Abstract
Cell migration is a crucial event for physiological processes, such as embryonic development and wound healing, as well as for pathological processes, such as cancer dissemination and metastasis formation. Cancer cell migration is a result of the concerted action of matrix metalloproteinases (MMPs), expressed by cancer cells to degrade the surrounding matrix, and integrins, the transmembrane receptors responsible for cell binding to matrix proteins. While it is known that cell-microenvironment interactions are essential for migration, the role of the physical state of such interactions remains still unclear. In this study we investigated human fibrosarcoma cell migration in two-dimensional (2D) and three-dimensional (3D) fibronectin (FN) microenvironments. By using antibody blocking approach and cell-binding site mutation, we determined that \(\upalpha _{5}\upbeta _{1}\)-integrin is the main mediator of fibrosarcoma cell migration in 2D FN, whereas in 3D fibrillar FN, the binding of \(\upalpha _{5}\upbeta _{1}\)- and \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrins is not necessary for cell movement in the fibrillar network. Furthermore, while the general inhibition of MMPs with GM6001 has no effect on cell migration in both 2D and 3D FN matrices, we observed opposing effect after targeted silencing of a membrane-bound MMP, namely MT1-MMP. In 2D fibronectin, silencing of MT1-MMP results in decreased migration speed and loss of directionality, whereas in 3D FN matrices, cell migration speed is increased and integrin-mediated signaling for actin dynamics is promoted. Our results suggest that the fibrillar nature of the matrix governs the migratory behavior of fibrosarcoma cells. Therefore, to hinder migration and dissemination of diseased cells, matrix molecules should be directly targeted, rather than specific subtypes of receptors at the cell membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The interaction of cancer cells with their local microenvironment is crucial for the onset of cell migration during metastasis. The migration path results from the concerted action of cell adhesion and extracellular proteolysis [1]. Hence, understanding how distinct properties of the extracellular environment regulate cell migration locally may provide means to develop specific therapeutic interventions. In this context, extracellular proteases such as matrix metalloproteinases (MMPs) have been identified as significant regulators of extracellular matrix (ECM) properties.
The family of MMPs comprises several multidomain zinc-dependent endopeptidases, which exert proteolytic activities and regulate tissue remodeling under physiological and pathological conditions [2, 3]. In particular, the cell surface associated MT1-MMP has been identified as a key player in tumor progression, since it promotes cell migration, invasion and metastasis [4]. Due to its ability to activate e.g. pro-MMP-2 and pro-MMP-13, MT1-MMP acts as a pacemaker of proteolytic cascades [5, 6]. Furthermore, MT1-MMP cleaves several ECM proteins, such as collagen, laminin, fibrin, vitronectin and fibronectin, as well as cell surface receptors, such as CD44 and integrins, which mediate cell adhesion to the ECM [7, 8].
Integrins are heterodimeric transmembrane proteins that bind to specific motifs of ECM proteins and regulate cell migration, as well as adhesion-dependent functions, such as differentiation, proliferation and survival [9]. Upon binding to the ECM, integrins initiate the assembly of large protein complexes, named focal adhesions, where several kinases (e.g. FAK, Src and p130Cas) are recruited, thereby acting as signaling hubs [10]. Although expressed in cells embedded in 3D environments, focal adhesion proteins are not assembled in aggregates but are rather diffusely distributed in the cytoplasm. As such, it has recently been shown that upon migration, cell speed and directionality are regulated by different focal adhesion proteins which act on protrusion activity and matrix deformation in 3D systems, whereas these processes are redundant in the control of cell speed in 2D systems [11]. Furthermore, reports on blocking integrin interactions with their respective ligands and its effect on cell migration suggest that integrin-dependent adhesion is the main mode of cell-ECM fiber binding in cancer cells [12, 13].
Matrix degradation is considered as a prerequisite for the migration and invasion of neoplastic cells in tissues and MT1-MMP has been shown to be crucial for collagenolysis, whereas secreted MMPs are not [14, 15]. Matrix degradation mediated by MT1-MMP takes place at focal adhesion sites and is dependent on FAK-p130Cas complexes due to the physical interaction between MT1-MMP and FAK [16]. In collagen matrices cancer cells display a mesenchymal mode of migration, where matrix is degraded by MT1-MMP activity and is dependent on the formation of \(\beta _{1}\)-integrin-mediated lamellipodial protrusions in the direction of motility [17, 18]. At the leading edge, integrins and MT1-MMP localize at sites of interactions with collagen fibers thereby orchestrating cell traction and matrix degradation [17, 18]. Whether these two separate but interdependent processes are co-localized, is still a matter of debate [17, 19].
For tumor progression, the relevance of cell migration within an ECM network has been highlighted in studies, which report on qualitative and quantitative differences in migration between 2D and 3D environments [20, 21]. Regarding the role of ECM proteins in modulating the interactions between MT1-MMP and integrins, surfaces coated with matrix proteins have been shown to differentially regulate the localization and activity of MT1-MMP, \(\upalpha _\mathrm{v}\upbeta _{3}\)- and \(\beta _{1}\)-integrins [20]. Fibronectin, which serves as ligand for \(\upalpha _\mathrm{v}\upbeta _{3}\)- and \(\upalpha _{5}\upbeta _{1}\)-integrins, is an ECM glycoprotein involved in several physiological processes such as embryonic development, cell migration, and vasculogenesis [22]. Upregulation and increased assembly of fibronectin matrix might be crucial for the regulation of architecture of the premetastatic cancer cell niche [23, 24]. In vitro studies using surfaces coated with fibronectin indicated that protein degradation and reduced stability in focal adhesion formation at the leading edge are key events to trigger migration in invasive cancer cells which express MT1-MMP [25, 26].
