Abstract
Determining the etiology of end-stage renal disease (ESRD) constitutes a great challenge in the context of renal transplantation. Evidence is lacking on the genetic findings for adult renal transplant recipients through exome sequencing (ES). Adult patients on kidney transplant waitlist were recruited from 2017 to 2019. Trio-ES was conducted for the families who had multiple affected individuals with nephropathy or clinical suspicion of a genetic kidney disease owing to early onset or extrarenal features. Pathogenic variants were confirmed in 62 from 115 families post sequencing for 421 individuals including 195 health family members as potential living donors. Seventeen distinct genetic disorders were identified confirming the priori diagnosis in 33 (28.7%) families, modified or reclassified the clinical diagnosis in 27 (23.5%) families, and established a diagnosis in two families with ESRD of unknown etiology. In 14.8% of the families, we detected promising variants of uncertain significance in candidate genes associated with renal development or renal disease. Furthermore, we reported the secondary findings of oncogenes in 4.4% of the patients and known single-nucleotide polymorphisms associated with pharmacokinetics in our cohort to predict the drug levels of tacrolimus and mycophenolate. The diagnostic utility of the genetic findings has provided new clinical insight in most families that help with preplanned renal transplantation.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) affects over 850 million individuals worldwide. Recent predictions by the Institute of Health Metrics and Evaluations indicated that by 2040 CKD will be the fifth leading cause of life years lost on a global scale1,2. A positive family history is reported by around 30% of patients with CKD, and familial clustering is a common phenomenon in patients with end-stage renal disease (ESRD)3,4,5. Approximately 15% of patients with ESRD do not have a primary renal disease diagnosis and are therefore labeled as CKD with unknown origin6. Making a correct diagnosis in these patients may have therapeutic implications. Knowledge of the underlying kidney disease is crucial for ESRD management in the context of transplantation, as the primary etiology may affect graft survival in terms of recurrence and or rejection7,8,9,10. Moreover, a genetic diagnosis may be of pivotal importance for family counseling and in the setting of kidney transplantation, particularly when living related donation is involved9,11.
In recent years, we have gained a better understanding of the genetic landscape of CKD in children and young adults through next-generation sequencing. Approximately 500 monogenic causes of CKD have been identified12. It has been shown that a monogenic disease-causing variant can be identified in 10–36% of adults with CKD7,12,13. A few studies reported the initial experience on genetic diagnostic panel for kidney transplantation cohort8,14. However, genetic testing is currently not performed on a regular basis in patients with ESRD waiting for kidney transplantation. In this study, we aimed to unravel the genetic diagnosis for patients with familial ESRD on the kidney transplantation waitlist. We hypothesize that genetic causes in adults are underrecognized, particularly in patents with a positive family history or patient cohorts with unknown etiology. Improving the diagnosis of primary disease in patients with ESRD can therefore have implications for adequate clinical decisions for kidney transplantation.
Results
Clinical characteristics
A total of 115 families with 226 affected individuals (male: female 1.4:1) were recruited from 576 family cohorts with records in the kidney transplantation registry since 2017–2019. All the information of the 576 families came from the 64 dialysis centers in 18 provinces in China. The demographic data and the geographic distribution of cases are shown in Supplementary Fig. 1. Consanguinity was observed in one family.
Among the 115 families in this cohort, 104 families had multiple affected individuals checked for the records of urinalysis, renal function, and imaging studies of the kidneys. There were three families with extrarenal features and a negative family history of renal disease. There were eight patients who had early-onset kidney disease without any extrarenal features or family history of renal disease. Among the 226 affected individuals, 75.7% (171/226) had proteinuria including 11.5% (26/226) with nephrotic proteinuria at disease onset. Abnormal image findings such as renal size or echogenicity were reported by renal ultrasound in 42.9% (97/226) of the cases. Renal biopsy was performed in 30.1% (68/226) of the affected individuals. Hearing loss was recorded in 16.8% (38/226) and vision deficiency was recorded in 4.4% (10/226). The median age at diagnosis of renal disease was 22.0 years (interquartile range [IQR] 12.8–32.0 years). The median age at genetic test was 31.0 years (IQR 22.5–48.0 years). For these families, at least one of the family members was on the transplant waitlist. At the time of transplant registration, 125 (55.3%) probands developed into ESRD, four affected family members developed into CKD stage 4, six individuals developed into CKD stage 3, and six individuals developed into CKD stage 2. The median age at first renal replacement therapy was 28.2 years (range, 7.0–79.4 years). Overall, 11.9% (27/226) of the patients in this cohort underwent renal transplantation.
