Abstract
Background
Approximately 50% of children with steroid-sensitive nephrotic syndrome (SSNS) will suffer from frequent relapses or steroid dependency, prompting the use of so-called steroid-sparing drugs. In this pilot study, we compare the efficacy and safety of rituximab to oral cyclophosphamide as first-line steroid-sparing medications.
Methods
A prospective open-label non-randomized study of children with frequent relapsing or steroid-dependant SSNS. Exclusion criteria were steroid-resistant disease, prescription of immunosuppressive agents other than prednisolone or levamisole, evidence of impaired kidney function, leucopenia, or active infection. The recruited children were allocated either to the oral cyclophosphamide (3 mg/kg/day for 8 weeks) or intravenous rituximab treatment (two doses of 375 mg/m2/dose, 2 weeks apart) and were monitored for relapses and side effects for 12 months.
Results
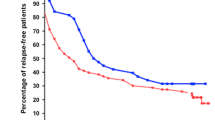
Forty-six subjects were included from two centers; 27 received cyclophosphamide and 19 received rituximab. One-year relapse-free survival was reached in 17 (58.6%) patients treated with cyclophosphamide compared to 16 (84.2%) with rituximab (adjusted HR 0.36; 95% CI 0.09–1.45; p = 0.151). The mean interval to relapse was 6.9 months in the cyclophosphamide group (N = 10) and 6.3 months in the rituximab group (N = 3). Both treatments were associated with a significant (p < 0.001) reduction in prescribed dose of oral alternate-day steroid from 1.02 to 0.36 mg/kg (cyclophosphamide) and 0.86 to 0.08 mg/kg (rituximab). Importantly, a significantly (p = 0.003) higher percentage of patients achieved complete withdrawal of steroid within 3 months of commencing study treatment in the rituximab (73.7%) versus cyclophosphamide (29.6%) group. Transient leucopenia was the most frequent adverse effect observed in the cyclophosphamide group (18.5%) and one patient (3.4%) had acute hepatotoxicity besides severe leucopenia and neutropenia in the 7th week of treatment with complete recovery with the withdrawal of cyclophosphamide and maintenance of remission. A minor infusion-related reaction in the form of a generalized macular skin rash was observed in one patient (5%) in the rituximab group.
Conclusions
Rituximab is non-inferior to cyclophosphamide and safe as a first-line steroid-sparing agent in children with SSNS. A larger multicenter study is required to assess superiority over cyclophosphamide.

Graphical abstract




Similar content being viewed by others
References
Vivarelli M, Massella L, Ruggiero B, Emma F (2017) Minimal change disease. Clin.J.Am.Soc.Nephrol. 12:332–345
Pravitsitthikul N, Willis NS, Hodson EM, Craig JC (2013), Non-corticosteroid immunosuppressive medications for steroid-sensitive nephroticsyndrome in children, Cochrane.Database.Syst.Rev. 29;(10):CD002290- PMID: 24166716 https://doi.org/10.1002/14651858.CD002290.pub4
Garin EH, Pryor ND, Fennell RS III, Richard GA (1978) Pattern of response to prednisone in idiopathic, minimal lesion nephrotic syndrome as a criterion in selecting patients for cyclophosphamide therapy. J Pediatr 92:304–308
Fu HD, Qian GL, Jiang ZY (2017) Comparison of second-line immunosuppressants for childhood refractory nephrotic syndrome: a systematic review and network meta-analysis. J Investig Med 65:65–71
Kyrieleis HA, Lowik MM, Pronk I, Cruysberg HR, Kremer JA, Oyen WJ, van den Heuvel BL, Wetzels JF, Levtchenko EN (2009) Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 4:1593–1600
Benz K, Dotsch J, Rascher W, Stachel D (2004) Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr.Nephrol. 19:794–797
Sun L, Xu H, Shen Q, Cao Q, Rao J, Liu HM, Fang XY, Zhou LJ (2014) Efficacy of rituximab therapy in children with refractory nephrotic syndrome: a prospective observational study in Shanghai. World J Pediatr 10:59–63
Wang X, Xu H (2013) New insights into treatment of nephrotic syndrome in children. Contrib.Nephrol. 181:119–130
Chaiwatanatorn K, Lee N, Grigg A, Filshie R, Firkin F (2003) Delayed-onset neutropenia associated with rituximab therapy. Br J Haematol 121:913–918
Dunleavy K, Hakim F, Kim HK, Janik JE, Grant N, Nakayama T, White T, Wright G, Kwak L, Gress R, Tosato G, Wilson WH (2005) B-cell recovery following rituximab-based therapy is associated with perturbations in stromal derived factor-1 and granulocyte homeostasis. Blood 106:795–802
Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Rava L, Ruggiero B, Emma F, Vivarelli M (2016) B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 27:1811–1822
Kattah AG, Fervenza FC, Roccatello D (2013) Rituximab-based novel strategies for the treatment of immune-mediated glomerular diseases. Autoimmun.Rev. 12:854–859
Dotsch J, Muller-Wiefel DE, Kemper MJ (2008) Rituximab: is replacement of cyclophosphamide and calcineurin inhibitors in steroid-dependent nephrotic syndrome possible? Pediatr.Nephrol. 23:3–7
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am 34:571–590
Sinha A, Bagga A (2012) Nephrotic syndrome. Indian J Pediatr 79:1045–1055