The invasion of tumor cells in 3D matrix systems has been described using two main model systems of the ECM, namely reconstituted basement membranes or fibrillar networks of type I collagen. However, the diversity of matrix molecules and biomechanical properties encountered by cancers cells in tissues is significantly different from the environments currently mimicked by conventional in vitro approaches. Recently, the physiological impact of MT1-MMP proteolysis in the regulation of fibronectin matrix turnover and \(\upalpha _{5}\upbeta _{1}\)-integrin endocytosis in fibrillar matrices assembled by fibroblasts has been investigated [27].
However, the functional mechanism, which underlies cancer cell sensing of the structural properties of fibronectin matrices has not been determined yet. In particular, it is not clear whether MT1-MMP in turn, contributes to integrin-mediated tumor cell migration in 3D fibrillar fibronectin matrices. In this work, we examine the function of integrins and MMPs in human HT1080 fibrosarcoma cells using blocking or inhibition approaches, and demonstrate that integrin binding and MT1-MMP expression are essential for cell migration in 2D fibronectin environment, but not in 3D fibrillar fibronectin matrix. Given these findings, we address whether MT1-MMP is an important regulator of integrin-mediated fibrosarcoma cell migration in 3D fibrillar fibronectin matrices. Our results show that inhibition of MT1-MMP activity has an opposite effect on integrin-mediated signaling in cells cultured on 2D fibronectin or 3D fibrillar fibronectin matrices, suggesting that in the latter, an amoeboid, rather than a mesenchymal mode of migration, is dominant.
2 Materials and methods
2.1 Cell cultures
Stably transfected NIH3T3 fibroblasts expressing fluorescent fibronectin (FN) (kindly provided by T. Ohashi and H.P. Erickson, Duke University, USA) were generated by inserting a FN-YPet/Neo expression vector, as described in Ohashi and Erickson [28]. Clones were selected with G418 (0.7 mg/ml, Life Technologies) and screened for visible FN matrix fibrils by fluorescence microscopy. Cells were maintained in culture in DMEM supplemented with 10 % fetal calf serum (FCS).
\(\hbox {FN}^\mathrm{RGE/RGE }\) fibroblasts from mouse embryos, in which functional RGD (Arg-Gly-Asp) motif in fibronectin is mutated to RGE (Arg-Gly-Glu) were kindly provided by R. Fässler (Max Planck Institute for Biochemistry, Martinsried, Germany). The generation of the knockin mice and the establishment of the cell line were described in [29]. Cells were cultured in DMEM supplemented with 10 % fetal bovine serum (FBS). Cells were adapted to grow in serum replacement medium (DMEM /Aim-V Medium /RPMI1640/ non-essential amino acids; all from Life Technologies) and then used for FN matrix assembly.
Human fibrosarcoma cells HT1080 (ATCC, CCL-121) were maintained in DMEM supplemented with 1 % L-glutamine, 1 % penicillin/streptomycin and 10 % FBS.
2.2 Preparation of fibronectin substrates
2.2.1 Fibronectin coated surfaces
Cellular fibronectin from human foreskin fibroblasts (Sigma-Aldrich) was reconstituted in distilled sterile water at a concentration of 0.5 mg/ml. For surface coating, the solution was diluted in sterile PBS at a concentration of \(10\upmu \hbox {g}/\hbox {ml}\). 35 mm dishes or 8-well \(\mu \)-slide chambers (Ibidi GmbH) were then incubated with fibronectin solution at a concentration of \(1 2 \upmu \hbox {g}/\hbox {cm}^{2}\). The samples were kept for 45 min at room temperature (RT) and allowed to air dry.
2.2.2 Fibrillar fibronectin matrices
The procedure for the preparation of cell-derived fibrillar FN matrices is depicted in Fig. 1. The dishes were coated with 2 % 3-aminopropyltriethoxysilane (Sigma-Aldrich) diluted in ethanol for 15 min at RT. After rinsing, the surfaces were incubated in a 2 % glutaraldehyde aqueous solution (Sigma-Aldrich) for 30 min at RT. Surfaces were then coated with fibronectin as described above. FN-YPet or \(\hbox {FN}^\mathrm{RGE/RGE }\) fibroblasts were trypsinized with 0.25 % (w/v) Trypsin/EDTA and plated on the surfaces at a density of \(10{,}000\hbox { cells}/\hbox {cm}^{2}\) until reaching confluence. Cell lysis and matrix preservation were performed as described in Mao and Schwarzbauer [30]. In brief, cells were washed once with a buffer consisting of 100 mM \(\hbox {Na}_{2} \hbox {HPO}_{4}\), 2 mM \(\hbox {MgCl}_{2}\), 2 mM EGTA. After adding cell lysis buffer (8 mM \(\hbox {Na}_{2}\hbox { HPO}_{4,}1\) % NP-40; pH 9.6), the samples were incubated at \(37^{\circ }\hbox {C}\) for 10 min; the lysis buffer was then refreshed and samples were incubated for 20 min at \(37^{\circ }\hbox {C}\).
Preparation of FN fibrillar matrices for studies on cancer cell migration. a Globular FN is crosslinked to the surface. b Fibroblasts are seeded on the substrate and c cultured until reaching confluence. d upon formation of a FN fibrillar network, fibroblasts are then removed by lysis. e HT1080 human fibrosarcoma cells are seeded on FN fibrillar matrices
The FN matrix was washed once with a buffer containing 300 mM KCl, 10 mM \(\hbox {Na}_{2}\hbox { HPO}_{4}\); pH 7.5, followed by rinsing with water and PBS. Fresh matrices were prepared for each set of experiments and their integrity was inspected by fluorescence microscopy before and after lysis (Fig. 2). For the visualization of the matrix produced by \(\hbox {FN}^\mathrm{RGE/RGE}\) fibroblasts, fibronectin was immunostained. FN-RGE matrices were blocked with 1 % (w/v) BSA/PBS for 30 min, and incubated with anti-fibronectin antibody (Millipore) and then Alexa Fluor 488 conjugated goat anti-rabbit IgG (Life Technologies).