Subgroups were defined as a priori clinical diagnosis and details are presented in the Methods section. The probands from 103 families had a primary clinical diagnosis including steroid-resistant nephrotic syndrome (SRNS, 42/115), glomerulonephritis (GN, 49/115), tubulointerstitial kidney disease (TIKD, 4/115), and congenital anomalies of the kidney and urinary tract (CAKUT, 8/115). In 12 out of the 115 families, the cause of ESRD was unknown (ESRDu). A total of 55.7% (64/115) of the families were diagnosed based on pathological findings.
Genetic diagnosis established by exome sequencing
We performed exome sequencing (ES) in 421 individuals from the 115 families enrolled in this study. Besides 115 probands for genetic diagnosis, 111 affected individuals with CKD as family members received genetic detection, and 195 health family members underwent genetic screening for potential living kidney donors. A molecular genetic diagnosis was identified in 53.9% (62/115) of familes with suspected genetic kidney disease on the transplant waitlist. Of these families, 13 (11.3%) had an autosomal recessive (AR) disease, 18 (15.7%) had an autosomal dominant (AD) disease, and 31 (27.0%) had an X-linked dominant (XLD) disease. Seventeen different monogenic causes of kidney disease were confirmed in the families with a primary clinical diagnosis of GN (28), SRNS (27), CAKUT (3), TIKD (2), and ESRDu (2). ES confirmed a specific underlying cause within the broader category of clinical suspected disease in 33 (28.7%) families, modified the diagnosis in 24 (20.9%) families, reclassified the primary disease in three families, and establish a diagnosis in two families with ESRDu. (Fig. 1, Table 1, and Supplementary Table 2)
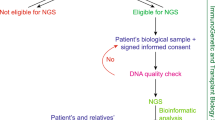
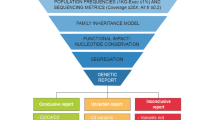
a Study design and stratification. Through evaluation of the registry information in the waiting list for transplantation, patients with a positive family history or patients with clinical suspicion of a genetic kidney disease owing to childhood early onset or extrarenal features were enrolled into the study. All the recruited families (n = 115) with ESRD were divided into five subgroups according to the prior clinical diagnosis of renal disease. ES was performed in 421 individuals from the 115 families (226 patients with CKD). Family-ES identified a specific underlying cause within the broader category of clinical suspected disease in 33 families, modified or reclassified the clinical diagnosis in 27 families, and established a diagnosis in two families referred with ESRD of unknown origin. In 17 families, we detective promising variants of uncertain significance (VUS) in candidate genes associated with renal development. b Circos-style plot of genetic diagnosis in 62 families of ESRD. Ten categories of kidney diseases are indicated outside the widest arc of the circle, chromosome numbers are labeled outside the smaller arc, and gene symbols with patient numbers (patients with pathogenic or likely pathogenic variants) are listed inside. Links are colored by ten categories. c From clinical diagnosis to genetic diagnosis for renal disease based on ES study in 115 families. Left and middle: division of the priori clinical diagnosis and change in final diagnosis. Middle and right: division of change in final diagnosis and genetic findings (pathogenic, likely pathogenic variants, and VUS). The width of the lines in the Sankey plot is proportional to the relative quantity of families within each group. ADTKD autosomal dominant tubulointerstitial kidney disease, CAKUT congenital anomalies of the kidney and urinary tract, ESRD end-stage renal disease, GN glomerulonephritis, HSPN Henoch–Schonlein purpura nephritis, SRNS steroid-resistant nephrotic syndrome, TIKD tubulointerstitial kidney disease, NPHP nephronophthisis.
