Abstract
Calcium (Ca)-containing phosphate binders have been recommended for the treatment of hyperphosphatemia in children with chronic kidney disease. To study the effects of high Ca levels on trabecular bone volume (BV) and osteoprotegerin (OPG) expression in uremic young rats, a model of marked overcorrection of secondary hyperparathyroidism was created by providing a diet of high Ca to 5/6 nephrectomized young rats (Nx-Ca) for 4 weeks. The results of chondrocyte proliferation and apoptosis, osteoclastic activity, OPG expression and BV were compared among intact rats given the control diet, intact rats given a high Ca diet and 5/6 nephrectomized rats given the control diet (Nx-Control) and the high Ca diet (Nx-Ca). Ionized Ca levels were higher and parathyroid hormone levels were lower in Nx-Ca rats than in the other groups. Final weight, final length and final tibial length of Nx-Ca rats were significantly less than those of the other groups, although the length gain did not differ among the groups. The hypertrophic zone width was markedly enlarged in Nx-Ca rats. Chondrocyte proliferation rates did not differ among the groups, whereas osteoclastic activity was decreased in Nx-Ca rats compared with the Nx-Control animals. The OPG expression and BV were increased in Nx-Ca rats compared with the Nx-Control rats. Increased BV should improve bone strength, whereas disturbance of osteoclastogenesis interferes with bone remodeling. Bone quality has yet to be determined in high Ca-fed uremic young rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease mineral and bone disorders (CKD-MBD) are abnormalities of bone and mineral metabolism found in patients with CKD. Bone pathology occurs as an aspect of CKD-MBD and is called renal osteodystrophy (ROD). ROD is characterized by bone lesions ranging from high-turnover lesions of secondary hyperparathyroidism (SHPT) to low-turnover lesions of osteomalacia and adynamic bone (AD). Overall, SHPT remains the most common type of ROD afflicting children with CKD [1]. However, the incidence of AD has been increasing over the past decade. This increase has been attributed to aggressive interventions in managing SHPT patients with parathyroidectomy and active vitamin D and calcium (Ca) supplementation [2].
Dietary phosphate reduction, active vitamin D supplementation and Ca-containing phosphate binders are recommended for the treatment of hyperphosphatemia in patients with CKD to prevent SHPT. Growth impairment along with a reduction of tibial length have been observed in nephrectomized rats given high Ca. The hypertrophic zone of the growth plates in these animals were enlarged and associated with a decrease of matrix degradation and osteoclastogenesis [3].
At the molecular level, osteoclastogenesis is regulated by the receptor–activator of nuclear factor kappa beta (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) system. OPG is a soluble decoy receptor competing with RANK, which is found on the surface of osteoclasts, in bound form to RANKL which is expressed on osteoblasts. The competitive binding of OPG to RANKL results in the inhibition of osteoclast proliferation and differentiation [4]. In nephrectomized rats given high Ca, widening growth plates could potentially be the result of increased OPG expression by hypertrophic chondrocytes, and these might prevent osteoclastic breakdown of growth plate calcified cartilage. However, OPG expression in the growth plates of nephrectomized rats given high Ca has not yet been characterized. The aim of this study was to assess OPG expression in the growth cartilage of uremic rats receiving high Ca. We also measured trabecular bone volume (BV) in this animal model as BV is known to be increased in mice overexpressing the OPG gene [5] and in ovariectomized animals given recombinant OPG [6].
Material and methods
Forty-seven male weanling Sprague-Dawley rats each weighing approximately 110 gm (Harlan Laboratories, San Diego, CA) were obtained at 4 weeks of age and housed under similar conditions (12/12-h light/dark cycle, ambient temperature 22 ± 3°C). All study procedures were reviewed and approved by the Animal Protection Committee of UCLA (Los Angeles, CA). The rats were supplied with water and standard rat chow containing 18.6% protein, 0.8% Ca, 0.7% phosphorus (P), 0.2% magnesium (Mg) and 4.2 IU/g vitamin D (Purina Mills, Indianapolis, IN) ad libitum. After 1 week of acclimatization, 25 rats underwent a two-stage 5/6 nephrectomy to produce renal failure, as previously described [7]. Twenty-two rats underwent sham nephrectomy in two stages corresponding to the time of the actual two-stage 5/6 nephrectomy. The day after completing the second set of surgical procedures, all of the animals were weighed and the body length measured from the tip of the noses to the end of the tails.