2.3 Integrin blocking
HT1080 cells in culture were treated with cell dissociation buffer (Life Technologies) for detachment. Then, 150,000 cells were resuspended in \(50\,\upmu \hbox {l}\) serum-free DMEM containing 2 mg/ml BSA and 1:20 dilutions of primary antibodies against \(\upalpha _\mathrm{v}\upbeta _{3}\)- and \(\upalpha _{5}\upbeta _{1}\)-integrins (Millipore). Cells were incubated for 30 min on ice, centrifuged at 500 \(\times \) g for 5 min at \(4^{\circ }\hbox {C}\) and plated onto either FN coatings or FN fibrillar matrices for further analysis.
2.4 MMP inhibition and MT1-MMP silencing
For general MMP inhibition, the broad-spectrum hydroxamate inhibitor of matrix metalloproteinases GM6001 and its respective GM6001 control (both from Merck KgaA) were added to the culture media to a final concentration of \(10\,\upmu \hbox {M}\). MT1-MMP gene expression was knocked down with ON-TARGETplus Human MT1-MMP siRNA SMARTpool (Thermo Fisher Scientific); the knocked down cells are indicated as “siMT1-MMP” or siM. The non-targeting control is indicated as “siControl” or siC. The siRNA-transfection was performed with DharmaFECT transfection reagent (Thermo Fisher Scientific) according to manufacturers’ instructions.
Cell contractility was inhibited by adding \(50~\upmu \hbox {M}\) of the specific myosin II inhibitor Blebbistatin (Sigma) to the culture medium directly before starting the migration assays.
2.5 Microscopy and image analysis
Following detachment from the culture flask with an EDTA-based dissociation buffer, HT1080 human fibrosarcoma cells were suspended in Opti-MEM (Life Technologies) containing \(10\,\upmu \hbox {M}\) of cytoplasmic dye (\(\hbox {CellTracker}^\mathrm{TM}\) Red CMTPX, Life Technologies) for 30 min at \(37^{\circ }\hbox {C}\). Cells were then pelleted, resuspended in culture medium and plated on the surfaces at a density of 25,000 cells/well for migration experiments. Cells were monitored using an Olympus IX inverted fluorescence microscope and an UPlanFL 20x/0.50 Ph1 or a PlanApo 60x/1.40 oil objective (all Olympus Europa Holding GmbH) with a Delta Vision system (Applied Precision Inc.). Time-lapse imaging was performed at \(37^{\circ }\hbox {C}\) under a \(5 \%\hbox { CO}_{2}\) atmosphere; images of at least three different fields per sample were acquired every 10 min for 8 h.
2.6 Analysis of protein expression
Detection of proteins was performed by SDS-PAGE gel electrophoresis and western blot analysis. Briefly, HT1080 cells were seeded at a density of \(120,000\hbox { cells}/\hbox {cm}^{2}\) either on FN coating or on fibrillar FN matrix. Cells were lysed 1 hr after plating on these substrates, in 1 % IGEPAL CA-630, 0.25 % sodium deoxycholate, 667 mM EDTA, 100 mM PMSF, 200 mM \(\hbox {Na}_{3}\!\hbox {VO}_{4}\), 50 mM Tris–HCl, 150 mM NaCl containing protease inhibitors (Complete Mini, Roche). Equal protein amounts were separated by SDS–PAGE and transferred to a PVDF membrane. The membranes were then probed with the primary antibodies overnight at \(4^{\circ }\hbox {C}\). After washing and incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz), bands were detected using the ECL Plus Detection Kit (GE Healthcare) and the Fujifilm LAS-3000 System (Fuji Photo Film, Europe).
2.7 Analysis of cell migration speed and directional persistence
The centroid position (x,y) of each migrating cell was calculated at each time step (10 min) for the entire duration of the experiments using the particle analyzer of ImageJ software [31]. For the analysis, cells were not considered if they (i) left the image field, (ii) were dividing, (iii) formed clusters, (iv) underwent apoptosis or (v) migrated in regions where matrix was not present or very sparse. The distance that the centroid of the cell moved (over each time step value in space) was then used to calculate cell migration speed during the 8 h time period. Migration speed data for each cell at all time points were then compiled to obtain the mean cell migration speed for the entire duration by dividing the contour length of the trajectory by the duration between the end points.
Furthermore, the end-to-end distance and the directional change \((\Theta )\) between consecutive steps were evaluated. Persistence was defined by the ratio of the end-to-end distance and the contour length as described in [32]. In order to estimate time dependent behavior, the trajectories were grouped into 2 h-long segments, for which the end-to-end distance, contour length and average velocity were also calculated.
2.8 Statistical analysis
Experiments were repeated three to five times and approximately 100 cells from three different observation fields for each treatment group were analyzed. All statistical processing and box-and-whisker plots were done using R, the statistical programming language. The polar histograms of step directions and the histograms of average velocities were plotted using Gnuplot. For the velocity and persistence box-and-whisker plots, each box is defined by the 1st and 3rd quartile of the data, the line in the box indicates the median. Whiskers extend to the extreme of the data, or maximum to 1.5 times the interquartile range of the box. The black squares indicate the remaining data points, thus the outliers.
The angular persistence is presented using the normalized histogram of the step angles (the angle between consecutive steps), generated for 10 degree broad pockets (36 values between \(-\pi \)–\(\pi \)), plotted in a polar plot. The bars point in the direction of the middle value of the histogram pockets, and the length is proportional to the probability density at that angle. The velocity histograms were produced calculating a histogram using 50 pockets between the minimum and maximum of the data set. For better visibility, the histograms were plotted as scatter plots and a line produced by a cubic spline interpolation to guide the eye in Gnuplot (see supporting material Fig. S1).
