Abstract
Introduction
RefluxStop is an implantable device for laparoscopic surgical treatment of gastroesophageal reflux disease (GERD) to restore and maintain lower esophageal sphincter and angle of His anatomy without encircling and putting pressure on the food passageway, thereby avoiding side effects such as dysphagia and bloating seen with traditional fundoplication. This study reports the clinical outcomes with RefluxStop at 4 years following implantation of the device.
Methods
A prospective, single arm, multicenter clinical investigation analyzing safety and effectiveness of the RefluxStop device in 50 patients with chronic GERD.
Results
Available data are presented for 44 patients at 4 years with the addition of three patients at 3 years carried forward. At 4 years, median GERD-HRQL score was 90% reduced compared to baseline. Two patients (2/44) used regular daily proton pump inhibitors (PPIs) despite subsequent 24-h pH monitoring off PPI therapy yielding normal results. There were no device-related adverse events (AEs), esophageal dilations, migrations, or explants during the entire study period. AEs reported between 1 and 4 years were as follows: one subject with heartburn and a pathologic pH result with device positioned too low at surgery; one subject with dysphagia, thus, 46/47 patients reported no dysphagia-related AEs between years 1 and 4. Two patients (2/47) were dissatisfied with treatment despite normal 24-h pH monitoring, of whom one had manometry-verified dysmotility at 6 months, indicating dissatisfaction for reasons other than acid reflux.
Conclusion
These results confirm the excellent and already published 1-year results as stable in the long-term, supporting the safety and effectiveness of the RefluxStop device in treating GERD for over 4 years. GERD-HRQL score, pH testing, and PPI usage indicate treatment success without dysphagia or gas-bloating and only minimal incidence of other AEs. This favorably low rate of AEs is likely attributable to RefluxStop’s dynamic physiologic interaction and non-encircling nature.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Background
Individuals with gastroesophageal reflux disease (GERD) can experience profoundly impaired quality of life [1] and are at increased risk of developing esophageal adenocarcinoma [2, 3]. The mainstay of medical treatment involves reducing the acidity of refluxate using proton pump inhibitors (PPIs), while surgical options seek to address the underlying cause of reflux itself, through hiatal hernia reduction, crural repair, and partial or complete fundoplication. However, fundoplication techniques compress the distal esophagus and create an increased risk of adverse effects, such as dysphagia, inability to belch or vomit, and gas-bloating [4].
In contrast, RefluxStop surgery avoids encircling and putting pressure on the food passageway, which is typical of fundoplication procedures, such as Nissen or Toupet. The device sits in an invaginated pouch on the outside of the stomach, where it is implanted following hiatal hernia repair (Fig. 1). The procedure works by treating the cause of acid reflux via all three aspects of the anti-reflux reflux barrier (ARB) [5]; namely, restoring the flap valve by recreation of the acute angle of His, restoring the lower esophageal sphincter (LES) in its functional position, as well as a hiatal repair. By reinstating the normal physiologic status of the ARB, acid reflux is effectively managed for the hypothetical long term.
Obtaining sufficient intraabdominal esophageal length and restoration of the angle of His to its anatomically normal and acute angle are integral aspects of the procedure. Performed correctly, implantation of this device ensures that the LES remains within the abdominal cavity at an appropriate distance from the hiatal orifice, even during dynamic movements of respiration, with adequate surrounding pressures to enable LES contractility and closure as well as a restored angle of His, flap valve, and diaphragmatic hiatus to prevent regurgitation of gastric contents [6, 7].
This article presents the most up-to-date clinical results at 4 years of the ongoing RefluxStop prospective clinical investigation to evaluate long-term clinical outcomes and discusses aspects of the technique to ensure the best prospect of treatment success.
Methods
Study design and objectives
This was a prospective, single arm, multicenter clinical investigation with trial registration number NCT02759094 (https://clinicaltrials.gov/ct2/show/NCT02759094). The study design has been reported in detail in an earlier publication [8]. Fifty patients with chronic GERD were included in the study. Patients had symptoms for at least 6 months, confirmed with 24-h pH monitoring, defined as pathologic when distal esophageal pH was < 4 for ≥ 4.5% of the 24-h period. Patients with hiatal hernia greater than 3 cm were not eligible for this study. Surgery was performed between December 2016 and September 2017 at four hospitals. For further details, see the publication of earlier follow-up results [8]. The study data is in general presented both as Per Protocol (PP) and as Full Analysis Set (FAS).