Eleven sham-nephrectomized control (Intact-Control, n = 11) animals and 11 nephrectomized control (Nx-Control, n = 11) animals were fed a standard rat chow as described above. By contrast, 14 nephrectomized rats were placed on a high Ca diet containing 2.5% Ca and 0.7% P, 0.2% Mg and 4.2 IU/g vitamin D (Nx-Ca, n = 14). Eleven other sham-nephrectomized rats were also fed a high Ca diet (Intact-Ca, n = 11). Animals from each group were maintained on the specified diets for 4 weeks. In order to ensure an equivalent caloric intake between the control and the nephrectomized animals, the rats were pair-fed by feeding the control rats the same amount of food that had been consumed the previous day by their nephrectomized counterparts.
After 4 weeks, bromodeoxyuridine (BrdU, 100 mg/kg; Zymed Laboratories, San Francisco, CA) was administered intraperitoneally approximately 2 h prior to sacrifice. At sacrifice, blood was obtained by cardiac puncture and tissues were fixed by trans-cardiac perfusion as previously described [3]. Blood was centrifuged and serum was stored at -70°C until further use. Tibiae were excised, and the final tibial length was reported as the average of the triplicate measurements between the proximal and distal articular surfaces using an electronic caliper. Bones were then immersed in 4% paraformaldehyde/phosphate buffered saline (PFA/PBS) for 48 h, and the tibiae decalcified and embedded in paraffin [3]. Sections of bone (5 μm) obtained using a Leica RM 2165 microtome (Leica Microsystems, Nussloch, Germany) were mounted on Superfrost Plus slides (Fisher Scientific, Springfield, NJ) and used for morphometric analysis, in situ hybridization, bone histochemistry and bone immunohistochemistry. Femurs were dehydrated in increasing concentrations of alcohol, embedded in methylmethacrylate, sectioned into 5-μm sections and mounted on slides for bone histomorphometry and bone mineralized matrix assessment.
Serum biochemical determinations
Blood ionized Ca levels were determined using a Ca-sensitive electrode (ICA Radiometer, Copenhagen, Denmark). Serum P, creatinine and blood urea nitrogen (BUN) levels were measured by standard laboratory methods. Serum parathyroid (PTH) levels were measured using an immunoradiometric assay for rat intact PTH (Immunotopics, San Clemente, CA).
Morphometric assessment of growth plate cartilage
Morphometric assessment of the growth plate cartilage was performed on sections stained with hematoxylin and eosin and counter-stained with azure A [7]. The total width of the growth plate cartilage at the proximal end of each tibia was measured. An average of 30 width measurements from each growth plate represented the final width determinations in individual animals. The width of the zone occupied by hypertrophic chondrocytes was measured by the same method.
In situ hybridization
In situ hybridization was performed on sections of decalcified tibiae. [35S]-labeled riboprobes with a specific activity of 1–2 × 109 cpm/μg in combination with the Gemini transcription kit (Promega, Madison, WI) was used for MMP-9/gelatinase B. The Digoxigenin (DIG)-labeled riboprobe (Roche Diagnostics, Mannheim, Germany) was used for OPG. The number of cells expressing the specific mRNA was counted and expressed as a percentage of the number of positive cells to the total number of cells in the appropriate growth plate zone where the mRNA expression was localized.
Immunohistochemistry (BrdU labeling, TUNEL reaction, TRAP)
Chondrocyte proliferation was assessed by the BrdU staining kit (Zymed Laboratories). Immunoreactive cells were counted, and data were expressed as the percentage of cells that were positive within the proliferative zone and hypertrophic zone immediately adjacent to the proliferative zone. Chondrocyte apoptosis was detected using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reaction (ApopTag In Situ Apoptosis Detection kits; Intergen, Purchase, NY). The rate of apoptosis was calculated from the percentage of lower hypertrophic chondrocytes that were positively stained.
The tartrate resistant acid phosphatase (TRAP) stain was performed using α-naphthyl acid phosphate and sodium tartrate salt followed by counterstaining with nuclear fast green. TRAP-positive cells were counted in a rectangular region defined by the area extending from the growth plate to approximately 300 μm below the chondro-osseous junction in the primary spongiosa. Results were expressed as the number of TRAP-positive cells per area of the growth plate.