To probe the similarities between the mean values of data sets (velocities or angles), the Wilcoxon rank sum (or Mann-Whitney-Wilcoxon) test was used. The null hypothesis was that the means are equal (their difference is zero), and the alternative hypothesis was that they differ with a non-zero value. The p-values were calculated using a normal approximation, for the data sets containing more than 50 elements. The similarity of sample distributions were tested using the two-sided Kolmogorov-Smirnov test. The null hypothesis is that the two data sets are pulled from the same distribution (one has a distribution not less and not greater than the other). Exact p-values can not be calculated for data with ties, which was the case for all of our samples, and asymptotic distributions were used.
3 Results and discussion
3.1 Tumor cell migration is increased in presence of fibrillar fibronectin matrix and is dependent on the RGD sequence in cell-binding domain
Since the nature of cell adhesive interactions with the extracellular matrix (ECM) regulates migration, we first compared the effects of 2D and 3D fibronectin (FN) environments on human fibrosarcoma cell speed and directionality. Cells adhering and migrating on surfaces coated with cellular FN served as a 2D system (indicated as “FN coating”). This form of FN is in a globular conformation, hence not preassembled in fibers and it is adsorbed on the surface of the dish. Upon adhesion, cells assemble it into fibrils via cytoskeletal reorganization [33]. For the formation of 3D fibrillar FN matrices, embryonic fibroblasts were maintained in culture for several days to assemble the matrix (indicated as “FN matrix”) and then lysed as described in materials and methods. Furthermore, to determine the specific involvement of the FN cell-binding domain on tumor cell migration, fibroblasts, which express a mutated cell-binding domain (RGE instead of RGD sequence), were used to produce the fibrillar matrix (indicated as “FN-RGE matrix”). Hence, while the assembly of a fibrillar microstructure is maintained in this type of matrix, its cell-adhesive properties are drastically reduced [29]. Cell migration on these different FN environments was monitored by time-lapse fluorescence microscopy (Fig. 3a, Video S1 and Video S2). To visualize and track the migrating cells, a live cell labeling was used (Fig. 3a, in red) whereas the fibrillar FN matrix could be directly visualized by imaging the YFP-fusion protein expression (Fig. 3a, in green). As shown in the box-and-whisker plots (Fig. 3b), the migration speed of fibrosarcoma cells on FN coating is low and remains unvaried during the entire observation time. In contrast, on fibrillar FN matrix, cells migrate at a higher speed, which also remains constant over time. Upon mutation of the RGD sequence in the cell-binding domain of fibrillar FN-RGE matrix, cell migration is drastically decreased in comparison to the FN matrix group. The average migration velocity of cells on FN matrix is statistically significant different, in comparison to the FN coating and the FN-RGE matrix groups, as indicated in the probability distribution plots (Fig. S1A). These differences in migration could be observed for a longer time period, i.e. 8 h after seeding on different FN environments (Fig. S2). It should be noted that the velocity distribution remained unchanged for the 2 h and up to the 8 h observation time.
Migration speed of fibrosarcoma cells in fibronectin environments. a Fluorescence microscopy images of HT1080 cells stained with a cytoplasmic labeling dye (red) on FN coatings (first row), on fibrillar FN matrices (green, second row) and on fibrillar FN-RGE matrices (third row). The colored lines show the tracking paths at the indicated time points. Scale bar, \(50\,\upmu \hbox {m}\). b Box-and-whisker plots of cell migration speed on FN coating (in red), FN matrix (in green) and FN-RGE matrix (in cyan). The first box of each group indicates the analysis of cell speed for 0–2 h, the second box for 2–4 h. The box plot shows median and quartiles, with data points outside the whiskers being outliers. c and d Analysis of directionality of migration, evaluated as persistence at 0–2 and 2–4 h in the box plot (in c), and as persistence angle in the polar plots (in d). (Color figure online)
Since the structure of the ECM modulates directional migration, we quantified migration persistence over time as described in materials and methods. On both fibrillar FN matrices, namely FN matrix and FN-RGE matrix, the directionality of cell migration is higher than on FN coating (Fig. 3c). The relative distance and direction of migrating cells is shown in the polar plots in Fig. 3d. Here the starting point of all cells is assigned to the middle of the plot and the relative positions of cells 4 h after the beginning of the migration experiment are shown. Note that the directionality in migration is evident only for the FN matrix (Fig. 3d in green) and the FN-RGE matrix (Fig. 3d in cyan) groups.
The differences in cell migration speed on FN coating and fibrillar FN matrix are in agreement with other studies on NIH3T3 fibroblasts and human keratinocytes migration in fibrillar FN [20]. Therefore, the increased migration speed cannot be specifically attributed to cancer cells, but it is rather a general phenomenon, which takes place in this type of extracellular environment. The reduced migration speed of cells plated on FN-RGE matrices suggests that the immediate interaction of integrins with the RGD site of FN is important for binding and regulation of migration.
The increase in migration persistence we observed for both FN and FN-RGE matrices stems from the fibrillar nature of these environments. Here, cells exhibit an elongated phenotype as they align along FN fibers and follow their inherent paths (Video S1). In contrast, on FN coatings, migration is random and cells present a round shape. Therefore, directionality is mainly regulated by the fibrils independently of the cell-binding domain, suggesting contact guidance as possible mechanism for efficient cell migration.