The surgical procedure performed was described in publication of earlier results [8]. Further considerations to ensure optimal surgical outcomes are addressed in the discussion of this article, as some learning points have been recognized via clinical practice since the introduction of the technique.
The procedure includes extensive mediastinal dissection to achieve 4–5 cm of abdominal esophagus, loose crural repair after hernia repositioning, fundus dissection dividing at least three short gastric arteries, and posterior dissection inferiorly on the fundus to achieve a floppy tension-free fundus. This is necessary to match the length of the freely dissected esophagus. Plication is performed between the fundus and esophagus on the patient’s left side between the vagal nerves (trunks) on at least 90° of the esophageal circumference. Thereafter, the device is loosely but fully invaginated in a fundic pouch in the cranial-most portion of the gastric fundus.
All adverse events (AEs) were recorded both annually at years 1–4 and at any additional visits. Annually, the GERD Health-Related Quality of Life (GERD-HRQL) and foregut symptom questionnaires (regurgitation) were completed. Patients were also asked about PPI usage. The GERD-HRQL questionnaire gives a total score (0–50) indicative of symptom severity with a separate question apropos patient satisfaction. Patients who were considered to have potential failure of treatment, that is, less than 50% improvement in GERD-HRQL score or regular daily PPI usage, were further investigated with 24-h pH monitoring and contrast-swallow imaging, followed by possible gastroscopy (if pH monitoring normal) and manometry (according to judgement of the surgeon).
The primary outcome was change in GERD-HRQL total score from baseline.
An independent Data Monitoring Committee (DMC) evaluated the safety data and selected effectiveness data.
Statistical analysis
The following sets were used in the statistical analysis:
-
Full analysis set (FAS): all subjects who received the device implant (n = 50), based on modified intention-to-treat (mITT).
-
Per protocol (PP) analysis set: all subjects at follow-up.
The effectiveness variables were analyzed for both the FAS and the PP set. When timely results as per protocol were not available, previous latest results were used.
Distribution of parametric data was inspected by histograms, box plots, and scatterplots. The 95% confidence intervals (CIs) for the relative GERD-HRQL score reductions were calculated via the Clopper-Pearson exact method formula. The change in GERD-HRQL satisfaction assessments were inspected via alluvial plots. To test for significant differences between data of multiple follow-ups, data were first tested for equality of variances via Levene’s test. In case of equal variances, differences were assessed via analysis of variance (ANOVA); otherwise, via the Kruskal–Wallis test. To test for significant differences between individual visits, Wilcoxon tests were applied. p-values for multiple testing were adjusted via Benjamini–Hochberg procedure; the significance level was 0.05. Statistical analysis was performed using R version 4.2.2 under the Microsoft Windows operating system.
Ethics approval
The study was carried out in accordance with the Declaration of Helsinki and the Regional Ethics Committees approved the study protocol: the Medical Research Council (MRC), Scientific and Research Ethics Committee (SREC), Budapest, Hungary, and the Ethics Committee of Serbia (EOS), Belgrade, Serbia. All patients provided written informed consent to participate, which included consent for the publication of anonymized data.
Results
Study population
The RefluxStop device was implanted in 50 subjects. As mentioned in more detail above, 44 subjects were included in the PP analysis. For clarity, some previously published results are summarized and included in the present article with the addition of new 4-year outcomes.
At baseline, mean (SD) age was 51.5 (11.8) years, 56% were male, and mean (SD) weight was 78.2 (14.7) kg.
Missing patients
Follow-up data were available on 44/50 subjects at 4 years. The missing patients were as follows:
-
One subject died from COVID-19 and two missed the 4-year follow-up, of whom:
-
All three missing patients (one dead and two missed) were well-treated at 3 years: all three were satisfied, with exceptionally low GERD-HRQL scores (i.e., average score 1.3), no PPI usage, and regurgitation classification of none.
-
-
Three patients terminated the study in the first year: one at 3 months and two at 6 months, of whom:
-
Two patients were satisfied, did not take PPIs, had a low average GERD-HRQL score of 2.5, and no regurgitation.
-
One patient had a broken needle left subcutaneously, removed under local anesthesia. This patient terminated dissatisfied, with a high GERD-HRQL score, however, no regular daily PPI usage at the 6-month visit, and regurgitation classified as none. This patient refused 24-h pH testing.