Webb H, Jaureguiberry G, Dufek S, Tullus K, Bockenhauer D (2016) Cyclophosphamide and rituximab in frequently relapsing/steroid-dependent nephrotic syndrome. Pediatr.Nephrol. 31:589–594
Hogan J, Deschenes G (2019) How to improve response to rituximab treatment in children with steroid-dependent nephrotic syndrome: answer to Drs. Fujinaga and Nishino. Pediatr.Nephrol. 34:361–362
Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM (2013) Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84:1025–1033
Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM (2011) Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol 6:1308–1315
Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, Srivastava RN, Moudgil A, Bagga A (2010) Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 5:2207–2212
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Bonanni A, Calatroni M, D'Alessandro M, Signa S, Bertelli E, Cioni M, Di Marco E, Biassoni R, Caridi G, Ingrasciotta G, Bertelli R, Di Donato A, Bruschi M, Canepa A, Piaggio G, Ravani P, Ghiggeri GM (2018) Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 2018(03/25):1238–1249
Berger JR, Malik V, Lacey S, Brunetta P, Lehane PB (2018) Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J Neuro-Oncol 2018(03/05):323–331
Cleland BD, Pokorny CS (1993) Cyclophosphamide related hepatotoxicity. Aust N Z J Med 23:408
Subramaniam SR, Cader RA, Mohd R, Yen KW, Ghafor HA (2013) Low-dose cyclophosphamide-induced acute hepatotoxicity. Am J Case Rep 14:345–349
Snyder LS, Heigh RI, Anderson ML (1993) Cyclophosphamide-induced hepatotoxicity in a patient with Wegener's granulomatosis. Mayo Clin Proc 68:1203–1204
Haubitz M (2007) Acute and long-term toxicity of cyclophosphamide. Transplantationsmedizin19:S26–S31
Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, Jayne DRW (2015) Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun 2014(12/31):60–65
Takei T, Nitta K (2011) Rituximab and minimal change nephrotic syndrome: a therapeutic option. Clin.Exp.Nephrol. 15:641–647
Prytuإ AA, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, Taylor M, Marks SD, Tuchman S, Camilla R, Ognjanovic M, Filler G, Smith G, Tullus K (2010) Rituximab in refractory nephrotic syndrome. Pediatr Nephrol (Berlin, Germany) 2009/12/23:461–468
Ahn YH, Kim SH, Han KH, Choi HJ, Cho H, Lee JW, Shin JI, Cho MH, Lee JH, Park YS, Ha IS, Cheong HI, Kim SY, Lee SJ, Kang HG (2018) Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: a multicenter open-label trial in Korea. Medicine 97:e13157
Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasأ M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group (2014), Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome, J Am Soc Nephrol 2014/01/30: 850–863
Nakagawa T, Shiratori A, Kawaba Y, Kanda K, Tanaka R (2016) Efficacy of rituximab therapy against intractable steroid-resistant nephrotic syndrome. Pediatr Int 2016(06/23):1003–1008
Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, Pasini A, Montini G, Edefonti A, Belingheri M, De Giovanni D, Barbano G, Degl'Innocenti L, Scolari F, Murer L, Reiser J, Fornoni A, Ghiggeri GM (2015) Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 2015(01/15):2259–2266
Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, Saito M, Yoneko M, Iijima K (2009) Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol (Berlin, Germany) 2009/05/07:1321–1328
Scolari F, Dallera N, Gesualdo L, Santoro D, Pani A, Santostefano M, Feriozzi S, Mani LY, Boscutti G, Messa P, Magistroni R, Quaglia M, Ponticelli C, Ravani P (2019) Rituximab versus steroids and cyclophosphamide for the treatment of primary membranous nephropathy: protocol of a pilot randomised controlled trial. BMJ Open 9:e029232
Chan EY-H, Webb H, Yu E, Ghiggeri GM, Kemper MJ, Ma AL-T, Yamamura T, Sinha A, Bagga A, Hogan J, Dossier C, Vivarelli M, Liu ID, Kamei K, Ishikura K, Saini P, Tullus K (2019) Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int 2020(10/31):393–401
Acknowledgments
The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Funding
This Project was funded by the Deanship of Scientific Research (DSR), at King Abdulaziz University, Jeddah, under grant no. G:33-140-1441.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research ethics committees at both contributing centers approved the study. Written informed consents were obtained from one of the parents of all included children. The study was performed according to the principles of the declaration of Helsinki.
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jameela A. Kari and Khalid A. Alhasan are joint first authors
Electronic supplementary material
ESM 1
(PPTX 1078 kb)
Rights and permissions
About this article
Cite this article
Kari, J.A., Alhasan, K.A., Albanna, A.S. et al. Rituximab versus cyclophosphamide as first steroid-sparing agent in childhood frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr Nephrol 35, 1445–1453 (2020). https://doi.org/10.1007/s00467-020-04570-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04570-y