Assessment of the bone mineralized matrix and quantitative bone histomorphometry
Sections of plastic-embedded non-decalcified femurs were used for assessment of the bone mineralized matrix by Von Kossa staining for mineralization. Quantitative bone histomorphometry was performed by the Goldner stain using a Merz ocular grid [8] and the Osteoplan II Image Analysis System (Kontron, Ecking, Germany). One hundred adjacent fields of the examined region, which was defined by the area in the primary spongiosa between 300 and 500 μm below the growth plate, were counted under a microscope at a magnification of 160×. Areas and perimeters were measured by point counting and by linear intersection methods. The results were reported using the standardized methods proposed by Parfitt et al. [9]. BV was expressed as a percentage of total tissue volume (TV), and osteoid volume (OV) was expressed as a percentage of BV. The perimeter measurements for the osteoid surface (OS) and the eroded surface (ES) were expressed as a percentage of the total bone surface (BS).
Statistical analysis
All results are expressed as mean values ± standard deviation (SD). Data were evaluated by one-way analysis of variance (ANOVA) with the Tukey HSD post-hoc test for comparisons between groups using Statistical Package for the Social Sciences ver. 13 software (SPSS, Chicago, IL). Probability values of less than 5% were considered to be significant.
Results
Serum creatinine and BUN were significantly higher in nephrectomized rats than in the intact rats which had normal renal function. Although the serum creatinine levels in intact rats were elevated, they remained significantly lower than those in nephrectomized rats. The results from the creatinine assay must have been a deviation since the BUN levels were within the normal ranges, which indicated that the intact rats had normal renal function. Serum creatinine levels in nephrectomized rats were about 2 mg/dL, reflecting a model of relatively severe uremia. Blood ionized Ca concentrations were significantly higher and serum PTH values were significantly lower in Nx-Ca rats than in Nx-Control rats. Serum PTH values were significantly higher in the Nx-Control rats than in the other groups. Serum P levels did not differ among groups (Table 1).
Body weight and length did not differ among groups at the beginning of the 4-week study period. The final weight of rats fed a high Ca diet was significantly less than that of their control counterparts and was lowest in Nx-Ca. Final length was significantly less in Nx-Ca than in Intact-Control rats but did not differ from the other groups. Despite Nx-Ca rats having the shortest final length, the length gain did not differ among the four groups. Final tibial length was significantly less in the nephrectomized animals than in their intact counterparts. The tibial length of Nx-Ca rats was significantly shorter than that in the other groups; however, there was no significant difference in tibial length between Nx-Ca and Nx-Control rats (Table 2).
The width of the epiphyseal growth plate and the hypertrophic zone in the proximal tibia did not differ among the Intact-Control, Intact-Ca and Nx-Control rats. By contrast, the epiphyseal growth plate was significantly wider in Nx-Ca rats than in the other groups. This difference was the result of an increase in the hypertrophic zone width immediately adjacent to the primary spongiosa (Fig. 1).
The width of total growth plate and hypertrophic zone in Intact versus nephrectomized rats (Nx) on normal (Control) and high calcium (Ca) diets (magnification 40×). GP Growth plate, Intact-Control, sham-nephrectomized control rats on standard diet, Intact-Ca sham-nephrectomized rats on high Ca diet, Nx-Control nephrectomized control rats on standard diet, Nx-Ca nephrectomized rats on high Ca diet
To determine the underlying processes contributing to the widening of the growth plate in Nx-Ca rats, chondrocyte proliferation rate, chondrocyte apoptosis rate, matrix degradation and osteoclastic activity were assessed. Chondrocyte proliferation and apoptosis rate were not different among the four groups (Table 3). By contrast, matrix degradation and osteoclastic activity were significantly lower in Nx-Ca rats than in the other groups (Figs. 2, 3, respectively). The mRNA expression for OPG was significantly increased in Nx-Ca rats compared to the other groups (Fig. 4).
