Abstract
Background
Food insecurity has been linked to higher rates of obesity. It has also been shown to diminish the effectiveness of weight loss strategies, including intensive lifestyle interventions. One essential component of food insecurity is having a geospatial disadvantage in access to healthy, affordable food, such as living within a food desert. This study aims to determine if food insecurity also impacts weight loss and nutritional outcomes in patients who underwent Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG).
Methods
Clinical outcomes of patients who underwent RYGB or SG at Cleveland Clinic or affiliate regional hospitals in the United States from 2010 to 2018 were collected. Modified Retail Food Environmental Index (mRFEI) data was collected from the Center for Disease Control and merged with patient census tract data, allowing the patient cohort to be divided into those living in areas identified as food secure (mRFEI > 10%), food swamps (mRFEI = 1–10%), or food deserts (mRFEI = 0). Postoperative weight change was evaluated with quadratic growth mixture models and stratified by surgery type.
Results
A total of 5097 patients were included in this study cohort, including 3424 patients who underwent RYGB and 1673 who underwent SG. The median duration of follow-up was 2.3 years (IQR 0.89–3.6 years). Food security status was not associated with postoperative weight change (RYGB p = 0.73, SG p = 0.60), weight loss nadir (RYGB p = 0.60, SG p = 0.79), or weight regain (RYGB p = 0.93, SG p = 0.85). Deficiencies in nutritional markers at 1–2 years after surgery were also not significantly different between food security groups.
Conclusion
Despite the established relationship between food insecurity and obesity, food insecurity does not negatively impact weight loss or nutritional outcomes following RYGB or SG, demonstrating metabolic surgery as a powerful and equitable tool for treating obesity.
Level of Evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity is a serious chronic condition of increasing prevalence [1]. Prior studies have linked higher rates of obesity with residing in food deserts due to barriers prohibiting access to healthy food. A food desert is defined by the U.S. Department of Agriculture (USDA) as an “area characterized by relatively poor access to healthy and affordable food” [2]. Others have linked high densities of supermarkets within 0.5 miles of one’s residence to lower body mass index (BMI) [3]. Local groups have identified multiple food deserts with the Cleveland metropolitan area as well as within broader Cuyahoga County. These locations coincide with historically redlined areas, with markedly increased rates of poverty. Prior collaboration between state, county and city initiatives with boards of health have indicated that greater than 59% of all Cleveland residents and 35% of all Cuyahoga County residents live in food deserts, which have disproportionate representations of non-white residents [4].
Metabolic surgery has proven to be the most efficacious and durable intervention for weight loss and metabolic syndrome remission in patients with severe obesity [5]. Several studies have demonstrated that decreased adherence to the recommended post-metabolic surgery diet can be associated with weight regain and nutritional deficiencies. While poor self-discipline and lack of motivation can be intrinsic factors associated with poor diet adherence after metabolic surgery, limited availability of healthy foods has been suggested as an important extrinsic factor [6].
Previous studies have shown negative associations between food insecurity and successful weight loss interventions in patients with obesity. Myers et al. found that food insecurity modulates weight loss following high intensity lifestyle-based weight loss interventions, with decreased weight loss in patients facing lack of sufficient healthy food sources [7]. A limited number of studies have examined the impact of poor access to nutritious foods on weight loss and nutritional outcomes following metabolic surgery [8]. This study aims to compare postoperative outcomes of patients who underwent metabolic surgery by utilizing geospatial data to determine if patients who reside in food insecure areas experience reduced weight loss and/or worse nutritional outcomes than patients who reside in food secure neighborhoods.
Materials and methods
Data description
This is an Institutional Review Board-approved retrospective cohort study in which data were queried from our institutional contributions to the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) to identify patients 18 years or older who underwent sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) within Cleveland Clinic hospitals in Ohio from 2010 to 2018. Patients who underwent a revision or conversion surgery, and patients who did not live in Ohio or whose census tract could not be identified were excluded (n = 1243). Body weight on all postoperative visits across four follow-up years was exported from the electronic health record and merged with preoperative MBSAQIP data. Census tracts for individual patients were identified using patient addresses at the time of surgery. Modified Retail Food Environmental Index (mRFEI) data was collected from the Center for Disease Control (CDC) and merged with patient data by census tract. The mRFEI is a measure which indicates the percentage of healthy food retailers within a census tract or ½ mile from the tract boundary relative to all food retailers within the area [9]. It has been widely used across studies which have established relationships between food insecurity and various outcomes such as glycemic control [10], obesity rates [11], and physical activity [12]. Healthy food retailers include supermarkets, larger grocery stores, supercenters, and produce stores. Less healthy food retailers include fast food restaurants, small grocery stores, and convenience stores [9].