The genetic study confirmed the molecular diagnosis for patients’ underdiagnosis kidney disease. First, genetic diagnosis was confirmed for the 22 families whose clinical suspicion of Alport syndrome was lack of the pathological evidence of collagen IV deficiency. Pathogenic variants in COL4A5 (19, XLD) and COL4A3 (AR,2; AD 1) were identified respectively in the 22/49 families from GN group without performing the renal histological study of collagen or glomerular basement membrane (GBM). Second, pathogenic variants of known genes were identified for FSGS in 12 families from GN/SRNS group apart from Alport syndrome. The genetic causes of FSGS were shown including COQ8B (AR, 3), TRPC6 (AD, 2), PAX2 (AD,3), NPHS2 (AR,1), NUP160 (AR,1), WT1 (AD, 1) and UMOD (AD, 1). In four families, we detected pathogenic variants in UMOD and PAX2 as the phenocopy genes for FSGS separately. The affected individuals from these families were primary diagnosed of SRNS without abnormal findings of ultrasound. Three of the 91 families referred with GN or FSGS were confirmed the genetic diagnosis of PAX2-associated FSGS post multidisciplinary board discussion. All the patients from the three families presented with proteinuria during adolescence. They did not have extrarenal nor syndromic features, renal dysplasia, or histological features of CAKUT. The PAX2 variant co-segregated across all affected individuals available to us in two families, whereas one de novo variant was found in the third family.
Furthermore, the genetic findings modified the final diagnosis (Table 1). Out of the SRNS group without any abnormal findings of in GBM, pathogenic variants in COL4A5 (XLD, 13), COL4A3 (AR, 3), or COL4A4 (AR, 1) were identified in the 17/42 families. We genetically diagnosed the rare diseases including lipoprotein nephropathy with APOE variant (AD, 2), nephronophthisis (NPHP, AR, 2), Fabry disease with GLA variant (XLR,1), renal coloboma syndrome (AD, 2), and mitochondrial disease (1). After an multidisciplinary team (MDT) discussion, genetic diagnosis was confirmed in two families with lipoprotein nephropathy, one family with Fabry disease, one with mitochondrial disease, one with NPHP, and one with ADTKD. Pathogenic variants in APOE-Kyoto(p.Arg25Cys) and APOE-Chicago (p.Arg147Pro) were identified in two families. Subsequently, re-staining slides of the kidney biopsy confirmed the histochemical diagnosis of lipoprotein nephropathy. A pathogenic variant of GLA for Fabry disease was diagnosed in two affected individuals from one family who presented non-nephrotic proteinuria during adolescence and developed ESRD in their 50s. Rechecking the electron microscope images found the Myelin-like bodies as the typical pathologic features in Fabry disease. In another family with affected siblings, we found biallelic variants in RMND1 (trans) that have been reported in rare cases of mitochondrial disease with renal defects15. The two siblings presented initially with hyperuricemia, non-nephrotic proteinuria, and progressed into renal dysfunction. Hearing impairment and tubulointerstitial nephritis shown by renal biopsy were reported in one of them. The older brother had a successful renal transplant at the age of 18. Reverse phenocoping allowed us to correct the diagnosis of primary disease in 5.2%(6/115) families through clinical reassessment and unexpected genetic findings in PAX2, UMOD and TTC21B. Among the eight families with CAKUT, we detected pathogenic variants in known disease-causing genes in three families. A heterogenic variant of isolated CAKUT genes (ITGB4, 1) was detected in one CAKUT family. The biallelic variants in NPHP genes (TTC21B, 1) were detected in the second CAKUT family. And a heterogenic variant of UMOD was confirmed in the third CAKUT family with the final diagnosis of hyperuricemic nephropathy. Out of the 12 families of ESRDu, we detected the biallelic variants of NPHP3 in one family and the heterogeneous variant in PAX2 in the second family confirming the diagnosis of renal coloboma syndrome.
Variants of uncertain significance and secondary findings
In 5.2% of the families (6 of 115), we detected variants of uncertain significance (VUS) in a gene known to cause kidney disease including LAMA5 (4), NPHS1 (1), and TTC21B (1). The variants did not meet our criteria for definite confirmation of pathogenicity according to American College of Medical Genetics and Genomics (ACMG) guideline16. We also detected the VUS based on the candidate gene list associated with renal development in 9.6% of families (Supplementary Table 3). Phenotype–genotype correlation and co-segregation had been analyzed in candidate genes, including DSCAM, MAZ, KDM2B, VAV2, GLI2, GLI3, PLXNB2, LAMP2, and SHROOM3. Variants of LAMP2 were identified in the male twins who presented with proteinuria and hypertension during adolescence. Both had mild cardiomyopathy, but reported no cardiac symptoms. Danon disease17 was suspected for this family.