In situ hybridization of MMP-9/Gelatinase B mRNA expresssion (arrowheads) in the chondro–osseous junction in Intact versus nephrectomized rats (Nx) on normal (Control) and high calcium (Ca) diets, respectively. The average number of MMP-9/Gelatinase B expressing chondrocytes per total number of chondrocytes in the chondro–osseous junction in each group is shown in the graph (magnification 40×)
Tartrate resistant acid phosphatase (TRAP) staining reveals osteoclasts (arrowheads) in the chondro–osseous junction in Intact versus nephrectomized rats (Nx) on normal (Control) and high calcium (Ca) diets, respectively. Osteoclastic activity was determined by the average number of osteoclasts per area of the growth plate in the chondro–osseous junction (magnification 175×)
In situ hybridization of OPG mRNA expresssion in the chondro–osseous junction in Intact versus nephrectomized rats (Nx) on normal (Control) and high calcium (Ca) diets, respectively. The average number of OPG expressing chondrocytes per total number of chondrocytes in the chondro–osseous junction in each group was shown in the graph (magnification 175×)
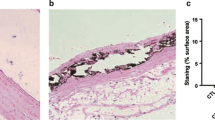
Quantitative bone histomorphometry analyses revealed that Nx-Ca rats had higher BV than the other groups (Table 4, Fig. 5). OV and OS were lower in Nx-Ca rats than in the Nx-Control rats. Although OV and OS were higher in Nx-Ca rats than in the intact rats, the difference did not reach statistical significance (Table 4). ES was significantly higher in the Nx-Control rats than in the other groups. There was no difference in ES between the Nx-Ca and intact rats.
The bar graph demonstrates trabecular bone area measurement expressed as a percentage of total tissue area in the Intact versus nephrectomized rats (Nx) on normal (Control) and high calcium (Ca) diets. The lower panel shows the bone mineralized matrix assessed by Von Kossa staining of sections of non-decalcified femur (magnification 6.3×)
Discussion
In this study, the final weight was significantly less in rats fed a high Ca diet than in their control counterparts and was lowest in Nx-Ca rats. The poor weight gain in Nx-Ca rats may have been confounded by the smaller amount of food consumed by these rats as we did not ensure an equivalent amount of caloric intake between the high Ca groups and their control counterparts. The shorter final length of Nx-Ca rats could partly be attributable to poor weight gain. Therefore, a high Ca diet could potentially aggravate the growth impairment in uremic rats. Nevertheless, tibial length and length gain did not differ between Nx-Ca rats and the Nx-Control rats.
The epiphyseal growth plates in Nx-Ca rats were markedly enlarged due to an increased hypertrophic zone width. The proliferative zone width in these animals remained unchanged. The chondrocyte proliferation rate was normal in our animal model compared with the decreased chondrocyte proliferation in younger nephrectomized rats given a high Ca diet reported by Sanchez et al. (initial weight 90 g vs. 60 g) [10]. However, the decrease in matrix degradation and osteoclastic activity were similar in both studies [10].
OPG expression was significantly elevated in the nephrectomized animals given a high Ca diet but not in intact rats on the same diet. OPG inhibits osteoclastogenesis by competitively binding to RANKL, thereby preventing RANKL from binding and activating RANK [4]. OPG expression and secretion are modulated by various metabolic regulators, including PTH [11]. Studies in murine models revealed that osteoclast differentiation is induced as the result of an increased RANKL/OPG ratio in bone marrow stromal cells following PTH exposure [12, 13]. Similar findings were noted in humans receiving intermittent PTH treatment: serum RANKL level increased while the serum OPG level decreased [14]. Thus, the increased OPG expression in uremic rats receiving a high Ca diet could be attributable to significantly reduced PTH levels in this group of rats.
A suppressed PTH level similar that observed in our animal model was also found in nephrectomized rats receiving calcitriol therapy. Unlike our animal model, their growth plate showed a normal width, decreased chondrocyte proliferation and normal BV [15]. This evidence raises the possibility that high Ca may cause disturbances in the growth plate and bone in uremic rats through other additional mechanisms that are distinct from PTH suppression, such as by directly inhibiting MMP-9/gelatinase B expression [10], or calcitriol could possibly counteract the direct effect of PTH on OPG expression [16].
A decreased expression of MMP-9/gelatinase B could interfere with angiogenesis, a crucial process in endochondral ossification, as MMP-9/gelatinase B plays a major role in hypertrophic zone vascularization and ossification. An enlargement of the hypertrophic zone similar to the change in the growth plate observed in our animal model was also reported in homozygous mice with a null mutation in MMP-9/gelatinase B. These animals showed delayed angiogenesis and apoptosis of hypertrophic chondrocytes [17]. Further investigations on angiogenic factors, including the evaluation of vascular endothelial growth factor (VEGF) expression in uremic rats fed a high Ca diet should better elucidate the effects of Ca on alterations in growth plate morphology.
The widening growth plates observed in our animal model could be the result of increased OPG expression by hypertrophic chondrocytes, resulting in an inhibition of osteoclastic breakdown of the growth plate calcified cartilage. Although increased growth plate width has been observed in fetal rats exposed to bisphosphonates, the underlying mechanism of alterations in growth plate morphology from this antiresorptive agent is likely to be the result of the inhibition of collagen fiber breakdown, as shown in the study by Kim et al. [18]. Moreover, bisphosphonates prevent bone resorption via different mechanisms independent of the OPG pathway, as supported by the fact that bisphosphonates effectively inhibit bone resorption and prevent bone loss even in the OPG knockout mice [19].
