Abstract
Background
Hypomagnesemia in patients with congenital anomalies of the kidneys and urinary tract or autosomal dominant tubulointerstitial kidney disease is highly suggestive of HNF1B-associated disease. Intriguingly, the frequency of low serum Mg2+ (sMg) level varies and is lower in children than in adults with HNF1B mutations that could be partially due to application of inaccurate normal limit of sMg, irrespective of age and gender. We aimed to re-assess cross-sectionally and longitudinally the frequency of hypomagnesemia in HNF1B disease by using locally derived reference values of sMg.
Methods
Fourteen children with HNF1B-associated kidney disease were included. Control group comprising 110 subjects served to generate 2.5th percentiles of sMg as the lower limits of normal.
Results
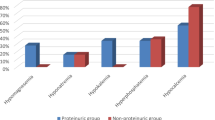
In both controls and patients, sMg correlated with age, gender, and fractional excretion of Mg2+. In girls, sMg concentration was higher than in boys when analyzed in the entire age spectrum (p < 0.05). In HNF1B patients, mean sMg was lower than in controls as compared with respective gender- and age-specific interval (p < 0.001). Low sMg levels (< 0.7 mmol/l) were found in 21.4% of patients at diagnosis and 36.4% at last visit, which rose to 85.7% and 72.7% respectively when using the age- and gender-adjusted reference data. Similarly, in the longitudinal observation, 23% of sMg measurements were < 0.7 mmol/l versus 79.7% when applying respective references.
Conclusions
Hypomagnesemia is underdiagnosed in children with HNF1B disease. sMg levels are age- and gender-dependent; thus, the use of appropriate reference data is crucial to hypomagnesemia in children.


Similar content being viewed by others
References
Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri G, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska A, Mehls O, Antignac C, Schaefer F, Salomon R (2006) Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 17:2864–2870. https://doi.org/10.1681/asn.2006030277
Vivante A, Kohl S, Hwang D, Dworschak G, Hildebrandt F (2014) Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol 29:695–704. https://doi.org/10.1007/s00467-013-2684-4
Clissold R, Hamilton A, Hattersley A, Ellard S, Bingham C (2014) HNF1B-associated renal and extra-renal disease—an expanding clinical spectrum. Nat Rev Nephrol 11:102–112. https://doi.org/10.1038/nrneph.2014.232
Adalat S, Bockenhauer D, Ledermann S, Hennekam R, Woolf A (2010) Renal malformations associated with mutations of developmental genes: messages from the clinic. Pediatr Nephrol 25:2247–2255. https://doi.org/10.1007/s00467-010-1578-y
Faguer S, Decramer S, Chassaing N, Bellanné-Chantelot C, Calvas P, Beaufils S, Bessenay L, Lengelé J, Dahan K, Ronco P, Devuyst O, Chauveau D (2011) Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int 80:768–776. https://doi.org/10.1038/ki.2011.225
Heidet L, Decramer S, Pawtowski A, Morinière V, Bandin F, Knebelmann B, Lebre A, Faguer S, Guigonis V, Antignac C, Salomon R (2010) Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol 5:1079–1090. https://doi.org/10.2215/cjn.06810909
Faguer S, Chassaing N, Bandin F, Prouheze C, Garnier A, Casemayou A, Huart A, Schanstra J, Calvas P, Decramer S, Chauveau D (2014) The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int 86:1007–1015. https://doi.org/10.1038/ki.2014.202
Raaijmakers A, Corveleyn A, Devriendt K, van Tienoven T, Allegaert K, Van Dyck M, van den Heuvel L, Kuypers D, Claes K, Mekahli D, Levtchenko E (2014) Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transplant 30:835–842. https://doi.org/10.1093/ndt/gfu370
van der Made C, Hoorn E, de la Faille R, Karaaslan H, Knoers N, Hoenderop J, Vargas Poussou R, de Baaij J (2015) Hypomagnesemia as first clinical manifestation of ADTKD-HNF1B: a case series and literature review. Am J Nephrol 42:85–90. https://doi.org/10.1159/000439286
Adalat S, Woolf A, Johnstone K, Wirsing A, Harries L, Long D, Hennekam R, Ledermann S, Rees L, van't Hoff W, Marks S, Trompeter R, Tullus K, Winyard P, Cansick J, Mushtaq I, Dhillon H, Bingham C, Edghill E, Shroff R, Stanescu H, Ryffel G, Ellard S, Bockenhauer D (2009) HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20:1123–1131. https://doi.org/10.1681/asn.2008060633