Although our focus was the genetic diagnosis on primary disease, we also examine the genes recommended by the ACMG published guidelines for secondary findings/incidental findings (Supplementary Table 4). Ten variants of oncogenes were reported as potentially medically actionable and appropriate for return in 4.4% of patients. Further genetic counseling was performed for the two families evaluating and discussing the potential risks for individuals with BRCA2 pathogenic variants. In addition, polymorphisms variants as genetic determinants for tacrolimus or mycophenolate pharmacokinetics were screened in the 226 patients by ES (Supplementary Table 5 and Supplementary Fig. 6). It provided genetic information to optimize the personalized immunosuppressant dosage in kidney transplantation.
Discussion
In this study, we show the potential diagnostic role of ES in adult patients with familial kidney disease ready for renal transplantation. ES of parent–child trios provided a molecular genetic diagnosis for 53.9% families with suspected genetic kidney disease on the waitlist for transplantation.
Familial testing through ES has improved the diagnostic accuracy in patients with CKD, especially in patients with familial undetermined kidney disease. Patients with genetic disorders can develop ESRD and receive transplantation without a correct diagnosis of causal nephropathy, and that these disorders can cluster and reach a higher-than-expected prevalence in this setting. It has been shown to have a higher diagnostic yield in patients with a positive family history of CKD, patients with extrarenal manifestations, or patients with early-onset age12,13,18. Targeted ES in a broad CKD population identified diagnostic variants in 307 of 3315 (9.3%) adult patients including 64.7% of the patients with ESRD12. Genetic diagnosis was identified in 37% (50/153) of the adult patients with undetermined ESRD through a kidney-specific gene panel9. Here we reported a molecular genetic diagnosis was confirmed in 54% of the 115 families with suspected genetic kidney disease. The dissimilarities in diagnostic yield between these studies likely result from differences in the sample size, inclusion criteria, sequencing technique approach, and different selection of genes. It is crucial to identify the genetic diagnosis and to access the potential risks for recipients and donors for kidney transplantation. Therefore, we recruited the patients from the waitlist after accessing the need for genetic analysis for kidney disease suspected to have an inherited basis in the setting of multiple affected family members, extrarenal phenotype, or early-onset age. We excluded the AD polycystic kidney disease (ADPKD) patients with a clear family history in which modified PCR conditions were efficient for sequencing of the PKD1 gene. The excluded patients with ADPKD accounted for 4.5% of our waitlist for transplantation.
The diagnostic utility of the genetic findings provided new clinical insight in most families that helps to preplanned renal transplantation. A high index of suspicion is needed to detect the key phenotype that may be hidden in patient history suggesting the presence of genetic kidney disease. Reverse phenotyping following ES among patients clinically diagnosed based on nonspecific findings of renal histology or radiology could rescue wrong diagnosis to around 30% in kidney disease19. Here we corrected the diagnosis of the primary disease in 5% of the 115 families through reverse phenotyping. The missing diagnosis can have a serious impact on graft survival and in general on management transplant patients and on other affected family members. A timely diagnosis of certain rare disease, such as Fabry disease and mitochondrial disease identified in our study, is paramount for patient care. As is known enzyme replacement therapy is considered safe after kidney transplantation, and protective in terms of graft and patient survival, continuing even after the transplant to carry out a protective action on the extrarenal aspects of the disease.
It is necessary for appropriate donor selection for transplantation and surveillance of at-risk family members9. A significant proportion of ESRD patients who subsequently received transplantation have a presumptive diagnosis of nephropathy that turns out to be wrong. The increased risk of ESRD post donation in related living donors may reflect a missed genetic disease. Of the 17 monogenic disorders detected in our study, Alport syndrome due to COL4A5/COL4A3 accounted for one third of all genetic diagnoses. These patients were underdiagnosed because of a lack of the pathological evidence of collagen IV deficiency or abnormal findings by electron microscopy. The genetic implications are different for the affected individuals and other family members with X-linked or AR Alport syndrome. All disorders arising from abnormalities of the collagen IV α345 molecule as forms of Alport syndrome can present with highly variable phenotypes. Selection of living related donors for patients with Alport syndrome requires careful consideration of the risk for CKD on the basis of the genotype20. In total, we confirmed the genetic diagnosis in 15.7% of families with AD inherited disease, and 27% with XLD inherited disease. Owing to incomplete penetrance in AD inherited disease, not all individuals with pathogenic variants will be symptomatic. AD causes of FSGS often manifest later in life and can be associated with variable expressivity. Additional clinical evaluation was carried out for the potential donors with pathogenic variants considering the frequency of secondary genetic findings in the general population5.The family members who carried a variant in one of those genes should be preclude from living kidney donation, given their risks for developing disease. Hence, genetic screening should be offered for all at-risk living donors.