-
Effectiveness results at 4 years
Table 1 summarizes the outcomes at 4 years.
Primary effectiveness outcome
Reduction in GERD-HRQL score
Median (IQR) GERD-HRQL score decreased by 90% (Table 2) from a baseline of 29.5 (24–33) to 3.0 (0–9.2) at 4-year follow-up. It should be noted that, excluding subjects who had < 50% improvement in GERD-HRQL score for reasons other than acid reflux as verified by a normal 24-h pH monitoring result, 98% of the patients had ≥ 50% reduction in GERD-HRQL score at year 4.
Secondary effectiveness outcomes
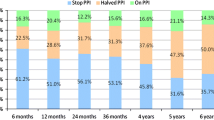
PPI use
Before surgery, all 50 subjects were taking PPIs daily. At the 4-year follow-up, two subjects (2/44; 4.5%) regularly took daily PPIs, however, both had normal 24-h pH monitoring results (Table 3). None of the three subjects with the last visit at 3 years or the three subjects who terminated the study early were taking PPIs at their last follow-up visit.
Subject satisfaction (GERD-HRQL questionnaire item)
At 4 years, two subjects (2/44) were dissatisfied, however, both had subsequent normal 24-h pH monitoring results. One of these subjects had undergone assessment at 2- and 4-year follow-up with both showing normal 24-h pH results (Table 3). The second subject underwent 24-h pH monitoring after 4-year follow-up and 24-h pH monitoring remained normal. These results demonstrate that neither of the two dissatisfied subjects at 4 years had GERD as the likely cause of their dissatisfaction.
Five of the six subjects who did not have 4-year follow-up data were satisfied at their latest follow-up visit (Table 3).
Regurgitation
Daily regurgitation occurred in 43 subjects (43/50, 86%) at baseline. At 4 years, 95.5% (42/44) of subjects had no or minimal/occasional episodes of regurgitation, and all (100%) subjects experienced improvement in regurgitation compared to baseline (Table 4 and Fig. 2). The three missing subjects with 3-year results reported regurgitation as none, thus together 45/47 had none or minimal regurgitation.
Gas-bloating
At 4 years, gas-bloating symptoms disappeared in 30 subjects (68%), improved in 11 subjects (25%), remained unchanged in two subjects (5%), and worsened in one subject (2%) (Table 5).
Safety results at 4 years
From RefluxStop implantation until the 4-year follow-up visit:
-
One patient died due to COVID-19.
-
No AEs related to the RefluxStop device occurred during the entire study period.
-
No device deficiencies.
-
No device explants.
-
No migration/erosion.
-
No esophageal dilatation was required.
Adverse device effects (ADEs), serious adverse device effects (SADEs), adverse events (AEs) and serious adverse events (SAEs)
No adverse device effects or serious adverse device effects (ADEs, SADEs) occurred during the 4-year follow-up period. During surgery, as previously reported [8], two severe SAEs related to the procedure were reported for two (2/50) subjects; namely, one infection with abscess and one hematoma, both within 30 days, and both treated to resolution.
Between 1 and 4 years (Full Analysis Set), no further SAEs occurred other than the COVID-19 death. One procedure-related AE occurred: recurrence of acid reflux symptoms verified by a pathologic 24-h pH monitoring result. On postoperative contrast-swallow X-ray, the device was identified as being positioned too low, at least partly hindering its function, placing it in the Failure Risk category and resulting in a borderline result. One subject reported mild dysphagia at 3-year follow-up, however, the dysphagia score substantially improved from baseline (see below).
Dysphagia and odynophagia
One subject reported mild dysphagia at 3-year follow-up and had a dysphagia score of 2 on the GERD-HRQL dysphagia-specific question; however, this subject had a score of 5 (severe dysphagia) preoperatively and thus, their dysphagia de facto improved. Forty-six (46/47) patients reported no dysphagia. Table 6 shows the reported dysphagia and odynophagia at baseline, AEs in the first year, and between 1- and 4-year follow-ups. The total number of spontaneously reported AEs for odynophagia and dysphagia correlated with the number of patients reporting a severity of > 2 on the GERD-HRQL questionnaire.