3.2 \(\upalpha _{5}\upbeta _{1}\)-integrin binding to fibronectin modulates tumor cell migration
Several integrin types bind to FN [34]. More specifically, the RGD motif in the cell-binding domain of FN is a ligand for both \(\upalpha _{5}\upbeta _{1}\)- and \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrins [35, 36]. In cancer, the expression of integrins, in particular in terms of specificity and affinity, is regulated by several intracellular and extracellular factors. We confirmed the expression of \(\upalpha _{5}\upbeta _{1}\)- and \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrins in HT1080 fibrosarcoma cells at both gene and protein level (data not shown). To determine the role of integrins in modulating cell migration speed, we performed receptor blocking experiments using specific antibodies against \(\upalpha _{5}\upbeta _{1}\)- and \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrins prior to seeding cells on FN coatings or on FN matrices. The quantitative analysis of cell migration speed is shown in Fig. 4a. On FN coatings, cancer cell migration is significantly decreased when \(\upalpha _{5}\upbeta _{1}\)- integrins are blocked, whereas \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrin blocking does not affect migration (see also Fig. S1B). Interestingly, for fibrosarcoma cells seeded on FN matrices, migration is slightly increased when \(\upalpha _{5}\upbeta _{1}\)-integrin is blocked, whereas blocking of \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrin has no effect. On all types of FN environments, combining both treatments elicits similar responses after \(\upalpha _{5}\upbeta _{1}\)-integrin blocking; therefore migration is not completely suppressed (data not shown). Furthermore, directionality is not regulated by either integrin binding to FN (Fig. 4b), corroborating the finding that fibrillar topography is the main regulator of migration persistence. It should be noted that on FN coating blocking of \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrin increases directionality, whereas blocking of \(\upalpha _{5}\upbeta _{1}\)-integrin increases random motility. Taken together, these results indicate that \(\upalpha _{5}\upbeta _{1}\)-integrin differentially modulates cell migration in 2D and 3D FN environments.
Effects of inhibition of integrin binding on cell migration in fibronectin environments. a HT1080 cells that were pre-treated with either \(\upalpha _{5}\upbeta _{1}\)- or \(\upalpha _\mathrm{v}\upbeta _{3}\)- integrin blocking antibodies were then seeded on FN coated surfaces (groups in red) or on fibrillar FN matrices (groups in green). Migration speed is shown for 0–2 and 2–4 h time intervals after cell seeding. b Polar plots indicating the persistence angle of migration in cells plated on FN coated surfaces (left, in red) and on fibrillar FN matrices (right, in green). (Color figure online)
It has been previously shown that the strength of HT1080 cell adhesion to either FN coatings or fibrillar FN matrices is drastically decreased upon antibody blocking of \(\upalpha _{5}\upbeta _{1}\)-integrin [37, 38]. In contrast, blocking of \(\upalpha _\mathrm{v}\upbeta _{3}\)-integrin does not significantly affect fibrosarcoma cell adhesion [38, 39], although this integrin type binds to fibrillar FN [40] and is important for adhesion to FN in other cell lines. Upon \(\upalpha _{5}\upbeta _{1}\)-integrin blocking, the reduced migration speed on FN coatings suggests that cell binding via \(\upalpha _{5}\upbeta _{1}\)-integrin is necessary for promoting migration in 2D. For matrix remodeling and fibrillogenesis, the nature of interaction between cells and the globular form of FN proteins is mostly mediated by \(\upalpha _{5}\upbeta _{1}\)-integrins, thereby resulting in receptor clustering, focal adhesion assembly and actin stress fiber bundling [36]. Following the activation of downstream signaling to the cytoskeleton and further regulation of forces at adhesion sites, traction forces exerted by the cell result into a forward movement [33]. In 2D systems, like surface coating with FN, blocking of \(\upalpha _{5}\upbeta _{1}\)-integrins and preventing their interaction with FN might negatively regulate force generation [41], thereby causing a decrease in migration speed. In contrast, adhesion of fibrosarcoma cells via \(\upalpha _{5}\upbeta _{1}\)-integrins seems to hinder their migration within the fibrillar FN matrix to some extent. In fact, among the different integrins which are known to interact with FN, \(\upalpha _{5}\upbeta _{1}\)-integrin has the highest binding affinity [42]. Additionally, the fibrillar conformation of FN might facilitate the binding of other integrin types [34], although these are not the main mediators of mature focal adhesion assembly and cytoskeleton signaling. Furthermore, as suggested in other reports, physical and topographical cues of the 3D fibrillar matrix, and the resulting variation in stiffness of the environment, might affect the nature of interaction of integrins with the matrix [21]. In particular, matrix topography regulates cell migration rate regardless of ligand density and linear topographical cues on surfaces, which mimic aligned matrix fibers, represent an important regulator of directionality of migration via actomyosin contractility. Additionally, higher speed could be due to low adhesion structures in response to reduced matrix stiffness in comparison to the coating. As such, when integrin binding is blocked, the biochemical information conveyed by the receptor is absent and matrix stiffness governs cell migratory behavior [43]. It is also possible that fibrosarcoma cells switch their migration mode from mesenchymal to amoeboid when interacting with 3D fibrillar FN matrices. The former involves focal adhesions and actin stress fiber formation, the latter implies weak adhesive interactions to the substrate [44].
3.3 Different effects of MT1-MMP on integrin-mediated signaling in 2D and 3D fibronectin matrices
For invasion, cancer cells not only activate integrin-dependent migration pathways, but also upregulate the expression of proteolytic enzymes to penetrate and simultaneously reorganize interstitial tissues [45]. Contact-dependent proteolysis is tightly connected to ECM topography and the corresponding receptors which bind to the ECM [46, 47]. Additionally, the close relationship between proteolysis, cell adhesion and force generation has been recently reported [48, 49]. To determine the role of protease activity on cancer cell migration in FN environments, we focused on matrix metalloproteinases (MMPs), which are known to be the major determinants of matrix degradation [44]. We first investigated the contribution of MMPs to fibrosarcoma migration processes in fibrillar FN matrices by using a general inhibitor of MMPs, the broad-spectrum hydroxamate inhibitor GM6001 [50, 51]. Our analysis of migration speed and directionality indicates that the general inhibitor has no effect on cells plated on either FN coating or fibrillar FN matrix (Fig. 5a, b). Since it has been reported that cancer cells can switch between protease-driven and actomyosin-based motility [52, 53], we also determined the effects of myosin II inhibition on fibrosarcoma cell migration (Fig. 5). Here, migration is efficiently inhibited by blebbistatin treatment of cells on fibrillar FN matrices, whereas on FN coatings migration speed is low but still comparable to the control group (Fig. 5a). Treatment with blebbistatin has however no distinct effect on the directionality of migration (Fig. 5b).