A genetic diagnosis may also validate a risk estimation of post-transplant recurrence. Genetic FSGS, except for NPHS1-nepropathy, is considered to have a low risk of recurrence in the post-transplant setting. We reported the modification of diagnosis in 30% families, including 14 families from primary diagnosis of FSGS to Alport syndrome. We also demonstrated pathogenic variants in the phenocopy genes such as UMOD21, TTC21B22, or PAX223 associated FSGS. The genetic subtype of FSGS established collectively accounted for 10% (12/115) of the genetic causes in our cohort. Furthermore, although allograft survival is improved after living donation when compared with deceased donation, there may be a lot of hesitance in pursuing living donor transplantation in patients with FSGS because of recurrent disease. There is a high risk of post-transplant disease reoccurrence in the idiopathic group that is postulated to be caused by circulating factors other than monogenetic cause. Among the 39.6% (36/91) of the families referred of GN or SRNS in our study, pathogenic variants were absent that may be an alarm for high risk of post-transplant recurrence.
The disclosure of the VUS of candidate disease-causative genes may help to identify much of the missing pathogenicity for renal disease. VUS should generally only be considered for reporting where there is a high level of supporting evidence24. In this study we reported the VUS of known causative genes for kidney disease in 5.2% of the families and VUS in candidate genes associated with renal development in 9.6% of the families. Solving the problem of VUS interpretation relies on further functional study on the candidate genes. As deficient mouse models of these candidate genes (such as GLI225, GLI326, PLXNB227, and SHROOM328) have shown phenotypes of renal disorders, the promising VUS could provide much information for research teams.
We reported, for the first time, the secondary findings following ES for ESRD patients. The germline variants of oncogenes in 4.4% of the 115 patients preparing for transplant should be interpreted more carefully. It was reported that 4.8% (7/145) of ES screening referrals for a variety of rare genetic diseases had secondary findings related to cancer29. Kidney transplant recipients are at least two times more likely to develop or die of cancer than the general population18,30. Transplant candidates and potential donors should be screened for cancer as part of the assessment process30. Secondary findings of cancer predisposing genes may provide more evidence to tailor cancer screening in transplant candidates. In addition, the disclosure of secondary findings on pharmacogenetics is of great significance for transplantation31. Attempts have been made to create dosing models that based on these genetic factors as well as clinical factors to predict the dosage of tacrolimus or mycophenolate32. Genetic findings on pharmacogenetics through ES may contribute to the preplanning for transplantation.
The limitations of our study included a modest cohort size of relative ethnic homogeneity. There may be a selection of patients with stable situations enrolled in the transplantation center. According to the geographic distribution of the 64 dialysis centers in 18 provinces, we suspected that our findings are relevant to the other parts of China. Most families (90.4%) of our study had multiple effects, resulting in three-quarters of them with a genetic diagnosis of AD or XL disease. Some of the families who have pathogenic variants of incomplete penetrance could had been missed in our study. Such as complement genes contribute to atypical hemolytic uremic syndrome (aHUS) were absent in our study. aHUS requires perioperative and lifelong complement blockage to prevent post-transplant recurrence. The high diagnostic rate on ES clearly highlights the potential medical-economic savings to patients and insurance companies paying for testing. Further genetic screening should be conducted for all the ESRD cases without secondary causes before transplant. Second, deep intronic variants, variants within variable number tandem repeats, copy number variations, and missing sequence omitted to exome capture kit (i.e., GREB1L, PKD1) would have been missed by our approach. Our study probably underestimates the overall burden of genetic disorders among patients with ESRD. Genome sequencing is an increasingly important comprehensive method with which to investigate the genetic causes of inherited renal disease. Furthermore, the inability to fully interpret all the variants limits the use of sequencing data for both the patients and their family members. Improved methods in which variants are interpreted in concert with clinical diagnoses may address this deficiency.