Study groups
Patients were divided into one of three food security groups based on the mRFEI of the census tracts in which they resided at the time of surgery. The CDC defines mRFEI scores of 0% as food deserts (FDesert). A food swamp (FSwamp) is defined by mRFEI scores from 1–10% in other publications. Food swamps correspond to areas where convenience stores or fast food restaurants represent most of the available food with little fresh produce available. The final group, with mRFEI scores greater than 10% is designated as Food Secure (FSecure). These are areas where more than 10% of food outlets offer healthy food options and fresh produce.
Weight loss outcomes
BMI was calculated based on weight and height data extracted from the electronic medical record. Preoperative BMI was assessed from patient weight recorded on the day of the index metabolic surgery. Given maximum weight loss is usually achieved in the first few years after metabolic surgery [13], BMI nadir was calculated as the lowest postoperative BMI achieved within four years post-surgery. BMI regain was calculated as the maximum postoperative BMI following BMI nadir within the four-year time frame following the index metabolic surgery. The time to achieving BMI nadir was calculated from date of surgery to the date of the lowest postoperative BMI. The time until maximum weight regain was calculated from the date of lowest BMI to the date of maximum BMI measured after nadir.
Nutritional data
Preoperative laboratory values for albumin, calcium, iron, vitamin D, and vitamin B12 were extracted for all patients via the electronic health record. All postoperative values for these labs, drawn within eleven and twenty-five months postoperatively, were also extracted. Nutritional deficiencies were identified in patients for whom resulted laboratory values were less than established criteria for normal range.
Statistical analysis
All analyses were stratified by surgery type (RYGB or SG). Continuous measures were summarized using means and 95% confidence intervals, or medians and interquartile ranges, and were compared between food security groups using ANOVA or Kruskal–Wallis tests, as appropriate. Categorical patient characteristics were summarized using counts and percentages and were compared using chi-square tests. Associations between food security groups and preoperative BMI were evaluated using the SAS procedure GLM to fit multivariable generalized linear regression models with contrasts statements pairwise comparisons between food security groups. To evaluate differences in postoperative change in BMI, quadratic growth mixed models with time as a quadratic random effect were fit [14, 15]. Contrast statements were written for pairwise comparisons between food security groups. To evaluate the time until BMI nadir and the time until maximum weight regain, Cox Proportional Hazards (PH) Models with contrast statements to test for differences in food security groups were used. The PH assumption was confirmed using Schoenfeld residuals. Finally, to visualize postoperative weight change among food security groups, restricted cubic splines were fit to the data using 5 knots [16, 17]. All models were adjusted for patient preoperative demographic characteristics and comorbidities. All models were robust against adjustment, and tests about food security did not change when non-significant covariates were removed. Results from the full models are reported. All analysis was performed in SAS 9.4 (SAS Institute, Cary, NC). All tests were two-tailed and performed at a significance level of α = 0.05.
Geospatial mapping of mRFEI values and percent weight loss following RYGB and SG was completed in R version 4.2.1 (R Core Team, 2022). To perform the mapping, data values were joined to a census tract shapefile of Cuyahoga County from the 2010 decennial Census.
Results
A total of 5097 patients underwent metabolic surgery within our health system from 2010 and 2018 and met the selection criteria in this study (Fig. 1). A total of 3424 patients underwent RYGB and 1673 underwent SG. The median follow-up was 2.3 years (IQR, 0.9–3.6 years). Total weight measurements recorded during four-year follow-up ranged from one to seven, with a median of three follow-up measurements. Weight measurements were distributed uniformly across the follow-up time frame.
