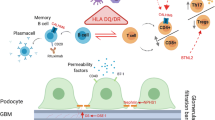
Abstract
Reports on genetically informative steroid-responsive (sensitive) idiopathic nephrotic syndrome (SSNS) families are lacking. We studied an extended SSNS Bedouin (B) family with a high rate of consanguinity. The clinical presentation and steroid response of its 11 affected individuals were similar to those of sporadic SSNS (spontaneous remission towards puberty and minimal change disease by kidney biopsy). Genome-wide linkage analysis, using a 382 microsatellite-markers mapping set and additional markers adjacent to 80 candidate genes of the index family, did not support linkage to any chromosomal locus. Retrospective analysis of all additional children with SSNS treated by our institution in the past 20 years (n = 96, 50% of them of Jewish origin) revealed another five non-related B families with 2–3 first-degree cousins affected with SSNS in each. The overall familial SSNS rate among the B population (excluding the index family) was 28%, compared with 4% among Jews (Js) (OR 1.8–64, P < 0.005). There were more Bs with simple SSNS than there were Js (71% and 40%, respectively; OR 3.58, 95% CI 1.41–9.23, P < 0.01). In summary, SSNS in this index family was not linked to any of the presently known chromosomal loci nor predicted to be caused by mutation in any one of a list of genes associated with nephrotic syndrome (NS). The presence of other B families affected by SSNS supports the role for susceptibility genes enrichment, exposing highly consanguineous populations to an increased incidence of SSNS.


Similar content being viewed by others
Abbreviations
- B:
-
bedouin
- FSGS:
-
focal segmental glomerulosclerosis
- GBM:
-
glomerular basement membrane
- J:
-
jew
- LOD:
-
logarithmic odds
- MCNS:
-
minimal change nephrotic syndrome
- NS:
-
nephrotic syndrome
- SSNS:
-
steroid sensitive idiopathic nephrotic syndrome
References
White RH (1973) The familial nephrotic syndrome. I: A European survey. Clin Nephrol 1:215–219
Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24:349–354
Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A (2004) Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13:2625–2632
Fuchshuber A, Gribouval O, Ronner V, Kroiss S, Karle S, Brandis M, Hildebrandt F (2001) Clinical and genetic evaluation of familial steroid-responsive nephritic syndrome in childhood. J Am Soc Nephrol 12:374–378
Negev Development Authority and Negev Center for Regional Development, Ben Gurion University. Statistical Report. https://doi.org/www.negev.co.il/statis/ch2.asp
Barratt TM, Avner ED, Harmon WE (1999) Pediatric nephrology, 4th edn. Lippincott, Williams & Wilkins, Baltimore, pp 731–747
Statistical abstract of Israel No. 57, CBS, Central Bureau of Statistics, Jerusalem, 2006 https://doi.org/www1.cbs.gov.il/reader/shnatonenew_site.htm
Fishelson M, Geiger D (2002) Exact genetic linkage computations for general pedigrees. Bioinformatics 18(Suppl 1):S189–198
Shalhoub RJ (1974) Pathogenesis of lipid nephrosis: a disorder on T-cell function. Lancet 2:556–560
Yoshizawa N, Kusumi Y, Matsumoto K, Oshima S, Takeuchi A, Kawamura O, Kubota T, Kondo S, Niwa H (1989) Studies of a glomerular permeability factor in patients with minimal-change nephrotic syndrome. Nephron 51:370–376
Schnaper HW, Aune TM (1987) Steroid-sensitive mechanism of soluble immune response suppressor production in steroid-responsive nephrotic syndrome. J Clin Invest 79:257–264
Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F (1996) Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 334:878–883
Le Berre L, Godfrin Y, Gunther E, Buzelin F, Perretto S, Smit H, Kerjaschki D, Usal C, Cuturi C, Soulillou JP, Dantal J (2002) Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J Clin Invest 109:491–498
Grimbert P, Audard V, Remy P, Lang P, Sahali D (2003) Recent approaches to the pathogenesis of minimal-change nephrotic syndrome. Nephrol Dial Transplant 18:245–248
Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P (2004) Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113:1390–1397
Ruf RG, Schultheiss M, Lichtenberger A, Karle SM, Zalewski I, Mucha B, Everding AS, Neuhaus T, Patzer L, Plank C, Haas JP, Ozaltin F, Imm A, Fuchshuber A, Bakkaloglu A, Hildebrandt F; APN Study Group (2004) Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney Int 66:564–570
Sako M, Nakanishi K, Obana M, Yata N, Hoshii S, Takahashi S, Wada N, Takahashi Y, Kaku Y, Satomura K, Ikeda M, Honda M, Iijima K, Yoshikawa N (2005) Analysis of NPHS1, NPHS2, ACTN4, and WT1 in Japanese patients with congenital nephrotic syndrome. Kidney Int 67:1248–1255
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256