Discussion
This study sought to determine whether the promising effectiveness and safety outcomes of the RefluxStop device seen at 6 months and 1 year [8] would be maintained over the long-term period of 4 years and help elucidate its future role in the treatment of GERD. The rationale for developing a new treatment approach stems from the acknowledged shortcomings of both medical and surgical standard therapies. Despite the efficacy and widespread use of PPIs, it is estimated that one-third of patients do not experience adequate disease control with medication [9]. Furthermore, standard anti-reflux surgery is plagued by adverse postoperative effects, such as gas-bloating, dysphagia, and the inability to belch or vomit, that are attributed in part to encirclement and compression of the distal esophagus [10, 11], an aspect that is avoided in the RefluxStop procedure.
The follow-up rates in the present study are high and the results are generally presented both as Per Protocol and Full Analysis Set. The main long-term effectiveness findings at 4 years demonstrate excellent outcomes, with 90% reduction in median GERD-HRQL score, sustained improvement from baseline as observed at interim follow-ups (i.e., 93.1% reduction in median GERD-HRQL score at 3 years), and low rates of regular daily PPI use (4.3%; 2/47, both of whom had normal 24-h pH testing results). Neither of the two patients who reported dissatisfaction at 4-year follow-up were found to have acid reflux based on objective 24-h pH monitoring.
Collectively, these outcomes support the premise that RefluxStop is a highly effective method of treating chronic GERD with long-term results up to 4 years. Importantly, these favorable effects occurred in the context of low rates of side effects in this time period: dysphagia in 1/47, odynophagia in 0/47, and gas-bloating improvement or resolution in 44/47. Such complications are highly relevant because they have a great effect on quality of life and patient satisfaction. For instance, Humphries et al. reported that dissatisfaction following laparoscopic fundoplication was most often due to new symptoms such as dysphagia and gas-bloating, despite improvement in GERD [12].
Other than surgery-related complications (with full recovery), as presented in earlier interim results [8], AEs between 1- and 4-year follow-up were minimal with no ADEs occurring during the entire study period. More specifically, no explantation, esophageal dilatation, device dislocation, or device migration occurred during the study. The complication profile reported to date is generally favorable in comparison with standard-of-care anti-reflux surgery.
After the 6-month 24-h pH monitoring (normal pH in 98% of subjects from PP analysis), all potential failure patients during follow-up to the current 4-year period underwent additional pH monitoring, contrast-swallow X-ray, and endoscopy studies in selected cases. Potential failure patients consisted of those who were dissatisfied, took regular daily PPIs, or had GERD-HRQL questionnaire results with < 50% improvement from baseline. Out of potential failure patients, only one had a pathologic pH test result; thus, only one subject was an objectively verified failure patient. This one patient also reported heartburn at 4-year follow-up, however, without requiring PPI usage. Moreover, the device was noted to be positioned too low on contrast-swallow imaging, both immediately following surgery and at 4-year follow-up, placed in a position categorized as “Failure Risk”. This patient is illustrative of the great importance of device positioning. This has been previously discussed in journal publication [8] and in several conference communications, but certain details must be reiterated, as an appropriate technique is essential to ensuring successful outcomes.
The most important factor in predicting success of the RefluxStop procedure is the positioning of the device: in proximity to the esophagus and with its entire body above the upper edge of the LES (Fig. 3). As such, the upper edge of the device should be positioned at least 4 cm above the angle of His. To achieve such a satisfactory position, certain steps must be followed. Hernia reduction and free dissection of the esophagus is performed initially without esophageal traction to ensure at least 2.5 cm of intraabdominal esophagus; then, gentle downward traction is applied to intraabdominally mobilize the esophagus 1.5–2.5 cm further down while dissection continues superiorly in the mediastinum (i.e., preferably all the way to the venae pulmonalis). Traction is only applied to simplify dissection and increase intraabdominal esophageal length, but not to stretch the esophagus for additional length. Thus, this procedure includes extensive mediastinal dissection to achieve 4–5 cm of tension-free intraabdominal esophagus.
The gastric fundus also requires dissection in achieving a floppy tension-free fundus to match the length of the freely dissected esophagus. It is recommended to divide at least three of the short gastric arteries, as well as perform a posterior dissection inferiorly on the fundus all the way around to the angle of His to achieve a floppy fundus, to be able to subsequently perform a tension-free plication. Plication of the fundus and esophagus is performed on the patient’s left side between the vagal trunks and on at least 90° of the esophageal circumference. When performing the plication, suturing of the fundus to the esophagus should be done with 1 cm of downward traction applied to the esophagus, if needed, to achieve 5 cm of plication length. Once this plication has been completed, all relevant structures usually descend 1 cm inferiorly to the desired locus that helps maintain the ARB in a normal physiologic position.