Flow chart for patient inclusion in the study. A total of 5097 patients met the selection criteria. BMI body mass index, MBSAQIP metabolic and bariatric surgery accreditation and quality improvement program, mRFEI modified food retail environmental index, RYGB Roux-en-Y gastric bypass, SG sleeve gastrectomy
Preoperative characteristics
Seventy-eight percent of patients were female. Median age at the time of surgery was 46.4 years (IQR, 37.6–55.9). Demographic characteristics for each food security group are reported in Table 1 (RYGB) and Table 2 (SG). There were fewer black patients in the FSecure subgroup compared with the FSwamp and FDesert subgroups. There was largely no association between food security, preoperative characteristics, and baseline nutritional variables. The median preoperative BMI was similar across food security groups for both RYGB and SG. There was no association between preoperative BMI and food security for either type of surgery (RYGB p = 0.54; SG p = 0.70, Tables 1 and 2).
Postoperative change in BMI
Food security was not associated with postoperative weight change (RYGB p = 0.73, SG p = 0.68); Table 3). Cubic smoothing splines fitting percent-changes in postoperative BMI following metabolic surgery were plotted for each food security group and showed similar patterns for percent-change in postoperative BMI across food security groups during a four-year follow-up period (Fig. 2). Finally, for patients residing in the city of Cleveland and, more broadly, Cuyahoga County, there was limited geographic overlap between patterns of food insecurity and percent weight loss following surgery [RYGB (Fig. 3A); SG (Fig. 3B)].
Smoothed trends in weight change after gastric bypass and sleeve gastrectomy stratified by food security status. Cubic smoothing splines with five knots fit to percent-changes in postoperative BMI following metabolic surgery. The y-axis is restricted to show the spline fit. Visual divergence between food security groups at year four is secondary to scarcity of data points at this time point
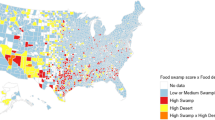
Geospatial mapping of “hot spots” of food deserts and food swamp areas by mREI and geospatial trends in percent weight loss following RYGB (A) and SG (B). Geomapping mRFEI and percent weight loss for patients in each surgery cohort demonstrate lack of overlap between food security hot spots and postoperative weight loss. mRFEI modified food retail environmental index, RYGB Roux-en-Y gastric bypass, SG sleeve gastrectomy
Weight loss nadir
Weight loss nadir occurred at a mean of 1.5 years after surgery for RYGB and 1.3 years postoperatively for SG. Most patients (82.2%) reached their weight loss nadir within 2 years. The overall median BMI nadir of 32 kg/m2 was achieved in each group: FSecure (32.1, IQR 27.9–37.7), FSwamp (32.0, IQR 28.0–37.1), FDesert (32.1, IQR 28.0–37.2). There was no association between BMI nadir and food security group (RYGB p = 0.60, SG p = 0.79). Results by surgery type are reported in Table 4. After adjusting for preoperative factors (sex, race, ethnicity, hypertension, and sleep apnea), the only statistically significant differences in weight loss nadir were seen in FSecure patients who underwent SG; these patients reached their weight loss nadir at slower rates compared to Fswamp patients (p = 0.02) at the time of surgery. Results were robust against adjustment for additional demographic factors.
Weight regain following nadir
The median percent weight regain after weight loss nadir was 9.3% in RYGB patients (IQR 4.9–15.3%) and 8.9% in SG patients (IQR 4.3–13.8%). There was no association between weight regain and food security group (RYGB p = 0.93, SG p = 0.85). There was also no association between time until maximum weight regain after nadir and food security. Weight regain results were also robust against adjustment for additional demographic factors (Table 3).
30-Day postoperative outcomes
There was no association between food security and 30-day readmission (RYGB p = 0.27, SG p = 0.10), reoperation (RYGB p = 0.88, SG p = 0.15), or mortality (RYGB p = 0.60, SG p = 0.99) (Table 1 and 2).
Nutritional deficiencies
There were no differences in nutritional deficiency rates for albumin, calcium, iron, vitamin B12, and vitamin D by food security group in both patients who underwent RYGB and SG surgery (Table 5). There were no notable differences in missingness of lab data between food security groups.
Sensitivity analysis
Following the calculated weight nadir based on available date, 1240 (44%) of RYGB patients and 665 (49%) of SG patients were lost to follow-up (LTF). A sensitivity analysis was conducted to evaluate differences in the patients utilized in the evaluation of weight regain versus those LTF. For both procedures, patients LTF were slightly younger (RYGB: 46 vs 47.9 years, p = 0.001; SG: 46.3 vs 48 years, p = 0.004). There were no differences between those who were followed and patients who were LTF by food security group (RYGB, p = 0.38; SG, p = 0.92).