Overall, our study demonstrates that ES reveals the underlying genetic causes in a significant proportion of adult patients with suspected genetic kidney disease ready for transplantation. The genetic findings allow for improving the diagnosis of primary disease that helps to assess the risk of post-transplant recurrence in the affected individuals and to surveil all at-risk live donors. It may promote the strategy of optimizing transplantation management in the future. More genetic work and long-term follow-up in a larger cohort could fully evaluate the benefit of genetic analysis before renal transplantation.
Methods
Study design and participants
Renal transplant candidates referred to the Organ Transplant Center at the First Affiliated Hospital of Zhengzhou University were recruited perspectively between January 2017 and December 2019. The patients were referred for the evaluation and management of kidney disease and consented to a general genetic research program. Written informed consent offered the possibility to opt yes or no for disclosure of secondary findings, unrelated to the referral condition. Patients could choose if they wished to have their samples and/or data for future research, both anonymously or not. This study is compliant with the “Guidance of the Ministry of Science and Technology (MOST) for the Review and Approval of Human Genetic Resources,” which requires formal approval for the export of human genetic material or data from China. This study was approved and monitored by the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (No. 2017-KY-106).
Our recruitment process is summarized in Fig. 1a. The eligibility criteria included the following: (i) age at registry on the waiting list of kidney transplantation more than 18 years; (ii) a family history of kidney disease that was defined as any family members with urinary abnormalities or impaired kidney function or undiagnosed kidney disease; or clinical suspicion of a genetic kidney disease owing to age onset less than 25 years or extrarenal features8,9,11. These families were referred for the evaluation and management of kidney disease and consented to a general genetic research. The following exclusion criteria were listed: (i) ADPKD that diagnosis based on radiological study and long-range PCR sequencing rather than ES; (ii) specific histological renal diagnosis (e.g., Alport syndrome diagnosed with collagen IV a3/a4/a5 abnormal pathological findings); and (iii) specific and plausible clinical diagnosis (e.g., long-term history of diabetes mellitus before ESRD, systemic lupus erythematosus, acquired obstructive uropathy, tumor, etc.).
The primary clinical diagnosis of each patient was determined via medical history review and the primary nephrologist’s referral into one of the following a priori clinical diagnosis categories33:
-
GN: encompassing membranoproliferative GN, crescentic GN, and hemolytic uremic syndrome.
-
SRNS, or nephrotic syndrome with biopsy findings of FSGS.
-
CAKUT, defined as any abnormality of number, size, shape, or anatomic position within the kidneys or urinary tract.
-
Cystic kidney disease including nephronophthisis (NPHP), medullary cystic disease, and other renal cystic ciliopathies.
-
TIKD: with biopsy findings of chronic tubulointerstitial nephritis without an obvious precipitating cause.
-
ESRD of unknown etiology (ESRDu): patients developed into ESRD with no information of renal histology or definite radiological diagnosis, or no other clinically plausible cause, for instance Fabry disease.
Exome sequencing and variant information
The samples of the affected individuals were subjected to ES of parent–child trios after the informed consent was obtained. Some of their unaffected family members as potential living donors were selected for ES post informed consent. ES procedure and variants annotation have been described in detail previously12. Genomic DNA was isolated from blood lymphocytes and was fragmented to an average size of 250 bp. End repair, adapter ligation, and PCR enrichment were performed following the protocol for VAHTS TM Universal DNA Library Prep Kit for Illumina V3 (Vazyme Biotech Co., Ltd, Nanjing, China). The enriched DNA libraries were subjected to exome capture using Agilent SureSelect Clinical Research Exome V2 or Human All Exon V7. The resulting libraries were sequenced on Illumina sequencers (HiSeq 4000 or Hiseq X) with the paired-end of 150 bp at Precision Medicine Center of Zhengzhou University. Variant interpretation was performed manually by a panel of nephrologists and clinical molecular geneticists. For clinical sequence interpretation the variants were classified according to the ACMG guidelines16.
Primary findings for the monogenic form of nephropathy
In brief, we prioritized variants that occurred in a known CKD-related gene panel7,9,12 (Supplementary Table 7) depending on the a priori clinical diagnosis and phenotype information. Diagnostic variants were defined as “pathogenic” or “likely pathogenic” according ACMG guidelines. For patients referred with a clinical diagnosis of nephrotic syndrome, we manually searched for the p.Arg229Gln variant in the NPHS2 gene, because this allele occurs at a frequency of >1%34. All diagnostic variants were confirmed by Sanger sequencing with segregation. Additional clinical evaluation and follow-up were performed for the affected and unaffected relatives with pathogenic variants confirmed, especially for the potential donors.