Since the RefluxStop procedure does not encircle and put pressure on the esophagus, dysphagia is normally entirely avoided as long as the crural repair is not performed too tightly. Thus, this step is conducted to achieve a somewhat looser hiatus closure than in Nissen fundoplication, wherein a bougie size 54, or bougie size 35 with insertion of a peanut through the opening, usually provides ample margin. While the device itself does not surround or compress the esophagus, it remains important that in performing the crural repair, the surgeon avoids closing the hiatus to too great a degree since this could result in the dysphagia that this technique seeks to avoid. The device must be entirely and loosely invaginated in a fundal pouch in proximity to the esophagus, in the cranial-most aspect on the gastric fundus. The proprietary deployment tool allows the surgeon to position and maintain the RefluxStop device in place.
The present study was a single arm investigation and direct head-to-head comparison against existing surgical standards cannot be made. However, compared to the literature on the standard-of-care (i.e., Nissen fundoplication as per most guidelines), the RefluxStop procedure generally presents superior results through indirect comparison. Tian et al. conducted a meta-analysis of laparoscopic fundoplication, reporting a postoperative dysphagia rate of 12.6% with 14% requiring endoscopic dilatation [13]. Furthermore, the overall prevalence of gas-related symptoms was 31.2% [13]. Other relevant literature includes a study by Nijjar et al. on 5-year follow-up of Nissen fundoplication [14], who reported: heartburn in 27%; PPI use in 4.5%; dysphagia for solids in 41%; and abdominal bloating in 59%. In a study by Draaisma et al. [15], 14% took PPIs and 15% had been reoperated on at 5-year follow-up. Furthermore, in a study by Ludemann et al., assessing 5-year follow-up of Nissen fundoplication, 12% took PPIs and 8% required reoperation [16]. If comparing these results with the RefluxStop study, the differences are substantial and a randomized study would be of benefit.
As with any novel surgical technique, a learning curve is unavoidable. In laparoscopic fundoplication, Salminen et al. reported that greater experience with procedures was associated with fewer complications, conversions, and early dysphagia episodes, highlighting the value of expert supervision, even after an initial learning phase of 20 individual procedures [17]. Prospectively, it seems likely that there will be centers with higher volumes and experience of the RefluxStop procedure that will have even lower rates of postoperative events than those presented here.
The strengths of the present CE mark study are its prospective multicenter design and provision of the longest clinical follow-up to date of this patient cohort—the first ever to undergo this procedure. Therefore, this study is of great novelty and value to those involved in the care of chronic GERD patients as well as those that may benefit from this surgery. Effectiveness was assessed using the GERD-HRQL questionnaire, which has been shown to be suitably valid and reasonably reliable in assessing the severity of GERD symptoms and response to treatment, including surgery [14], however, symptoms from other issues than GERD may influence the results. To objectively confirm outcomes, it is important to support patient-reported outcomes with the incorporation of objective measures such as 24-h pH monitoring; this was performed in those who had suspected treatment failure in our study. A failed questionnaire could have other causes besides GERD. For instance, gastritis is one such example with a prevalence approximating 25–35% [18], and in the broader context of Helicobacter pylori infection, more than half the world’s population [19].
The limitations of our study include the lack of a control group, which is a suggested key area for future study in determination of effectiveness and safety, using direct comparison to other existing surgical techniques. The sample size of 50 patients and the high experience level of most of the operating surgeons are also factors to consider in the generalizability of results. Furthermore, in clinical practice, there are many patients with complex clinical profiles, for example, those with large hiatal hernia (> 3 cm), high BMI (> 35 kg/m2), concurrent esophageal dysmotility, previous unsuccessful antireflux surgery, and atypical GERD symptoms, in whom the generalizability of these results may be limited. Dedicated studies or subanalyses of larger studies addressing these patient groups would be beneficial to guide treatment decisions in the real-world setting. Ongoing follow-up of the present patient cohort continues, with thorough evaluation including objective testing in the form of 24-h pH monitoring and contrast-swallow X-ray in all patients scheduled to take place at 5 years post-surgery.