Effects of matrix metalloproteinases and myosin II inhibition on cell migration in fibronectin environments. a HT1080 cells were seeded on FN coated surfaces (groups in red) or on FN matrices (groups in green) and incubated in the presence of the MMP inhibitor control (GM ctrl.), the MMP inhibitor GM6001 (GM) or blebbistatin (Blebb.). The migration speed is shown in the box plots, where the cell velocities for 0–2 h and for 2–4 h are indicated in the first and second box respectively. b Polar plots showing the persistence angle of migration of cells plated on FN coated surfaces (left in red) and on fibrillar FN matrices (right, in green), treated as described in (a). (Color figure online)
For breast cancer cells it has been shown that broad inhibition of MMPs doesn’t result in efficient blocking of cell migration in collagen matrices and that addition of ROCK1 inhibitors is required [54]. Therefore, inactivity of MMPs could cause tumor cells to switch to a migratory behavior which is dependent on cell contractility and upregulation of ROCK activity [55, 56]. We could also speculate here that active protrusive behavior, rather than local matrix degradation, is mostly responsible for migration in FN environment [57].
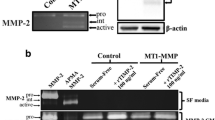
It has been previously shown that one of the membrane bound MMPs, namely MT1-MMP, regulates cell migration through modulation of focal adhesion stability on FN coated surfaces [25]. More in detail, the local lysis of FN at cell adhesion sites facilitates focal adhesion turnover, thereby promoting cell migration [26]. Therefore, to determine the specific role of MT1-MMP activity in cancer cell migration within fibrillar FN matrices, we employed an RNA silencing approach prior to cell seeding (Fig. 6a). Silencing of MT1-MMP significantly reduces migration speed of HT1080 cells on FN coatings (Fig. 6b and S1C) but not on fibrillar FN matrices, where even a slight increase in migration is observed (Fig. 6b and S1D). From gene expression analysis, we observed that silencing of MT1-MMP reduces expression of the \(\upalpha _{5}\)- integrin subunit (Fig. 6a), which could explain the increased migration speed in siMT1-MMP transfected cells, similar to \(\upalpha _{5}\upbeta _{1}\)-integrin blocking on fibrillar FN matrices. To confirm this hypothesis, MT1-MMP silenced cells were additionally blocked with \(\upalpha _{5}\upbeta _{1}\)-integrin antibodies and then plated on FN coatings or fibrillar FN matrices. As shown in Fig. 6b, on FN coatings MT1-MMP silenced and \(\upalpha _{5}\upbeta _{1}\)-integrin blocked fibrosarcoma cells present a significant decrease in migration speed in comparison to the control and the MT1-MMP silenced groups (Fig. S1C). On fibrillar FN, these cells migrate at a speed comparable to that of cells only silenced for MT1-MMP (Fig. S1D). The resepctive directionality as shown in the polar plots in Fig. 6c is not affected by abolishing the expression of MT1-MMP or by further inhibiting the binding of \(\upalpha _{5}\upbeta _{1}\)-integrins.
Effects of MT1-MMP silencing and \(\upalpha _5 \upbeta _1\)—integrin inhibition on migration and adhesion-mediated signaling in fibrosarcoma cells. a Western blot analysis of HT1080 cells which were either transfected with a non-targeting control (siC) or MT1-MMP (siM) siRNA and then seeded on FN coating or on FN matrix. Untreated cells are indicated as control (ctrl.) b and c HT1080 cells were plated on FN coating (groups in red) or on FN fibrillar matrix (groups in green) 48 h after siRNA transfection and migration speed and directionality were analyzed. Cells transfected with MT1-MMP siRNA were additionally incubated with the \(\upalpha _{5}\upbeta _{1}\)—integrin blocking antibody (siM/\(\upalpha _{5}\upbeta _{1})\) prior to seeding on fibronectin environments. d Representative western blots showing the expression and phosphorylation of focal adhesion proteins. \(\upalpha \)-Tubulin is used as loading control of the whole cell lysate. (Color figure online)
MT1-MMP regulates cell migration behavior on FN by influencing adhesion mediated signaling pathways [25]. Here, we determined the role of MT1-MMP expression on the phosphorylation of FAK (Tyr397), ERK1/2 (Tyr 204/187) and cofilin (Ser3) in fibrosarcoma cells cultured either on FN coating or on fibrillar FN matrix (Fig. 6d). Following MT1-MMP silencing, cells plated on FN coatings show no changes in the expression and phosphorylation of FAK and ERK1/2 in comparison to the controls, whereas the expression and phosphorylation of cofilin are increased (Fig. 6d left). On fibrillar FN matrices, MT1-MMP silencing leads to downregulation of FAK phosphorylation (1.2-fold), and more pronounced downregulation of cofilin (2.9-fold) compared to controls (Fig. 5d right). Phosphorylation of ERK1/2 is decreased in MT1-MMP silenced cells. To summarize these findings, MT1-MMP silencing has an opposite effect on protein phosphorylation and expression if cells are cultured on FN coatings or fibrillar FN matrices. If FN is presented in a folded conformation, MT1-MMP silencing enhances the phosphorylation of cofilin. If FN is presented in a fibrillar conformation, phosphorylation of FAK and expression of cofilin are both reduced.
