Abstract
Background
Genetic kidney disease is well established as an important cause of pediatric kidney failure, and genetic testing might increase diagnostic accuracy, but evidence is limited. This study was conducted to determine the diagnostic yield and clinical impact of genetic testing for children with kidney failure.
Methods
Patients who were diagnosed with kidney failure before 19 years of age at Children’s Hospital of Fudan University from 2009 to 2018 and received next-generation sequencing (NGS) were enrolled. The results for likely pathogenic variants in genes known to cause chronic kidney disease (CKD) were analyzed.
Results
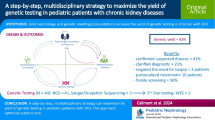
A molecular diagnosis was identified in 39.9% (75/188) of children with kidney failure. Specific subtype of clinical category was discerned in 54 (72.0%) patients, kidney disease was reclassified in 7 (9.3%) patients, the unknown etiology of 5 (6.7%) patients was molecularly diagnosed, and the clinical diagnoses of the other 9 (12.0%) patients were confirmed. In addition, genetic diagnosis was considered to have contributed to clinical management, including negating the need for kidney biopsy (26/75, 34.7%), avoiding immunosuppressive therapy (24/75, 32.0%), changing surveillance (48/75, 64.0%), guiding specific treatment (21/75, 28.0%), and guiding peri-transplant management and options for kidney transplantation (12/75, 16.0%). Furthermore, cascade testing was subsequently offered to 34.7% (26/75) of families.
Conclusions
Genetic testing identified a molecular diagnosis in nearly 40% of children with kidney failure. Our results confirm that in children with kidney failure, genetic testing can not only establish a specific molecular diagnosis, but has a significant impact on clinical management.
Graphical abstract





Similar content being viewed by others
Availability of data and materials
All data generated or analyzed are included in this paper.
Code availability
Not applicable.
References
Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C (2019) A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 34:1803–1805
Chua A, Cramer C, Moudgil A, Martz K, Smith J, Blydt-Hansen T, Neu A, Dharnidharka VR, NAPRTCS investigators (2019) Kidney transplant practice and outcome benchmarks over 30 years: The 2018 report of the NAPRTCS. Pediatr Transplant 23:e13597
Shen Q, Fang X, Man X, Zhai Y, Liu L, Wang C, Shang W, Feng G, Zhang L, Zeng L, Zhu Y, Chen J, Rao J, Warady BA, Schaefer F, Xu H (2021) Pediatric kidney transplantation in China: an analysis from the IPNA Global Kidney Replacement Therapy Registry. Pediatr Nephrol 36:685–692
de Haan A, Eijgelsheim M, Vogt L, Knoers NVAM, de Borst MH (2019) Diagnostic yield of next-generation sequencing in patients with chronic kidney disease of unknown etiology. Front Genet 10:1264
Jayasinghe K, Stark Z, Kerr PG, Gaff C, Martyn M, Whitlam J, Creighton B, Donaldson E, Hunter M, Jarmolowicz A, Johnstone L, Krzesinski E, Lunke S, Lynch E, Nicholls K, Patel C, Prawer Y, Ryan J, See EJ, Talbot A, Trainer A, Tytherleigh R, Valente G, Wallis M, Wardrop L, West KH, White SM, Wilkins E, Mallett AJ, Quinlan C (2021) Clinical impact of genomic testing in patients with suspected monogenic kidney disease. Genet Med 23:183–191
Mann N, Braun DA, Amann K, Tan W, Shril S, Connaughton DM, Nakayama M, Schneider R, Kitzler TM, van der Ven AT, Chen J, Ityel H, Vivante A, Majmundar AJ, Daga A, Warejko JK, Lovric S, Ashraf S, Jobst-Schwan T, Widmeier E, Hugo H, Mane SM, Spaneas L, Somers MJG, Ferguson MA, Traum AZ, Stein DR, Baum MA, Daouk GH, Lifton RP, Manzi S, Vakili K, Kim HB, Rodig NM, Hildebrandt F (2019) Whole-exome sequencing enables a precision medicine approach for kidney transplant recipients. J Am Soc Nephrol 30:201–215
Rao J, Liu X, Mao J, Tang X, Shen Q, Li G, Sun L, Bi Y, Wang X, Qian Y, Wu B, Wang H, Zhou W, Ma D, Zheng B, Shen Y, Chen Z, Luan J, Wang X, Wang M, Dang X, Wang Y, Wu Y, Hou L, Sun S, Li Q, Liu X, Bai H, Yang Y, Shao X, Li Y, Zheng S, Han M, Liu C, Cao G, Zhao L, Qiu S, Dong Y, Zhu Y, Wang F, Zhang D, Li Y, Zhao L, Yang C, Luo X, Chen L, Jiang X, Zhang A, Xu H, for “Internet Plus” Nephrology Alliance of National Center for Children's Care (2019) Genetic spectrum of renal disease for 1001 Chinese children based on a multicenter registration system. Clin Genet 96:402–410
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424
Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, Li Y, Zhang J, Nestor J, Krithivasan P, Lam WY, Mitrotti A, Piva S, Kil BH, Chatterjee D, Reingold R, Bradbury D, DiVecchia M, Snyder H, Mu X, Mehl K, Balderes O, Fasel DA, Weng C, Radhakrishnan J, Canetta P, Appel GB, Bomback AS, Ahn W, Uy NS, Alam S, Cohen DJ, Crew RJ, Dube GK, Rao MK, Kamalakaran S, Copeland B, Ren Z, Bridgers J, Malone CD, Mebane CM, Dagaonkar N, Fellström BC, Haefliger C, Mohan S, Sanna-Cherchi S, Kiryluk K, Fleckner J, March R, Platt A, Goldstein DB, Gharavi AG (2019) Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 380:142–151
Ottlewski I, Münch J, Wagner T, Schönauer R, Bachmann A, Weimann A, Hentschel J, Lindner TH, Seehofer D, Bergmann C, Jamra RA, Halbritter J (2019) Value of renal gene panel diagnostics in adults waiting for kidney transplantation due to undetermined end-stage renal disease. Kidney Int 96:222–230
Vivante A, Hildebrandt F (2016) Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12:133–146
Connaughton DM, Bukhari S, Conlon P, Cassidy E, O'Toole M, Mohamad M, Flanagan J, Butler T, O'Leary A, Wong L, O'Regan J, Moran S, O'Kelly P, Logan V, Griffin B, Griffin M, Lavin P, Little MA, Conlon P (2015) The Irish Kidney Gene Project—prevalence of family history in patients with kidney disease in Ireland. Nephron 130:293–301
Skrunes R, Svarstad E, Reisæter AV, Vikse BE (2014) Familial clustering of ESRD in the Norwegian population. Clin J Am Soc Nephrol 9:1692–1700
Akrawi DS, Li X, Sundquist J, Sundquist K, Zöller B (2014) Familial risks of kidney failure in Sweden: a nationwide family study. PLoS One 9:e113353
Braun DA, Schueler M, Halbritter J, Gee HY, Porath JD, Lawson JA, Airik R, Shril S, Allen SJ, Stein D, Al Kindy A, Beck BB, Cengiz N, Moorani KN, Ozaltin F, Hashmi S, Sayer JA, Bockenhauer D, Soliman NA, Otto EA, Lifton RP, Hildebrandt F (2016) Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity. Kidney Int 89:468–475
Hildebrandt F (2010) Genetic kidney diseases. Lancet 375:1287–1295
Niaudet P, Gubler MC (2006) WT1 and glomerular diseases. Pediatr Nephrol 21:1653–1660
Sun S, Xu L, Bi Y, Wang J, Zhang Z, Tang X, Cao Q, Zhai Y, Chen J, Fang X, Liu J, Fang Y, Xiang T, Qian Y, Wu B, Wang H, Zhou W, Shen J, Dong K, Liu X, Zheng B, Zhang A, Wang X, Wu Y, Ma D, Shen Q, Rao J, Xu H (2020) Early diagnosis of WT1 nephropathy and follow up in a Chinese multicenter cohort. Eur J Med Genet 63:104047
Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS (2012) Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 27:1729–1736
Robbiano A, Mandrile G, De Marchi M, Beck B, Baasner A, Murer L, Benetti E, Giachino D (2010) Novel human pathological mutations. Gene symbol: AGXT. Disease: hyperoxaluria. Hum Genet 127:468
Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Özkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Özcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F, PodoNet Consortium (2015) Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol 10:592–600
Ding WY, Koziell A, McCarthy HJ, Bierzynska A, Bhagavatula MK, Dudley JA, Inward CD, Coward RJ, Tizard J, Reid C, Antignac C, Boyer O, Saleem MA (2014) Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J Am Soc Nephrol 25:1342–1348
Acknowledgements
We thank all patients and their families who contributed to this study. We thank the coordinators of the Chigene (Beijing) Translational Medical Research Center Co. Ltd., WuXi NextCODE in Shanghai, and MyGenostics Co. Ltd. in Beijing, for sequencing technology support.
Author information
Authors and Affiliations
Contributions
J. Chen, F. Lin, Y. Zhai, Q. Shen, and H. Xu contributed to the research design; B. Wu, D. Ma, and H. Xu provided administrative, technical, and material support; F. Lin, C. Wang, J. Rao, Jiaojiao Liu, Jialu Liu, and M. Yu contributed to data acquisition; J. Chen, F. Lin, and Y. Zhai contributed to data analysis and interpretation; J. Chen, F. Lin, and M. Yu contributed to statistical analysis; J. Chen and Q. Shen drafted the manuscript; Q. Shen and H. Xu contributed critical revisions of the manuscript; Q. Shen and H. Xu contributed supervision and mentorship; and all authors approved the final version of the report.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Ethical Review Committee of the Children’s Hospital of Fudan University.
Consent to participate
Not applicable.
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 7667 kb).
Rights and permissions
About this article
Cite this article
Chen, J., Lin, F., Zhai, Y. et al. Diagnostic and clinical utility of genetic testing in children with kidney failure. Pediatr Nephrol 36, 3653–3662 (2021). https://doi.org/10.1007/s00467-021-05141-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05141-5