Based on the data from the present study, RefluxStop appears to be a strong candidate for effective surgical treatment of chronic GERD and should be considered as a treatment option in those seeking surgical intervention.
Conclusion
These 4-year results of the prolonged CE study evaluating RefluxStop™* confirm that the excellent published 1-year outcomes are stable long-term and support the safety and effectiveness of the RefluxStop device. GERD-HRQL scores (90% improvement), pH testing, and PPI usage (2/47 though normal pH monitoring result) indicate treatment success, in the context of minimal side effects of AE dysphagia (1/47) or gas-bloating. No cases of esophageal dilatation, explantation, device migration, or device-related AEs occurred during the entire study period. This low rate of AEs is likely attributable to RefluxStop’s loose fundus invagination and dynamic physiologic interaction that avoids encircling or exerting pressure on the food passageway. Considering these results, the RefluxStop procedure represents a strong alternative to standard anti-reflux surgery. Several further studies are both planned and underway; outcomes will be reported as they become available.
* RefluxStop® in US.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- GERD:
-
Gastroesophageal reflux disease
- HRQL:
-
Health-Related Quality of Life questionnaire
- CE Mark:
-
Conformité Européenne, a European certification mark
- LES:
-
Lower esophageal sphincter
- PPI:
-
Proton pump inhibitor
- FAS:
-
Full analysis set
- PP:
-
Per protocol
- ITT:
-
Intention-to-treat
- AE:
-
Adverse event
- SAE:
-
Serious adverse event
- SADE:
-
Serious adverse device effect
- ADE:
-
Adverse device effect
- DMC:
-
Data monitoring committee
- CI:
-
Confidence interval
- HH:
-
Hiatal hernia
References
Kamolz T, Pointner R, Velanovich V (2003) The impact of gastroesophageal reflux disease on quality of life. Surg Endosc 17(8):1193–1199. https://doi.org/10.1007/s00464-002-9229-4
Ho ALK, Smyth EC (2020) A global perspective on oesophageal cancer: two diseases in one. Lancet Gastroenterol Hepatol 5(6):521–522. https://doi.org/10.1016/s2468-1253(20)30047-9
Lagergren J, Bergström R, Lindgren A, Nyrén O (1999) Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340(11):825–831. https://doi.org/10.1056/nejm199903183401101
Ganz RA (2016) A review of new surgical and endoscopic therapies for gastroesophageal reflux disease. Gastroenterol Hepatol (N Y) 12(7):424–431
Nguyen NT, Thosani NC, Canto MI, Chang K, Lipham J, Abu Dayyeh B et al (2022) The American Foregut Society white paper on the endoscopic classification of esophagogastric junction integrity. Foregut J Am Foregut Soc 2(4):339–348. https://doi.org/10.1177/26345161221126961
Rosen RD, Winters R (2023) Physiology, lower esophageal sphincter. StatPearls [Internet]. StatPearls Publishing, St. Petersburg
Mousa H, Hassan M (2017) Gastroesophageal reflux disease. Pediatr Clin North Am 64(3):487–505. https://doi.org/10.1016/j.pcl.2017.01.003
Bjelović M, Harsányi L, Altorjay Á, Kincses Z, Forsell P (2020) Non-active implantable device treating acid reflux with a new dynamic treatment approach: 1-year results: RefluxStop™ device; a new method in acid reflux surgery obtaining CE mark. BMC Surg 20(1):159. https://doi.org/10.1186/s12893-020-00794-9
Yadlapati R, Hungness ES, Pandolfino JE (2018) Complications of antireflux surgery. Am J Gastroenterol 113(8):1137–1147. https://doi.org/10.1038/s41395-018-0115-7
Richter JE (2003) Let the patient beware: the evolving truth about laparoscopic antireflux surgery. Am J Med 114(1):71–73. https://doi.org/10.1016/s0002-9343(02)01389-x
Richter JE (2013) Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol 11(5):465–471. https://doi.org/10.1016/j.cgh.2012.12.006. (quiz e39)
Humphries LA, Hernandez JM, Clark W, Luberice K, Ross SB, Rosemurgy AS (2013) Causes of dissatisfaction after laparoscopic fundoplication: the impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc 27(5):1537–1545. https://doi.org/10.1007/s00464-012-2611-y
Tian ZC, Wang B, Shan CX, Zhang W, Jiang DZ, Qiu M (2015) A meta-analysis of randomized controlled trials to compare long-term outcomes of Nissen and toupet fundoplication for gastroesophageal reflux disease. PLoS One 10(6):e0127627. https://doi.org/10.1371/journal.pone.0127627