Concurrent with decreased osteoclastic activity and increased OPG expression, we found an increase in the bone mineralized matrix and BV in nephrectomized animals given a high Ca diet that resemble the findings presented in transgenic mice overexpressing the OPG gene [5]. More evidence supporting the bone protective effect of OPG has been noted in ovariectomized and orchiectomized animals receiving recombinant OPG administration as their BV and bone density were improved. These animals demonstrated decreased osteoclastogenesis and decreased bone loss [6, 20].
Osteoclastic activity assessed by TRAP staining was markedly decreased in Nx-Ca rats when compared with that in the other groups. By contrast, the bone resorption or ES, assessed by bone histomorphometry, did not significantly differ from that in the intact groups. However, the ES of Nx-Ca rats was significantly decreased when compared with that of the Nx-Control rts. Although the OV and OS were higher in Nx-Ca rats than in the intact groups, the difference did not reach statistical significance, but OV and OS were less in Nx-Ca rats than in the Nx-Control animals, suggesting that osteoblastic activity was decreased in the former when compared with the latter. Increased OPG expression may potentially modify osteoblastic function in uremic animals. However, the definite role of OPG on osteoblastic activity has to be further investigated. Increased BV in our Nx-Ca animals most likely resulted from decreased osteoclastic activity as the osteoblastic function was not significantly increased.
Contrary to studies in animal models, in hemodialysis patients, high serum OPG levels were found in those who had low bone mineral density. Serum OPG in this setting does not appear to protect the skeleton against the increased bone resorption of renal osteodystrophy [21]. It has been postulated that OPG in the serum is bound to plasma proteins and thus inactive [22]. Moreover, serum OPG levels may not entirely reflect OPG expression in the bone as elevation of serum OPG levels may be the result of reduced renal clearance and the lack of its elimination through the dialysis membrane [23].
In summary, uremic animals receiving a diet highly supplemented with Ca demonstrated decreased osteoclastic activity and increased BV associated with increased OPG expression. As RANK, RANKL and OPG play an integral role in the regulatory pathway of osteoclastogenesis, elucidating the regulation of the RANK/RANKL/OPG system in uremic animals receiving a high Ca diet could be initially performed by measuring only OPG expression. Even though we have shown that the OPG level was increased in such animals, the roles of RANK and RANKL on the bone remodeling process in uremic animals receiving a high Ca diet should be further examined. Disturbances of osteoclastogenesis have been shown to interfere with the bone remodeling process and result in an increased risk of recurrent fractures [24]. Conversely, increased BV should increase bone strength and prevent fractures, as has been shown in several reports [25, 26]. Although the bone quantity was increased in these animals, the bone quality is yet to be determined. To which extent nutrition interferes with the metabolism on the growth plates in uremic rats receiving a high Ca diet should be further assessed as the data on food intake and food conversion ratio were not available and the weight gain was significantly less in these animals.
As previously shown in Table 1, serum PTH levels in the Ca-supplemented nephrectomized rats in our experiment were severely suppressed to less than 50% of the mean value observed in the intact rats. Such an overcorrection of SHPT should be avoided in the clinical setting to allow normal bone remodeling to occur. This animal model reflects a rare but relevant clinical condition which needs to be prevented by all available means, including the use of calcium-free phosphate binders and low calcium dialysate concentrations.