If the genetic diagnosis remained unsolved for a family, we also evaluated the VUS of known disease-causative genes through discussion combined with the genotype and phenotype. In addition, we analyzed those variants in a virtual renal development gene panel based on all the pathways regulating kidney development as well as the regulators associated with renal transport and metabolism identified in human studies and animal models18 (Supplementary Table 8). Variants were manually evaluated for possible pathogenicity by a MDT of clinical geneticists, nephrologists, and pathologists after complete genotype–phenotype comparison.
Secondary findings
We assessed the sequence data for pathogenic variants in the 59 genes recommended by the ACMG to reveal any medically actionable secondary findings for individuals undergoing ES35. Per the ACMG recommendations for the analysis of secondary findings, only variants classified as known pathogenic or expected pathogenic were noted.
To investigate the individual pharmacogenetics in transplantation recipients, we collected the allelic variants with functional effects on the pharmacokinetics of tacrolimus or mycophenolate mofetil from the Pharmacokinetics Knowledgebase36. We performed the variant screening in 18 established single-nucleotide polymorphisms (Supplementary Table 4).
Reporting summary
Further information on variants data and research design is available in the Nature Research Reporting Summery linked to this article.
Data availability
The pathogenic variants have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and the submission number is SUB9604514. This study is compliant with the “Guidance of the Ministry of Science and Technology (MOST) for the Review and Approval of Human Genetic Resources,” which requires formal approval for the export of human genetic material or data from China. The phenotypic data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jager, K. J. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transpl. 34, 1803 (2019).
Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 392, 2052 (2018).
Akrawi, D. S., Li, X., Sundquist, J., Sundquist, K. & Zoller, B. Familial risks of kidney failure in Sweden: a nationwide family study. PLoS One 9, e113353 (2014).
Hildebrandt, F. Genetics of kidney diseases. Semin. Nephrol. 36, 472 (2016).
Rasouly, H. M. et al. The burden of candidate pathogenic variants for kidney and genitourinary disorders emerging from exome sequencing. Ann. Intern. Med. 170, 11 (2019).
Saran, R. et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 75, A6 (2020).
Connaughton, D. M. et al. Monogenic causes of chronic kidney disease in adults. Kidney Int. 95, 914 (2019).
Mann, N. et al. Whole-exome sequencing enables a precision medicine approach for kidney transplant recipients. J. Am. Soc. Nephrol. 30, 201 (2019).
Ottlewski, I. et al. Value of renal gene panel diagnostics in adults waiting for kidney transplantation due to undetermined end-stage renal disease. Kidney Int. 96, 222 (2019).
Stevens, P. E. & Levin, A., Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825 (2013).
Hays, T., Groopman, E. E. & Gharavi, A. G. Genetic testing for kidney disease of unknown etiology. Kidney Int. 98, 590 (2020).
Groopman, E. E. et al. Diagnostic utility of exome sequencing for kidney disease. N. Engl. J. Med. 380, 142 (2019).
Vivante, A. & Hildebrandt, F. Exploring the genetic basis of early-onset chronic kidney disease. Nat. Rev. Nephrol. 12, 133 (2016).
Thomas, C. P. et al. Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genet. Med. 22, 1025 (2020).
Shayota, B. J. et al. Characterization of the renal phenotype in RMND1-related mitochondrial disease. Mol. Genet. Genom. Med. 7, e973 (2019).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405 (2015).
Rowland, T. J., Sweet, M. E., Mestroni, L. & Taylor, M. R. Danon disease – dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J. Cell Sci. 129, 2135 (2016).
Warejko, J. K. et al. Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 13, 53 (2018).
Groopman, E. E., Povysil, G., Goldstein, D. B. & Gharavi, A. G. Rare genetic causes of complex kidney and urological diseases. Nat. Rev. Nephrol. 16, 641 (2020).
Kashtan, C. E. Renal transplantation in patients with Alport syndrome: patient selection, outcomes, and donor evaluation. Int J. Nephrol. Renovasc. Dis. 11, 267 (2018).