Velanovich V (2007) The development of the GERD-HRQL symptom severity instrument. Dis Esophagus 20(2):130–134. https://doi.org/10.1111/j.1442-2050.2007.00658.x
Draaisma WA, Rijnhart-de Jong HG, Broeders IA, Smout AJ, Furnee EJ, Gooszen HG (2006) Five-year subjective and objective results of laparoscopic and conventional Nissen fundoplication: a randomized trial. Ann Surg 244(1):34–41. https://doi.org/10.1097/01.sla.0000217667.55939.64
Ludemann R, Watson DI, Jamieson GG, Game PA, Devitt PG (2005) Five-year follow-up of a randomized clinical trial of laparoscopic total versus anterior 180 degrees fundoplication. Br J Surg 92(2):240–243. https://doi.org/10.1002/bjs.4762
Salminen P, Hiekkanen H, Laine S, Ovaska J (2007) Surgeons’ experience with laparoscopic fundoplication after the early personal experience: does it have an impact on the outcome? Surg Endosc 21(8):1377–1382. https://doi.org/10.1007/s00464-006-9156-x
Yin Y, Liang H, Wei N, Zheng Z (2022) Prevalence of chronic atrophic gastritis worldwide from 2010 to 2020: an updated systematic review and meta-analysis. Ann Palliat Med 11(12):3697–3703. https://doi.org/10.21037/apm-21-1464
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D et al (2017) Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153(2):420–429. https://doi.org/10.1053/j.gastro.2017.04.022
Acknowledgements
Special thanks to Prof. Milos Bjelović who substantially contributed to the study, however, now has left the University of Belgrade. Thanks also to the Sub-Investigators of the RefluxStop Clinical Investigation Study Group: Dragan Gunjić, Milan Veselinović, and Tamara Babič (University Hospital for Digestive Surgery—First Surgical Hospital, Clinical Center of Serbia; University of Belgrade, School of Medicine, Belgrade, Serbia); Péter Lukovich and Timea Kakucs (Semmelweis University, Budapest, Hungary); and Sándor Kathy (University of Debrecen Kenézy Gyula Teaching Hospital, Debrecen, Hungary).
Funding
Funding was provided by Implantica, Vaduz, Liechtenstein, and Zug, Switzerland, which financed the costs of the clinical investigation to achieve a CE mark for their new implantable device, RefluxStop™.
Author information
Authors and Affiliations
Contributions
LH was the lead investigator surgeon. LH, AA, and ZK recruited the subjects and collected the data. Third-party Link Medical AB handled the data collecting system Viedoc and collected and analyzed the data. LH wrote the article, summarized the results supplied, and contributed with intellectual content. JZ contributed drafting and critical review of manuscript. All authors were asked to provide feedback on the final manuscript.
Corresponding author
Ethics declarations
Disclosures
Authors László Harsányi, Zsolt Kincses and Áron Altorjay declare that they have no competing interests. Joerg Zehetner has received honoraria from Implantica and Johnson & Johnson and grants/contracts from Implantica and Johnson & Johnson, not in relation to the present manuscript.
Ethical approval
Ethics Committee: Hungary---The Medical Research Council (MRC), Scientific and Research Ethics Committee (SREC, An independent Central Ethics Committee (CEC) responsible for the independent review and approval for all medical device trials in Hungary. Trial approval reference number: 048734/2016/OTIG, issued by the Hungary Regulatory Authority the National Institute of Pharmacy and Nutrition), Alkotmány u. 25, H-1054 Budapest, Hungary. Ethics Committee: Serbia---Ethics Committee of Serbia (EOS), Vojvode Stepe 458, 110000 Belgrade, Serbia.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Consent for publication
All subjects have signed an Informed Consent Form in relation to their participation in the CE-mark clinical investigation RXI 001 of RefluxStop™, which provides consent for the publication of anonymized data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Harsányi, L., Kincses, Z., Zehetner, J. et al. Treating acid reflux without compressing the food passageway: 4-year safety and clinical outcomes with the RefluxStop device in a prospective multicenter study. Surg Endosc (2024). https://doi.org/10.1007/s00464-024-11114-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00464-024-11114-0