Discussion
In the most recent statement by the American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) on the indications for metabolic and bariatric surgery, food insecurity was highlighted as an important stressor that should be identified preoperatively given its potential to significantly impact postoperative outcomes [18]. However, very little was known about the degree to which food insecurity impacts weight loss outcomes after metabolic surgery, particularly SG and RYGB. Given that food insecurity poses a barrier to healthy food choices and is known to impede the impact of medical weight loss programs, we hypothesized that food insecurity would equally and negatively impact outcomes after SG and RYGB.
Our study demonstrated that food security status did not alter postoperative weight change after RYGB or SG. Food security neither impacted weight loss nadir, nor weight regain after nadir for either procedure. Furthermore, food security had no noticeable impact on 30-day readmission, reoperation rate, or mortality. Even more surprising was that regardless of the availability of healthy food options, food security also had no impact on key nutritional outcomes in our study. Deficiencies in albumin, vitamin D, iron, vitamin B12, and calcium were not significantly different between food security groups up to 2 years after surgery, when most weight loss occurred. Thus, these results suggest that both RYGB and SG can be safely and effectively used to treat obesity in patients facing food insecurity.
In reviewing our data, we assessed whether mRFEI scores were associated with area deprivation indices. The area deprivation index, of note, is an extremely well-validated measure established by the Health Resources & Services Administration which assigns national and state-level ranks to neighborhoods by socioeconomic deprivation or disadvantage. It incorporates domains such as income, education, employment, and housing quality [19, 20]. It is currently the most validated scientific tool for neighborhood-level disadvantage among US neighborhoods [21]. Since factors that drive food insecurity include poverty and unemployment [22], we felt ADI may effectively characterize the socioeconomic disadvantage which drives food insecurity on both the geospatial and personal level. In investigating an association between mRFEI and area deprivation index within our data, we found that areas classified as Food Deserts had higher deprivation rates (p = 0.045 for RYGB, p = 0.002 for SG). Similar to our results looking at metabolic surgical outcomes by mRFEI, we found that national ADI rank was also not associated with changes in post-op BMI, whether or not we adjusted for mRFEI.
Our findings are in line with the few studies that have also sought to evaluate similar questions. Mathson et al. studied the impact of food insecurity on early postoperative outcomes after metabolic surgery. While there was an association between food insecurity and longer length of stay, there was no impact on 30-day mortality [23]. Furthermore, a four-year study of an Appalachian state bariatric program demonstrated that food accessibility did not impact the weight lost at one-year, again highlighting the non-inferiority of metabolic surgery in subjects experiencing food insecurity [8]. To our knowledge, the current study represents the largest study with the longest follow-up time to date on this topic and reports the most comprehensive weight loss outcomes, measuring both weight loss nadir and weight gain after nadir. Another strength of our work is the use of the CDC’s definitions for food insecurity via mRFEI scores, making this work both reproducible and relevant to national policy discussions regarding the care of patients with obesity.
As expected, given the history of Cleveland, Ohio, and the impact of redlining on racial health disparities [24,25,26], fewer Black patients lived in areas identified as FSecure. In one of the largest studies of outcomes of metabolic surgery, African Americans had worse outcomes, including higher mortality rates regardless of whether RYGB or SG had been performed [27]. Though factors underlying these disparate outcomes warrant more study, geospatial disparities in food security do not appear to play a significant role.
Limitations
This study has several limitations. First, there are well established limitations to the mRFEI as a tool for characterizing food security, such as imprecision of retailer categories used to calculate proportion of healthy food options available within a census tract [28]. Nevertheless, it remains a widely accepted and accessible assessment tool, which increases replicability of our study methods. Second, there was a high number of patients lost to long-term follow-up. However, our sensitivity analysis showed that there were no differences in food insecurity status or relevant cofactors including race or comorbidities between those studied and those LTF. Thus, it is unlikely that those LTF influenced our data or the conclusions of this study. Third, a minority of patients moved during the study period, and thus may have switched food security groups. [29]. We reviewed our data and found that 14.5% of RYGB patients (85.5% were in the same zip code) and 12% of SG patients (88% stayed within the same zip code) moved to a different zip code within 2 years of their index metabolic surgical procedure. Fourth, food insecurity represents a significant stressor which can impact psychological outcomes after metabolic surgery, including depression, weight-based discrimination, and poor self-image, all of which can impact quality of life postoperatively [30]. This was not addressed in our study and remains an area of future study. Fifth, this study only addresses the impact of geospatial food insecurity on metabolic surgery outcomes from a population standpoint and does not address factors related to personal food insecurity, such as access to transportation, food assistance programs such as the Supplemental Nutrition Assistance Program (SNAP), or community food programs. This represents an area of interest for future study. Sixth, this study was a retrospective analysis at a single center, with geographic study area limited to the northeast Ohio region and demographics. Thus, further work in a variety of community settings is warranted to confirm our findings. Finally, it is a possibility that the rigorous assessment and patient selection process for metabolic surgery in and of itself inadvertently excludes some of the most socially and economically vulnerable patients of society and may select for relatively less socioeconomically disadvantaged patients, even among those living within food insecure areas. This is a potential reality of which surgeons should be cognizant and should prompt strategies for improved access to metabolic surgery for all patients.