Okorn C, Goertz A, Vester U, Beck B, Bergmann C, Habbig S, König J, Konrad M, Müller D, Oh J, Ortiz-Brüchle N, Patzer L, Schild R, Seeman T, Staude H, Thumfart J, Tönshoff B, Walden U, Weber L, Zaniew M, Zappel H, Hoyer P, Weber S (2019) HNF1B nephropathy has a slow-progressive phenotype in childhood—with the exception of very early onset cases: results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol 34:1065–1075. https://doi.org/10.1007/s00467-018-4188-8
Ridefelt P, Aldrimer M, Rödöö P, Niklasson F, Jansson L, Gustafsson J, Hellberg D (2012) Population-based pediatric reference intervals for general clinical chemistry analytes on the Abbott Architect ci8200 instrument. Clin Chem Lab Med 50:845–851. https://doi.org/10.1515/cclm-2011-0787
Colantonio D, Kyriakopoulou L, Chan M, Daly C, Brinc D, Venner A, Pasic M, Armbruster D, Adeli K (2012) Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58:854–868. https://doi.org/10.1373/clinchem.2011.177741
Fathallah-Shaykh SA, Cramer MT (2013) Uric acid and the kidney. Pediatr Nephrol 29:999–1008. https://doi.org/10.1007/s00467-013-2549-x
Elisaf M, Panteli K, Theodorou J, Siamopoulos KC (1997) Fractional excretion of magnesium in normal subjects and in patients with hypomagnesemia. Magnes Res 10:315–320
Schwartz G, Muñoz A, Schneider M, Mak R, Kaskel F, Warady B, Furth S (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/asn.2008030287
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–S266
Meites S, Buffone G (1989) Pediatric clinical chemistry, edition 3. AACC Press, Washington, D.C.
Jahnen-Dechent W, Ketteler M (2012) Magnesium basics. Clin Kidney J 5:i3–i14. https://doi.org/10.1093/ndtplus/sfr163
Baaij JHFD, Hoenderop JGJ, Bindels RJM (2012) Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J 5:i15–i24. https://doi.org/10.1093/ndtplus/sfr164
Elin RJ (1988) Magnesium metabolism in health and disease. Dis Mon 34:166–218. https://doi.org/10.1016/0011-5029(88)90013-2
Berner LA, Keast DR, Bailey RL, Dwyer JT (2014) Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J Acad Nutr Diet. https://doi.org/10.1016/j.jand.2013.10.012
Shimizu T, Takayanagi K, Iwashita T et al (2018) Down-regulation of magnesium transporting molecule, claudin-16, as a possible cause of hypermagnesiuria with the development of tubulo-interstitial nephropathy. Magnes Res 31:11–23. https://doi.org/10.1684/mrh.2018.0434
Coburn JW (1969) The physicochemical state and renal handling of divalent ions in chronic renal failure. Arch Intern Med 124:302–311. https://doi.org/10.1001/archinte.124.3.302
Seeman T, Fořtová M, Sopko B, Průša R, Pohl M, John U (2018) Hypomagnesaemia is absent in children with autosomal dominant polycystic kidney disease. Ann Clin Biochem 56:90–94. https://doi.org/10.1177/0004563218785190
Sikora P, Zaniew M, Haisch L, Pulcer B, Szczepanska M, Moczulska A, Rogowska-Kalisz A, Bienia B, Tkaczyk M, Ostalska-Nowicka D, Zachwieja K, Hyla-Klekot L, Schlingmann K, Konrad M (2014) Retrospective cohort study of familial hypomagnesaemia with hypercalciuria and nephrocalcinosis due to CLDN16 mutations. Nephrol Dial Transplant 30:636–644. https://doi.org/10.1093/ndt/gfu374
Liu T, Wang C, Lu J, Zhao X, Lang Y, Shao L (2016) Genotype/phenotype analysis in 67 Chinese patients with Gitelman’s syndrome. Am J Nephrol 44:159–168. https://doi.org/10.1159/000448694
Acknowledgments
The authors are grateful to Dr. Ewa Elenberg (Baylor College of Medicine, Houston, Texas, USA) for her critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Informed consent from the study subjects has been waived due to the retrospective nature of the study. The study was conducted in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kołbuc, M., Leßmeier, L., Salamon-Słowińska, D. et al. Hypomagnesemia is underestimated in children with HNF1B mutations. Pediatr Nephrol 35, 1877–1886 (2020). https://doi.org/10.1007/s00467-020-04576-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04576-6