FAK phosphorylation is required for integrin-dependent migration [58]. It has been demonstrated that FAK directs MT1-MMP to focal adhesions and in turn MT1-MMP cleaves FAK, thereby directly regulating focal adhesion stability and turnover on FN coated surfaces [25, 26]. In this context, the differences in phosphorylation of FAK and ERK in 2D and 3D fibronectin environments could be attributed to a different mode of migration adopted by fibrosarcoma cells. In agreement with Takino et al. [25, 26], on FN coatings \(\upalpha _{5}\upbeta _{1}\)-integrin binding and signaling, as well as MT1-MMP activity are required for efficient migration, which can be considered as mesenchymal migration. Here, it could be possible that at focal adhesions MT1-MMP and \(\upalpha _{5}\upbeta _{1}\)-integrin physically interact and random migration (Fig. 4b) is the result of cofilin phosphorylation. In presence of fibrillar FN matrices, cell migration is independent of integrin binding and of MT1-MMP proteolytic activity, therefore suggesting that in this case amoeboid migration mechanisms are predominant [18, 59]. In fact, the reduced phosphorylation of cofilin and therefore its higher activity in cells might signal for directionality sensing (Fig. 4b) along with acto-myosin based motility (Fig. 5).
4 Conclusion
Significant progress has been made in understanding the molecular mechanisms that regulate tumor cell migration. Many of the mechanisms that govern integrin-mediated adhesion also regulate local proteolytic activity. In this work we have shown that the presentation of fibronectin (FN) in its globular (2D) or fibrillar form (3D) regulates the concerted action of integrins and matrix metalloproteases necessary for cancer cell migration. In more detail:
-
1.
The directionality of migration is regulated by the structure of FN fibrils independently of its cell-binding domain.
-
2.
The mode of migration of fibrosarcoma cells within 3D FN matrices appears to be based on actomyosin contractility. In fact, \(\upalpha _{5}\upbeta _{1}\)-integrin hinders cancer cell migration within the 3D FN, since blocking this integrin type causes an increase in migration speed only in 3D FN. Furthermore, efficient blocking of cell migration in 3D FN environment is achieved by myosin II inhibition.
-
3.
Targeting of MT1-MMP significantly reduces migration speed of cells on FN coatings but not on fibrillar FN matrices. An opposite effect on protein phosphorylation and expression in cells cultured on 2D FN coatings or 3D fibrillar FN matrices is observed, suggesting that in 3D FN an amoeboid, rather than a mesenchymal mode of migration, is dominant.
Taken together, the results presented in this study emphasize the importance of the structural properties of the tumor microenvironment in modulating the migratory behavior of cancer cells. Therapeutic targeting of cryptic epitopes and cell binding sites of matrix molecules should be therefore considered as a possible strategy to efficiently hinder migration and dissemination of diseased cells.
What remains to be determined in future work is the switch between the formation of focal adhesions and the generation of invasive, matrix-degrading adhesion structures. It will also be important to characterize the spatial and temporal dynamics of adhesions in cells migrating in 3D matrices, and the adhesion-mediated signaling pathways in tumor cells during different modes of migration.
References
Bacac M, Stamenkovic I (2008) Metastatic cancer cell. Annu Rev Pathol 3:221–247. doi:10.1146/annurev.pathmechdis.3.121806.151523
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8(3):221–233. doi:10.1038/nrm2125
Seiki M (2003) Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett 194(1):1–11
Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G (1996) Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem 271(29):17124–17131
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M (1994) A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370(6484):61–65. doi:10.1038/370061a0
Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y (1997) Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem 272(4):2446–2451
Itoh Y, Seiki M (2004) MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem Sci 29(6):285–289. doi:10.1016/j.tibs.2004.04.001
Giancotti FG, Ruoslahti E (1999) Integrin signaling. Science 285(5430):1028–1032. doi:10.1126/science.285.5430.1028
McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC (2005) The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer 5(7):505–515. doi:10.1038/nrc1647
Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D (2010) A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12(6):598–604. doi:10.1038/ncb2062
Maaser K, Wolf K, Klein CE, Niggemann B, Zanker KS, Brocker EB, Friedl P (1999) Functional hierarchy of simultaneously expressed adhesion receptors: integrin alpha2beta1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices. Mol Biol Cell 10(10):3067–3079
Meyer AS, Hughes-Alford SK, Kay JE, Castillo A, Wells A, Gertler FB, Lauffenburger DA (2012) 2D protrusion but not motility predicts growth factor-induced cancer cell migration in 3D collagen. J Cell Biol 197(6):721–729. doi:10.1083/jcb.201201003
Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ (2006) A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev 20(19):2673–2686. doi:10.1101/gad.1451806
Li XY, Ota I, Yana I, Sabeh F, Weiss SJ (2008) Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase. Mol Biol Cell 19(8):3221–3233. doi:10.1091/mbc.E08-01-0016
Wang Y, McNiven MA (2012) Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol 196(3):375–385. doi:10.1083/jcb.201105153
Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 9(8):893–904. doi:10.1038/ncb1616
Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P (2003) Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160(2):267–277. doi:10.1083/jcb.200209006
Packard BZ, Artym VV, Komoriya A, Yamada KM (2009) Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol 28(1):3–10. doi:10.1016/j.matbio.2008.10.001
Doyle AD, Wang FW, Matsumoto K, Yamada KM (2009) One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol 184(4):481–490. doi:10.1083/jcb.200810041
Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P (2006) Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA 103(29):10889–10894. doi:10.1073/pnas.0604460103
Hynes RO (1990) Fibronectins. Springer series in molecular biology. Springer, New York
