Abstract
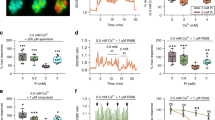
In mammals, neonatal positive calcium balance is required for adequate growth. Parathyroid hormone (PTH) plays a central role in this process mainly through its action on the distal nephron. We studied the effect of PTH on cytosolic calcium in distal segments from neonatal rat kidney. PTH elicited a concentration-dependent increase in cytosolic calcium in neonatal distal nephron (EC50=0.5 nM) but not in proximal tubules. At similar PTH concentrations the response was higher in the neonatal than in the adult tubules. The response was associated with protein kinase C (PKC), since phorbol myristate acetate (100 nM) increased [Ca2+]i, and staurosporin, an inhibitor of PKC, decreased (10 nM) or suppressed (100 nM) the PTH effect. cAMP analogues did not change [Ca2+]i. The response was diminished in low external calcium (0.1 mM) and absent at zero calcium, indicating dependency on external calcium. Resting calcium decreased from 80±10.8 to 28.6±2.6 nM at zero [Ca2+]e. PTH and nifedipine increased cytosolic calcium in an additive fashion. We show for the first time that PTH increased cytosolic calcium in the distal nephron of neonatal kidney, in a concentration-dependent pattern and in association with PKC activation. Higher sensitivity of the neonatal tubule might facilitate absorption of this cation during the neonatal period, when growth requires a positive balance of calcium.









Similar content being viewed by others
References
Karlen J, Rane S, Aperia A (1990) Tubular response to hormones is blunted in weanling rats. Acta Physiol Scand138:443–449
Sheu JN, Baum M, Harkins EW, Quigley R (1997) Maturational changes in rabbit renal cortical phospholipase A2 activity. Kidney Int 52:71–78
Friedman PA (1999) Calcium transport in the kidney. Curr Opin Nephrol Hypertens 8:589–595
Morel F (1992) Methods in kidney physiology: past, present, and future. Annu Rev Physiol 54:1–9
Morel F (1981) Sites of hormone action in the mammalian nephron. Am J Physiol 240:F159–F164
Burg M, Grantham J, Abramow M, Orloff J (1966) Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210:1293–1298
Reyes JL, Roch-Ramel F, Besseghir K (1987) Net sodium and water movements in the newborn rabbit collecting tubule: lack of modifications by indomethacin. Biol Neonate 51:212–216
Blaehr H (1991) Human renal biopsies as source of cells for glomerular and tubular cell cultures. Scand J Urol Nephrol 25:287–295
Vinay P, Gougoux A, Lemieux G (1981) Isolation of a pure suspension of rat proximal tubules. Am J Physiol 241:F403–F411
Valencia L, Bidet M, Martial S, Sanchez E, Melendez E, Tauc M, Poujeol C, Martin D, Namorado MD, Reyes JL, Poujeol P (2001) Nifedipine-activated Ca(2+) permeability in newborn rat cortical collecting duct cells in primary culture. Am J Physiol Cell Physiol 280:C1193–C1203
Reyes JL, Lamas M, Martin D, Del Carmen NM, Islas S, Luna J, Tauc M, Gonzalez-Mariscal L (2002) The renal segmental distribution of claudins changes with development. Kidney Int 62:476–487
Georgiadis N, Kardasopoulos A, Bufidis T (1999) The evaluation of corneal graft tissue by the use of trypan blue. Ophthalmologica 213:8–11
Reale E, Luciano L (1967) Critical electron microscopical studies on the localization of activity of alkaline phosphatase in the main portion of the kidney of mice. Histochemie 8:302–314
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Gesek FA, Friedman PA (1992) On the mechanism of parathyroid hormone stimulation of calcium uptake by mouse distal convoluted tubule cells. J Clin Invest 90:749–758
Hoenderop JG, De Pont JJ, Bindels RJ, Willems PH (1999) Hormone-stimulated Ca2+ reabsorption in rabbit kidney cortical collecting system is cAMP-independent and involves a phorbol ester- insensitive PKC isotype. Kidney Int 55:225–233
Reyes JL, Nava E, Namorado MC (1992) Receptor-mediated effect of a synthetic thromboxane-analogue on cytosolic calcium in isolated proximal tubules. Prostaglandins 44:145–154
Bouhtiauy I, Lajeunesse D, Brunette MG (1991) The mechanism of parathyroid hormone action on calcium reabsorption by the distal tubule. Endocrinology 128:251–258
Bindels RJ, Dempster JA, Ramakers PL, Willems PH, Os CH van (1993) Effect of protein kinase C activation and down-regulation on active calcium transport. Kidney Int 43:295–300
Champigneulle A, Siga E, Vassent G, Imbert-Teboul M (1997) Relationship between extra- and intracellular calcium in distal segments of the renal tubule. Role of the Ca2+ receptor RaKCaR. J Membr Biol 156:117–129
Iwata M, Herrington J, Zager RA (1995) Protein synthesis inhibition induces cytoresistance in cultured human proximal tubular (HK-2) cells. Am J Physiol 268:F1154–F1163
Gibney GT, Zhang JH, Douglas RM, Haddad GG, Xia Y (2002) Na(+)/Ca(2+) exchanger expression in the developing rat cortex. Neuroscience 112:65–73
Prakash YS, Seckin I, Hunter LW, Sieck GC (2002) Mechanisms underlying greater sensitivity of neonatal cardiac muscle to volatile anesthetics. Anesthesiology 96:893–906
Kempson SA, Lotscher M, Kaissling B, Biber J, Murer H, Levi M (1995) Parathyroid hormone action on phosphate transporter mRNA and protein in rat renal proximal tubules. Am J Physiol 268:F784–F791
Johnson V, Spitzer A (1986) Renal reabsorption of phosphate during development: whole-kidney events. Am J Physiol 251:F251–F256
Spitzer A, Barac-Nieto M (2001) Ontogeny of renal phosphate transport and the process of growth. Pediatr Nephrol 16:763–771
Bourdeau JE, Eby BK (1990) cAMP-stimulated rise of [Ca2+]i in rabbit connecting tubules: role of peritubular Ca. Am J Physiol 258:F751–F755
Lau K, Bourdeau JE (1995) Parathyroid hormone action in calcium transport in the distal nephron. Curr Opin Nephrol Hypertens 4:55–63
Linarelli LG (1972) Newborn urinary cyclic AMP and developmental renal responsiveness to parathyroid hormone. Pediatrics 50:14–23
Feng JQ, Clark NB (1994) Renal responses to parathyroid hormone in young chickens. Am J Physiol 267:R295–R302
Chattopadhyay N, Baum M, Bai M, Riccardi D, Hebert SC, Harris HW, Brown EM (1996) Ontogeny of the extracellular calcium-sensing receptor in rat kidney. Am J Physiol 271:F736–F743
Acknowledgements.
This study was partially supported by a grant from the Program ECOS-ANUIES for scientific collaboration France-Mexico (Project M96-B02). Dr. Laura Valencia was a recipient of a postgraduate fellowship from Secretaría de Educación Pública, México. The authors gratefully acknowledge the critical review of this material by Dr. M. Cereijido from the Department of Physiology and Biophysics at the Research Center for Advanced Studies. We also acknowledge the technical assistance of Gerardo Sierra and the secretarial support of Elvia Hernandez. Partial support of Conacyt 334511M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valencia, L., Melendez, E., Namorado, M.C. et al. Parathyroid hormone increases cytosolic calcium in neonatal nephron through protein kinase C pathway. Pediatr Nephrol 19, 1093–1101 (2004). https://doi.org/10.1007/s00467-004-1542-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1542-9