References
Salusky IB, Coburn JW, Brill J, Foley J, Slatopolsky E, Fine RN, Goodman WG (1988) Bone disease in pediatric patients undergoing dialysis with CAPD or CCPD. Kidney Int 33:975–982
Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, Goodman WG (1998) Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney Int 54:907–914
Sanchez CP, Kuizon BD, Abdella PA, Juppner H, Salusky IB, Goodman WG (2000) Impaired growth, delayed ossification, and reduced osteoclastic activity in the growth plate of calcium-supplemented rats with renal failure. Endocrinology 141:1536–1544
Wright HL, McCarthy HS, Middleton J, Marshall MJ (2009) RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med 2:56–64
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Shimizu-Ishiura M, Kawana F, Sasaki T (2002) Osteoprotegerin administration reduces femural bone loss in ovariectomized mice via impairment of osteoclast structure and function. J Electron Microsc (Tokyo) 51:315–325
Sanchez CP, Salusky IB, Kuizon BD, Abdella P, Juppner H, Goodman WG (1998) Growth of long bones in renal failure: roles of hyperparathyroidism, growth hormone and calcitriol. Kidney Int 54:1879–1887
Merz WA, Schenk RK (1970) A quantitative histological study on bone formation in human cancellous bone. Acta Anat (Basel) 76:1–15
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Sanchez CP, He YZ (2003) Effects of thyroparathyroidectomy, exogenous calcium, and short-term calcitriol therapy on the growth plate in renal failure. J Am Soc Nephrol 14:148–158
Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D (2004) The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 15:457–475
Lee SK, Lorenzo JA (1999) Parathyroid hormone stimulates TRANCE and inhibits osteoprotegerin messenger ribonucleic acid expression in murine bone marrow cultures: correlation with osteoclast-like cell formation. Endocrinology 140:3552–3561
Locklin RM, Khosla S, Turner RT, Riggs BL (2003) Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem 89:180–190
Buxton EC, Yao W, Lane NE (2004) Changes in serum receptor activator of nuclear factor-kappaB ligand, osteoprotegerin, and interleukin-6 levels in patients with glucocorticoid-induced osteoporosis treated with human parathyroid hormone (1–34). J Clin Endocrinol Metab 89:3332–3336
Lund RJ, Davies MR, Brown AJ, Hruska KA (2004) Successful treatment of an adynamic bone disorder with bone morphogenetic protein-7 in a renal ablation model. J Am Soc Nephrol 15:359–369
Kearns AE, Khosla S, Kostenuik PJ (2008) Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29:155–192
Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z (1998) MMP-9/Gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411–422
Kim MS, Kim JH, Lee MR, Kang JH, Kim HJ, Ko HM, Choi CH, Jung JY, Koh JT, Kim BK, Oh HK, Kim WJ, Lee EJ, Kim SH (2009) Effects of alendronate on a disintegrin and metalloproteinase with thrombospondin motifs expression in the developing epiphyseal cartilage in rats. Anat Histol Embryol 38:154–160
Liu J, Xu K, Wen G, Guo H, Li S, Wu X, Dai R, Sheng Z, Liao E (2008) Comparison of the effects of genistein and zoledronic acid on the bone loss in OPG-deficient mice. Bone 42:950–959
Li X, Ominsky MS, Stolina M, Warmington KS, Geng Z, Niu QT, Asuncion FJ, Tan HL, Grisanti M, Dwyer D, Adamu S, Ke HZ, Simonet WS, Kostenuik PJ (2009) Increased RANK ligand in bone marrow of orchiectomized rats and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerin. Bone 45:669–676
Doumouchtsis KK, Kostakis AI, Doumouchtsis SK, Tziamalis MP, Stathakis CP, Diamanti-Kandarakis E, Dimitroulis D, Perrea DN (2008) Associations between osteoprotegerin and femoral neck BMD in hemodialysis patients. J Bone Miner Metab 26:66–72
Collin-Osdoby P (2004) Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 95:1046–1057
Fukagawa M, Kazama JJ, Kurokawa K (2002) Renal osteodystrophy and secondary hyperparathyroidism. Nephrol Dial Transplant 17[Suppl 10]:2–5
Boyce BF, Xing L (2007) Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther 9[Suppl 1]:S1
Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, Opas EE, Seedor JG, Klein H, Frankenfield D (1993) The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest 92:2577–2586
Yang LC, Majeska RJ, Laudier DM, Mann R, Schaffler MB (2005) High-dose risedronate treatment partially preserves cancellous bone mass and microarchitecture during long-term disuse. Bone 37:287–295
Acknowledgments
We would like to thank the National Kidney Foundation of Southern California for supporting the Institutional Research Fellowship, Dr. William Goodman for kindly permitting the use of laboratory equipment for quantitative bone histomorphometry measurement and Dr. Isidro Salusky and Dr. Beatriz Kuizon for their constructive advice.
Transparency declarations
None to declare.
Conflict of interest statement
The results presented in this paper have not been published previously in whole or part, except in abstract form.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rianthavorn, P., Ettenger, R.B., Salusky, I.B. et al. Trabecular bone volume and osteoprotegerin expression in uremic rats given high calcium. Pediatr Nephrol 25, 2311–2319 (2010). https://doi.org/10.1007/s00467-010-1611-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1611-1