Boner G, Cox AJ, Kelly DJ, Tobar A, Bernheim J, Langham RG, Cooper ME, Gilbert RE (2003) Does vascular endothelial growth factor (VEGF) play a role in the pathogenesis of minimal change disease? Nephrol Dial Transplant 18:2293–2299
Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP (1995) The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet 10:400–406
Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS (1999) Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286:312–315
Clark AG, Vaughan RW, Stephens HA, Chantler C, Williams DG, Welsh KI (1990) Genes encoding the beta-chains of HLA-DR7 and HLA-DQw2 define major susceptibility. Clin Sci (Lond) 78:391–397
Tain YL, Liu CA, Yang KD (2002) Implications of blood soluble and cell surface tumor necrosis factor receptors in childhood nephrotic syndrome. Pediatr Nephrol 17:926–932
Uemichi T, Liepnieks JJ, Benson MD (1994) Hereditary renal amyloidosis with a novel variant fibrinogen. J Clin Invest 93:731–736
Costa PP, Figueira AS, Bravo FR (1978) Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci USA 75:4499–4503
Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M (2002) Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31:424–428
Neuhausen SL, Weizman Z, Camp NJ, Elbedour K, Sheffield VC, Zone JJ, Carmi R (2002) HLA-DQA1-DQB1 genotypes in Bedouin families with celiac disease. Hum Immunol 63:502–507
Alsana F, Porath A, Binsztok M, Weizman Z (2000) Factors affecting the incidence of celiac disease in two different populations. J Pediatr Gastroenterol Nutr 31:S211
Neuhausen SL, Feolo M, Camp NJ, Farnham J, Book L, Zone JJ (2002) Genome-wide linkage analysis for celiac disease in North American families. Am J Med Genet 111:1–9
Peltonen L, Palotie A, Lange K (2000) Use of population isolates for mapping complex traits. Nat Rev Genet 1:182–190
Weitzman D, Shoham-Vardi I, Elbedour K, Belmaker I, Siton Y, Carmi R (2000) Factors affecting the use of prenatal testing for fetal anomalies in a traditional society. Community Genet 3:61–70
Zlotogora J (2002) Molecular basis of autosomal recessive diseases among the Palestinian Arabs. Am J Med Genet 109:176–182
Zlotogora J, Shalev S, Habiballah H, Barjes S (2000) Genetic disorders among Palestinian Arabs: 3. Autosomal recessive disorders in a single village. Am J Med Genet 92:343–345
Ombra MN, Forabosco P, Casula S, Angius A, Maestrale G, Petretto E, Casu G, Colussi G, Usai E, Melis P, Pirastu M (2001) Identification of a new candidate locus for uric acid nephrolithiasis. Am J Hum Genet 68:1119–1129
Ruf RG, Fuchshuber A, Karle SM, Lemainque A, Huck K, Wienker T, Otto E, Hildebrandt F (2003) Identification of the first gene locus (SSNS1) for steroid-sensitive nephrotic syndrome on chromosome 2p. J Am Soc Nephrol 14:1897–1900
Kong X, Murphy K, Raj T, He C, White PS, Matise TC (2004) A combined linkage-physical map of the human genome. Am J Hum Genet 75:1143–1148
de Zoysa JR, Topham PS (2005) Podocyte biology in human disease. Nephrology 10:362–367
Acknowledgment
Dan Geiger’s research was supported by the Israeli Ministry of Science. This study was partially supported by a research grant from the National Institute of Biotechnology in the Negev. We thank Prof. Ilana Shoham-Vardi for her help with the statistical calculations.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at https://doi.org/10.1007/s00467-007-0448-8
Appendix
Appendix
Tables 2, 3, 4 and 5: markers were used for linkage analysis to the candidate genes. The genetic distance between the gene and the marker was calculated on the basis of the genetic map [37] and the physical distance to the marker in the Human UCSC genome browser https://doi.org/genome.ucsc.edu/cgi-bin/hgGateway), assuming 1 cM equals 0.5 Mb. Candidate genes were chosen on the basis of literature search. A detailed list of the references can be provided by D.L. upon request. See suggested review [38] for podocyte physiology and specific proteins potentially involved in NS. The genes are depicted by categories. Table 2: (a) Hereditary proteinuria syndromes and (b) chromosomal loci linked to NS or other glomerulopathies; Table 3: podocyte-related and GBM structural proteins; Table 4: (a) immunity-related genes, associated with SSNS severity and (b) immunity-related genes, associated with SSNS tendency; Table 5: genes associated with deterioration/fibrosis of renal function (all species).
Rights and permissions
About this article
Cite this article
Landau, D., Oved, T., Geiger, D. et al. Familial steroid-sensitive nephrotic syndrome in Southern Israel: clinical and genetic observations. Pediatr Nephrol 22, 661–669 (2007). https://doi.org/10.1007/s00467-006-0409-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0409-7