Conclusion
In conclusion, our work suggests that food security status may not significantly impact weight loss or nutritional outcomes following metabolic surgery. In fact, our data shows that even for those living in food insecure areas, desired weight loss can be achieved postoperatively and maintained at expected levels. Despite the association of food insecurity and obesity, food insecurity status should not preclude patients from eligibility for metabolic surgery. Metabolic surgery may offer one of the most most equitable solutions currently available to address the complex and ever challenging obesity epidemic in the United States.
References
Harris E (2023) US obesity prevalence surged over the past decade. JAMA 330:1515–1515. https://doi.org/10.1001/JAMA.2023.19201
Food Access Research Atlas – Documentation (2022) USDA Economic Research Service. https://www.ers.usda.gov/data-products/food-access-research-atlas/documentation/#definitions. Accessed 15 Apr 2024
Dubowitz T, Ghosh-Dastidar M, Eibner C et al (2012) The women’s health initiative: the food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity 20:862–871. https://doi.org/10.1038/OBY.2011.141
County C, Thrive W (2020) Performance evaluation & innovation (PEI) 2019 statistical performance report 2020. https://dsas.cuyahogacounty.us/pdf_dsas/en-. Accessed 19 Apr 2024
Gloy VL, Briel M, Bhatt DL et al (2013) Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. https://doi.org/10.1136/BMJ.F5934
Assakran BS, Widyan AM, Alhumaidan NA et al (2020) Dietary assessment and patient-perspective reasons for poor adherence to diet and exercise post bariatric surgery. BMC Res Notes. https://doi.org/10.1186/S13104-020-05373-Ynj
Myers CA, Martin CK, Apolzan JW et al (2021) Food insecurity and weight loss in an underserved primary care population: a post-hoc analysis of a cluster-randomized trial. Ann Intern Med 174:1032. https://doi.org/10.7326/M20-6326
Barr ML, Tabone LE, Brode C et al (2020) Successful weight loss after bariatric surgery in Appalachian state regardless of food access ranking score. Surg Obes Relat Dis 16:1737–1744. https://doi.org/10.1016/J.SOARD.2020.06.040
National Center for Chronic Disease Prevention and Health Promotion (2012) Census tract level state maps of the modified retail food environment index (mRFEI). Centers for Disease Control and Prevention. https://stacks.cdc.gov/view/cdc/61367/cdc_61367_DS1.pdf. Accessed 16 Apr 2024
Berkowitz SA, Karter AJ, Corbie-Smith G et al (2018) Food insecurity, food “deserts”, and glycemic control in patients with diabetes: a longitudinal analysis. Diabetes Care 41:1188–1195. https://doi.org/10.2337/DC17-1981/-/DC1
Cerceo E, Sharma E, Boguslavsky A et al (2023) Impact of food environments on obesity rates: a state-level analysis. J Obes. https://doi.org/10.1155/2023/5052613
Shupler M, Klompmaker JO, Leung M et al (2024) Association between density of food retailers and fitness centers and gestational diabetes mellitus in Eastern Massachusetts, USA: population-based study. Lancet Reg Health Am. https://doi.org/10.1016/J.LANA.2024.100775
Xu T, Wang C, Zhang H et al (2020) Timing of maximal weight reduction following bariatric surgery: a study in chinese patients. Front Endocrinol (Lausanne) 11:615. https://doi.org/10.3389/FENDO.2020.00615
Harrell FE (2015) Regression modeling strategies with applications to linear models, logistic and ordinal regression, and survival analysis second edition springer series in statistics, 2nd edn. Springer, New York
Johnson M (2002) Individual growth analysis using PROC MIXED. SAS Users Group Int 27:253–257
Gauthier J, Wu QV, Gooley TA (2019) Cubic splines to model relationships between continuous variables and outcomes: a guide for clinicians. Bone Marrow Transplant 55(4):675–680. https://doi.org/10.1038/s41409-019-0679-x
Croxford R (2016) Restricted cubic spline regression: a brief introduction. SAS Global Forum 2016 Proceedings, p 5621