Cretu A, Brooks PC (2007) Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. J Cell Physiol 213(2):391–402. doi:10.1002/jcp.21222
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293. doi:10.1038/nrc2621
Takino T, Watanabe Y, Matsui M, Miyamori H, Kudo T, Seiki M, Sato H (2006) Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp Cell Res 312(8):1381–1389. doi:10.1016/j.yexcr.2006.01.008
Takino T, Saeki H, Miyamori H, Kudo T, Sato H (2007) Inhibition of membrane-type 1 matrix metalloproteinase at cell-matrix adhesions. Cancer Res 67(24):11621–11629. doi:10.1158/0008-5472.CAN-07-5251
Shi F, Sottile J (2011) MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J Cell Sci 124(Pt 23):4039–4050. doi:10.1242/jcs.087858
Ohashi T, Erickson HP (2009) Revisiting the mystery of fibronectin multimers: the fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol 28(3):170–175. doi:10.1016/j.matbio.2009.03.002
Takahashi S, Leiss M, Moser M, Ohashi T, Kitao T, Heckmann D, Pfeifer A, Kessler H, Takagi J, Erickson HP, Fassler R (2007) The RGD motif in fibronectin is essential for development but dispensable for fibril assembly. J Cell Biol 178(1):167–178. doi:10.1083/jcb.200703021
Mao Y, Schwarzbauer JE (2005) Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci 118(Pt 19):4427–4436. doi:10.1242/jcs.02566
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods Hist Comment 9(7):671–675. doi:10.1038/nmeth.2089
Lambrechts A, Ampe C (eds) (2008) The motile actin system in health and disease. Transw Res Netw
Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K (1998) Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141(2):539–551
Yang JT, Hynes RO (1996) Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell 7(11):1737–1748
Ruoslahti E (1999) Fibronectin and its integrin receptors in cancer. Adv Cancer Res 76:1–20
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687. doi:10.1016/S0092-8674(02)00971-6
Bronner-Fraser M (1985) Alterations in neural crest migration by a monoclonal antibody that affects cell adhesion. J Cell Biol 101(2):610–617
Shi Q, Boettiger D (2003) A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol Biol Cell 14(10):4306–4315. doi:10.1091/mbc.E03-01-0046
Engler AJ, Chan M, Boettiger D, Schwarzbauer JE (2009) A novel mode of cell detachment from fibrillar fibronectin matrix under shear. J Cell Sci 122(Pt 10):1647–1653. doi:10.1242/jcs.040824
Mao Y, Schwarzbauer JE (2006) Accessibility to the fibronectin synergy site in a 3D matrix regulates engagement of alpha5beta1 versus alphavbeta3 integrin receptors. Cell Commun Adhes 13(5–6):267–277. doi:10.1080/15419060601072215
Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Thery M, Mann M, Fassler R (2013) beta1—and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 15(6):625–636. doi:10.1038/ncb2747
Singh P, Carraher C, Schwarzbauer JE (2010) Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26:397–419. doi:10.1146/annurev-cellbio-100109-104020
Baker EL, Bonnecaze RT, Zaman MH (2009) Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J 97(4):1013–1021. doi:10.1016/j.bpj.2009.05.054
Wolf K, Friedl P (2011) Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol 21(12):736–744. doi:10.1016/j.tcb.2011.09.006
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1):52–67. doi:10.1016/j.cell.2010.03.015
Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ (2000) Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol 149(6):1309–1323
Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE (2009) MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J Cell Sci 122(Pt 24):4558–4569. doi:10.1242/jcs.050724
Kirmse R, Otto H, Ludwig T (2011) Interdependency of cell adhesion, force generation and extracellular proteolysis in matrix remodeling. J Cell Sci 124(Pt 11):1857–1866. doi:10.1242/jcs.079343
Kirmse R, Otto H, Ludwig T (2012) The extracellular matrix remodeled: Interdependency of matrix proteolysis, cell adhesion, and force sensing. Commun Integr Biol 5(1):71–73
Grobelny D, Poncz L, Galardy RE (1992) Inhibition of human skin fibroblast collagenase, thermolysin, and pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry 31(31):7152–7154
Nishino N, Powers JC (1978) Peptide hydroxamic acids as inhibitors of thermolysin. Biochemistry 17(14):2846–2850
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374. doi:10.1038/nrc1075
Friedl P (2004) Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol 16(1):14–23. doi:10.1016/j.ceb.2003.11.001
Raviraj V, Fok S, Zhao J, Chien HY, Lyons JG, Thompson EW, Soon L (2012) Regulation of ROCK1 via Notch1 during breast cancer cell migration into dense matrices. BMC Cell Biol 13:12. doi:10.1186/1471-2121-13-12
Sahai E, Marshall CJ (2003) Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5(8):711–719. doi:10.1038/ncb1019
Friedl P, Wolf K (2003) Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp 70:277–285
Martin-Martin B, Tovell V, Dahlmann-Noor AH, Khaw PT, Bailly M (2011) The effect of MMP inhibitor GM6001 on early fibroblast-mediated collagen matrix contraction is correlated to a decrease in cell protrusive activity. Eur J Cell Biol 90(1):26–36. doi:10.1016/j.ejcb.2010.09.008
Sieg DJ, Hauck CR, Schlaepfer DD (1999) Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci 112(Pt 16):2677–2691
Friedl P, Zanker KS, Brocker EB (1998) Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech 43(5):369–378. doi:10.1002/(SICI)1097-0029(19981201)43:5<369:AID-JEMT3>3.0.CO;2-6
Acknowledgments
We are very thankful to K. Wolf (NCMLS, the Netherlands), H. Schroeder, R. Medda, K. Klein and D. Missirlis (University of Heidelberg) for fruitful discussions. We also thank A. Kopp-Schneider (DKFZ), G. Sawitzki, the Nikon Imaging Center and HBIGS program (University of Heidelberg) for their support. The financial support from Deutsche Forschungsgemeinschaft (DFG LU854/3-1), Max Planck Society and CellNetworks (Excellence cluster University of Heidelberg) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (mpg 2471 KB)
Supplementary material 2 (mpg 1196 KB)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Corall, S., Haraszti, T., Bartoschik, T. et al. \(\upalpha 5\upbeta \)1-integrin and MT1-MMP promote tumor cell migration in 2D but not in 3D fibronectin microenvironments. Comput Mech 53, 499–510 (2014). https://doi.org/10.1007/s00466-013-0960-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00466-013-0960-6