborative. Am J Transplant 21:2740–2748
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES (2016) A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90:212–221
Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, Askenazi DJ, Henderson T, Dill L, Somers MJG, Kerr J, Gilarde J, Zaritsky J, Bica V, Brophy PD, Misurac J, Hackbarth R, Steinke J, Mooney J, Ogrin S, Chadha V, Warady B, Ogden R, Hoebing W, Symons J, Yonekawa K, Menon S, Abrams L, Sutherland S, Weng P, Zhang F, Walsh K (2020) A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97:580–588
Lyren A, Coffey M, Shepherd M, Lashutka N, Muething S, Group SPSL (2018) We will not compete on safety: how children’s hospitals have come together to hasten harm reduction. Jt Comm J Qual Patient Saf 44:377–388
Denburg MR, Razzaghi H, Bailey LC, Soranno DE, Pollack AH, Dharnidharka VR, Mitsnefes MM, Smoyer WE, Somers MJG, Zaritsky JJ, Flynn JT, Claes DJ, Dixon BP, Benton M, Mariani LH, Forrest CB, Furth SL (2019) Using electronic health record data to rapidly identify children with glomerular disease for clinical research. J Am Soc Nephrol 30:2427–2435
Bertagnolli MM, Anderson B, Norsworthy K, Piantadosi S, Quina A, Schilsky RL, Miller RS, Khozin S (2020) Status update on data required to build a learning health system. J Clin Oncol 38:1602–1607
Forrest CB, Chesley FD Jr, Tregear ML, Mistry KB (2017) Development of the learning health system researcher core competencies. Health Serv Res 53:2615–2632
PEDSnet scholars: a career development program for junior faculty interested in learning health systems research. https://pedsnet.org/pedsnet-scholars/. Accessed 18 Dec 2020
Miles PV, Conway PH, Pawlson LG (2013) Physician professionalism and accountability: the role of collaborative improvement networks. Pediatrics 131(Suppl 4):S204–S209
Kennedy ST, Maddux MH, IMPROVECARENOW PEDIATRIC IBD LEARNING HEALTH SYSTEM (2019) Patient-clinician collaboration in the development of an IBD transfer toolkit. Pediatrics 144:e20190558
Batalden M, Batalden P, Margolis P, Seid M, Armstrong G, Opipari-Arrigan L, Hartung H (2016) Coproduction of healthcare service. BMJ Qual Saf 25:509–517
McGinnis JM, Fineberg HV, Dzau VJ (2021) Advancing the Learning Health System. N Engl J Med 385:1–5
Funding
CDV received support from NIH/NCATS 2KL2TR001426-05A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CDV has no conflicts of interest to report. PM is an inventor of technology licensed by Cincinnati Children’s to Hive Networks. JG has no conflicts of interest to report. DKH receives consulting fees from Magnolia Innovation, Bioporto, Kaneka.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varnell, C.D., Margolis, P., Goebel, J. et al. The learning health system for pediatric nephrology: building better systems to improve health. Pediatr Nephrol 38, 35–46 (2023). https://doi.org/10.1007/s00467-022-05526-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05526-0