Abstract
Background
Immunosuppression medication nonadherence has been associated with donor-specific antibodies and treatment-refractory rejection. Drug-level monitoring is a practical direct marker for nonadherence, as variations indicate erratic ingestion of medication. We previously reported that high variability in tacrolimus trough levels determined by the percent coefficient of variation (CV %) and standard deviation (SD) were associated with biopsy-proven rejection. We hypothesized that the CV % and SD in patients on a sirolimus/low-dose tacrolimus regimen may associate with self-reported medication nonadherence, rejection and donor-specific antibodies.
Methods
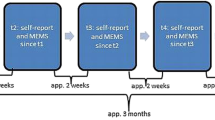
In this pilot feasibility study, we studied 37 biopsies in 23 pediatric renal transplant patients on both sirolimus and tacrolimus immunosuppression; CV %, SD, de novo donor-specific antibodies, rejection, and self-reported adherence were examined.
Results
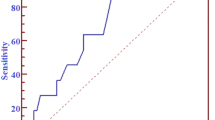
A cut-off sirolimus CV % of 25 maximized the percentage of biopsies correctly classified as rejection (32 of 37, or 86 %, p = 0.001). A cut-off tacrolimus CV % of 31 maximized the percentage of correctly classified biopsies (25 of 37, or 68 %, p = 0.09). Among patients with both high sirolimus and tacrolimus CV %, 67 % developed de novo donor-specific antibodies (p = 0.002) with a DQ predominance and 71 % reported nonadherence (p = 0.05).
Conclusions
In pediatric renal transplantation, sirolimus and tacrolimus CV % is a potential tool for monitoring patients at risk for allograft rejection and donor-specific antibodies secondary to medication nonadherence.




Similar content being viewed by others
References
Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN (2010) Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant 14:603–613
Sellares J, de Freitas D, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF (2012) Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12:388–399
Wiebe C, Gibson I, Blydt-Hansen T, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW (2012) Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12:1157–1167
Marcen R, Teruel J (2008) Patient outcomes after kidney allograft loss. Transplant Rev 22:62–72
Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR (2009) Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 9:2597–2606
Tsai EW, Wallace WD, Gjertson DW, Reed EF, Ettenger RB (2010) Significance of intragraft CD138+ lymphocytes and p-S6RP in pediatric kidney transplant biopsies. Transplantation 90:875–881
Schafer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S (2008) Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant 8(3):616–626
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Farmer K (1999) Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 21:1074–1090
Dobbels F, Berben L, De Geest S, Drent G, Lennerling A, Whittaker C, Kugler C, Transplant 360 Task Force (2010) The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation 90(2):205–219
Eisenberger U, Wuthrich R, Bock A, Ambuhl P, Steiger J, Intondi A, Kuranoff S, Maier T, Green D, DiCarlo L, Feutren G, De Geest S (2013) Medication adherence assessment: high accuracy of the new Ingestible Sensor System in kidney transplants. Transplantation 96:245–250
Shemesh E, Fine R (2010) Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant 14:940–943
Hsiau M, Fernandez H, Gjertson D, Ettenger RB, Tsai EW (2011) Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation 92:918–922
Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AL, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D (2010) Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant 14:968–975
Staatz CE, Tett SE (2007) Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 46:13–58
Garcia CD, Bittencourt VB, Alves AB, Garcia VD, Tumelero A, Antonello JS, Malheiros D (2006) Conversion to sirolimus in pediatric renal transplantation recipients. Transplant Proc 38(6):1901–1903
MacDonald AS (2003) Rapamycin in combination with cyclosporine or tacrolimus in liver, pancreas, and kidney transplantation. Transplant Proc 35:201S–208S
Schachter AD, Benfield MR, Wyatt RJ, Grimm PC, Fennell RS, Herrin JT, Lirenman DS, McDonald RA, Munoz-Arizpe R, Harmon WE (2006) Sirolimus pharmacokinetics in pediatric renal transplant recipients receiving calcineurin inhibitor co-therapy. Pediatr Transplant 10(8):914–919
El-Sabrout R, Delaney V, Qadir M, Butt F, Hanson P, Butt KM (2003) Sirolimus in combination with tacrolimus or mycophenolate mofetil for minimizing acute rejection risk in renal transplant recipients—a single center experience. Transplant Proc 35(3 Suppl):89S–94S.
Schubert M, Venkataramanan R, Holt D, Shaw LM, McGhee W, Reyes J, Webber S, Sindhi R (2004) Pharmacokinetics of sirolimus and tacrolimus in pediatric transplant patients. Am J Transplant 4:767–773
Butler J, Peveler R, Roderick P, Horne R, Mason J (2004) Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation 15:786–789
Bruce JM, Hancock LM, Lynch SG (2010) Objective adherence monitoring in multiple sclerosis: initial validation and association with self-report. Mult Scler 16:112–120
Blumberg J, Gritsch H, Reed E, Cecka J, Lipshutz G, Danovitch G, McGuire S, Gjertson DW, Veale JL (2013) Kidney paired donation in the presence of donor-specific antibodies. Kidney Int 84:1009–1016
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AH, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee (2014) Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14:272–283
Pape L, Ahlenstiel T (2014) mTOR inhibitors in pediatric kidney transplantation. Pediatr Nephrol 29(7):1119–1129
Ganschow R, Pape L, Sturm E, Bauer J, Melter M, Gerner P, Hocker B, Ahlenstiel T, Kemper M, Brinkert F, Sachse MM, Tonshoff B (2013) Growing experience with mTOR inhibitors in pediatric solid organ transplantation. Pediatr Transplant 17(7):694–706
Wu MJ, Shu KH, Lian JD, Yang CR, Cheng CH, Chen CH (2008) Impact of variability of sirolimus trough level on chronic allograft nephropathy. Transplant Proc 40:2202–2205
Wiebe C, Pochino D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, Goldberg A, Storsley LJ, Gibson IW, Rush DN, Nickerson PW (2013) Class II epitope matching—a strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13:3114–3122
Dunn TB, Browne BJ, Gillingham KJ, Kandaswamy R, Humar A, Payne WD, Sutherland DE, Matas AJ (2009) Selective retransplant after graft loss to nonadherence: success with a second chance. Am J Transplant 9:1337–1346
Pai ALH, Rausch J, Tackett A, Marsolo K, Drotar D, Goebel J (2012) System for integrated adherence monitoring: real-time non-adherence risk assessment in pediatric kidney transplantation. Pediatr Transplant 16:329–334
Acknowledgments
This work was supported by UCLA Children’s Discovery and Innovation Institute and Today and Tomorrow’s Children Fund. Additionally, Eileen Tsai and Helen Pizzo were supported by the Casey Lee Ball Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board at UCLA (IRB number 11–000153) and is in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pizzo, H.P., Ettenger, R.B., Gjertson, D.W. et al. Sirolimus and tacrolimus coefficient of variation is associated with rejection, donor-specific antibodies, and nonadherence. Pediatr Nephrol 31, 2345–2352 (2016). https://doi.org/10.1007/s00467-016-3422-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3422-5