Chun, J. et al. Autosomal dominant tubulointerstitial kidney disease-uromodulin misclassified as focal segmental glomerulosclerosis or hereditary glomerular disease. Kidney Int. Rep. 5, 519 (2020).
Huynh Cong, E. et al. A homozygous missense mutation in the ciliary gene TTC21B causes familial FSGS. J. Am. Soc. Nephrol. 25, 2435 (2014).
Barua, M. et al. Mutations in PAX2 associate with adult-onset FSGS. J. Am. Soc. Nephrol. 25, 1942 (2014).
Ellard, S., et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2020. (Recommendations ratified by ACGS Quality Subcommittee on 4 February 2020).
Fish, E. W., Murdaugh, L. B., Sulik, K. K., Williams, K. P. & Parnell, S. E. Genetic vulnerabilities to prenatal alcohol exposure: Limb defects in sonic hedgehog and GLI2 heterozygous mice. Birth Defects Res. 109, 860 (2017).
Hu, M. C. et al. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133, 569 (2006).
Perala, N. et al. Sema4C-Plexin B2 signalling modulates ureteric branching in developing kidney. Differentiation 81, 81 (2011).
Wei, C. et al. SHROOM3-FYN interaction regulates nephrin phosphorylation and affects albuminuria in allografts. J. Am. Soc. Nephrol. 29, 2641 (2018).
Chirita-Emandi, A. et al. Challenges in reporting pathogenic/potentially pathogenic variants in 94 cancer predisposing genes – in pediatric patients screened with NGS panels. Sci. Rep. 10, 223 (2020).
Au, E. H., et al. Overall and site-specific cancer mortality in patients on dialysis and after kidney transplant. J. Am. Soc. Nephrol. 30, 471–480 (2019).
Thauvin-Robinet, C. et al. Secondary actionable findings identified by exome sequencing: expected impact on the organisation of care from the study of 700 consecutive tests. Eur. J. Hum. Genet. 27, 1197 (2019).
Yang, J. Y. & Sarwal, M. M. Transplant genetics and genomics. Nat. Rev. Genet. 18, 309 (2017).
Venkat-Raman, G. et al. New primary renal diagnosis codes for the ERA-EDTA. Nephrol. Dial. Transpl. 27, 4414 (2012).
Tory, K. et al. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat. Genet. 46, 299 (2014).
Kalia, S. S. et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet. Med. 19, 249 (2017).
Barbarino, J. M., Whirl-Carrillo, M., Altman, R. B. & Klein, T. E. PharmGKB: a worldwide resource for pharmacogenomic information. Wiley Interdiscip. Rev. Syst. Biol. Med 10, e1417 (2018).
Acknowledgements
We sincerely thank the affected individuals and their families for participation in this study. We thank the 64 dialysis centers from 18 provinces in China. We thank our coordinators from the Supercomputing Center in Zhengzhou University (Zhengzhou) for data analysis support. J. R. is supported by a grant from National Natural Science Foundation of China (NSFC-8182207), a grant from Program of Shanghai Academic/Technology Research Leader (19XD1420600), and a grant from Chinese Academy of Medical Sciences (2019-RC-HL_020). W. J. S. is supported High-tech R&D Program of Henan science and Technology Department (192102310124).
Author information
Authors and Affiliations
Contributions
Conceptualization: W. J. S., J. R.; data curation: J. R., H. E. X., T. C. X.; formal analysis: W. J. S., J. R., Z. G. W., H. E. X., W. X. T., J. C. G.; funding acquisition: W. J. S. and J. R.; investigation: W. J. S., J. R.; methodology: J. R., H. E. X., T. C. X., and F. X.; project administration: W. J. S. and J. R.; resources: W. J. S., Z. G. W., G. W. F.; software: L. Z. X., J. Z. L., D. H. L., H. F. L., R. J. L., X. X. H., J. Y. G., and D. F.; validation: J. C. G., W. X. T., W. J. S., and J. R.; writing original draft: J. R., H. E. X., T. C. X.; writing review and editing: J. R., H. E. X., T. C. X., and W. J. S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Xu, H., Xiang, T. et al. An accessible insight into genetic findings for transplantation recipients with suspected genetic kidney disease. npj Genom. Med. 6, 57 (2021). https://doi.org/10.1038/s41525-021-00219-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41525-021-00219-3
- Springer Nature Limited