Eisenberg D, Shikora SA, Aarts E et al (2022) 2022 American society for metabolic and bariatric surgery (ASMBS) and international federation for the surgery of obesity and metabolic disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis 18:1345–1356. https://doi.org/10.1016/J.SOARD.2022.08.013
Kind AJH, Buckingham WR (2018) Making Neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med 378:2456. https://doi.org/10.1056/NEJMP1802313
University of Wisconsin School of Medicine Public Health. Area Deprivation Index v2.0 2015. https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed 10 July 2024
Powell WR, Sheehy AM, Kind AJH (2023) The area deprivation index is the most scientifically validated social Exposome tool available for policies advancing health equity. Health Affairs Forefront. https://doi.org/10.1377/FOREFRONT.20230714.676093
Bartfeld J, Dunifon R (2005) State-level predictors of food insecurity and hunger among households with children. USDA Economic Research Service. https://www.ers.usda.gov/publications/pub-details/?pubid=86013. Accessed 10 July 2024
Mathson LR, Lak KL, Gould JC et al (2024) The association of preoperative food insecurity with early postoperative outcomes after bariatric surgery. J Surg Res 294:51–57. https://doi.org/10.1016/J.JSS.2023.09.054
Nardone A, Chiang J, Corburn J (2020) Historic redlining and urban health today in U.S. cities. Environ Justice 13:109–119. https://doi.org/10.1089/ENV.2020.0011
Yankey O, Lee J, Gardenhire R et al (2023) Neighborhood racial segregation predict the spatial distribution of supermarkets and grocery stores better than socioeconomic factors in Cleveland, Ohio: a Bayesian spatial approach. J Racial Ethn Health Disparities. https://doi.org/10.1007/S40615-023-01669-4/FIGURES/3
Freedman DA, Bell BA, Clark J et al (2021) Small improvements in an urban food environment resulted in no changes in diet among residents. J Community Health 46:1–12. https://doi.org/10.1007/S10900-020-00805-Z
Hui BY, Roberts A, Thompson KJ et al (2020) Outcomes of bariatric surgery in African Americans: an analysis of the metabolic and bariatric surgery accreditation and quality improvement program (MBSAQIP) data registry. Obes Surg 30:4275. https://doi.org/10.1007/S11695-020-04820-W
Ferdinands AR, Brown JA, Nielsen CC et al (2023) What counts? Adding nuance to retail food environment measurement tools in a Canadian context. Public Health Nutr 26:1326–1337. https://doi.org/10.1017/S1368980023000733
Dutko P, Ploeg M Ver, Farrigan T (2012) Characteristics and influential factors of food deserts. http://www.ers.usda.gov/data-products/. Accessed 15 Apr 2024
Gastón-Panthaki A, Serrano A, Virani N et al (2023) Food insecurity, weight-based discrimination, weight self-stigma, and mental health in post-bariatric surgery patients. Body Image 45:46–53. https://doi.org/10.1016/J.BODYIM.2023.01.009
Acknowledgements
The authors would like to acknowledge The Cleveland Clinic Center for Populations Health Research for their assistance and support in executing and interpreting the results of this study as well as the Prevention Research Center for Healthy Neighborhoods at Case Western Reserve University School of Medicine for their support and essential work within the city of Cleveland.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Aminian has received research grants from Ethicon and Medtronic and in an advisory board for Eli Lilly, Ethicon, and Medtronic. Dr. Buchalter is supported by NIH Award Number T32CA094186. Other authors have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilkerson, A.D., Gentle, C., Dewey, E.N. et al. Association between geospatial disparities in food security with weight loss and nutritional outcomes of metabolic surgery. Surg Endosc (2024). https://doi.org/10.1007/s00464-024-11175-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00464-024-11175-1