Abstract
Background
Robotic technology is an important tool in surgical innovation, with robots increasingly being used in the clinical setting. Robots can be used to enhance accuracy, perform remote actions, or to automate tasks. One such surgical task is suturing, a repetitive, fundamental component of surgery that can be tedious and time consuming. Suturing is a promising automation target because of its ubiquity, repetitive nature, and defined constraints. This systematic review examines research to date on autonomous suturing.
Methods
A systematic review of the literature focused on autonomous suturing was conducted in accordance with PRISMA guidelines.
Results
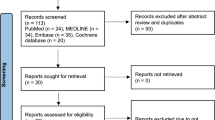
6850 articles were identified by searching PubMed, Embase, Compendex, and Inspec. Duplicates and non-English articles were removed. 4389 articles were screened and 4305 were excluded. Of the 84 remaining, 43 articles did not meet criteria, leaving 41 articles for final review. Among these, 34 (81%) were published after 2014. 31 (76%) were published in an engineering journal9 in a robotics journal, and 1 in a medical journal. The great majority of articles (33, 80%) did not have a specific clinical specialty focus, whereas 6 (15%) were focused on applications in MIS/laparoscopic surgery and 2 (5%) on applications in ophthalmology. Several suturing subtasks were identified, including knot tying, suture passing/needle insertion, needle passing, needle and suture grasping, needle tracking/kinesthesia, suture thread detection, suture needle shape production, instrument assignment, and suture accuracy. 14 articles were considered multi-component because they referred to several previously mentioned subtasks.
Conclusion
In this systematic review exploring research to date on autonomous suturing, 41 articles demonstrated significant progress in robotic suturing. This summary revealed significant heterogeneity of work, with authors focused on different aspects of suturing and a multitude of engineering problems. The review demonstrates increasing academic and commercial interest in surgical automation, with significant technological advances toward feasibility.

Similar content being viewed by others
References
Han J, Davids J, Ashrafian H, Darzi A, Elson DS, Sodergren M (2022) A systematic review of robotic surgery: from supervised paradigms to fully autonomous robotic approaches. Int J Med Robot Comput Assist Surg 18(2):e2358. https://doi.org/10.1002/rcs.2358
Leal Ghezzi T, Campos Corleta O (2016) 30 years of robotic surgery. World J Surg 40(10):2550–2557. https://doi.org/10.1007/s00268-016-3543-9
Kalan S, Chauhan S, Coelho RF, Orvieto MA, Camacho IR, Palmer KJ, Patel VR (2010) History of robotic surgery. J Robot Surg 4(3):141–147. https://doi.org/10.1007/s11701-010-0202-2
Kwoh YS, Hou J, Jonckheere EA, Hayati S (1988) A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng 35(2):153–160. https://doi.org/10.1109/10.1354
Emam TA, Frank TG, Hanna GB, Cuschieri A (2001) Influence of handle design on the surgeon’s upper limb movements, muscle recruitment, and fatigue during endoscopic suturing. Surg Endosc 15(7):667–672. https://doi.org/10.1007/s004640080141
Emam TA, Frank TG, Hanna GB, Stockham G, Cuschieri A (1999) Rocker handle for endoscopic needle drivers: technical and ergonomic evaluation by infrared motion analysis system. Surg Endosc 13(7):658–661. https://doi.org/10.1007/s004649901068
Byrne M, Aly A (2019) The surgical suture. Aesthet Surg J 39(Supplement 2):S67–S72. https://doi.org/10.1093/asj/sjz036
Paul HA, Bargar WL, Mittlestadt B, Musits B, Taylor RH, Kazanzides P, Zuhars J, Williamson B, Hanson W (1992) Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res 285:57–66
George EI, Brand TC, LaPorta A, Marescaux J, Satava RM (2018) Origins of robotic surgery: from skepticism to standard of care. J Soc Laparoendosc Surg 22(4):e2018.00039. https://doi.org/10.4293/JSLS.2018.00039
Attanasio A, Scaglioni B, De Momi E, Fiorini P, Valdastri P (2021) Autonomy in surgical robotics. Annu Rev Control Robot Autonomous Syst 4(1):651–679. https://doi.org/10.1146/annurev-control-062420-090543
Kang H, Wen JT (2002) Robotic knot tying in minimally invasive surgeries. IEEE/RSJ Int Conf Intell Robot Syst 2:1421–1426. https://doi.org/10.1109/IRDS.2002.1043954
van den Berg J, Miller S, Duckworth D, Hu H, Wan A, Fu X-Y, Goldberg K, Abbeel P (2010) Superhuman performance of surgical tasks by robots using iterative learning from human-guided demonstrations. In: 2010 IEEE international conference on robotics and automation, pp 2074–2081. https://doi.org/10.1109/ROBOT.2010.5509621
Chow D-L, Jackson RC, Çavuşoğlu MC, Newman W (2014) A novel vision guided knot-tying method for autonomous robotic surgery. In: 2014 IEEE international conference on automation science and engineering (CASE), pp 504–508. https://doi.org/10.1109/CoASE.2014.6899373
Knoll A, Mayer H, Staub C, Bauernschmitt R (2012) Selective automation and skill transfer in medical robotics: a demonstration on surgical knot-tying. Int J Med Robot Comput Assist Surg 8(4):384–397. https://doi.org/10.1002/rcs.1419
Lu B, Li B, Chen W, Jin Y, Zhao Z, Dou Q, Heng P-A, Liu Y (2022) Toward image-guided automated suture grasping under complex environments: a learning-enabled and optimization-based holistic framework. IEEE Trans Autom Sci Eng 19(4):3794–3808. https://doi.org/10.1109/TASE.2021.3136185
Jackson RC, Çavuşoğlu MC (2013) Needle path planning for autonomous robotic surgical suturing. In: 2013 IEEE international conference on robotics and automation (ICRA), pp 1669–1675. https://doi.org/10.1109/ICRA.2013.6630794
Liu T, Çavuşoğlu MC (2015) Optimal needle grasp selection for automatic execution of suturing tasks in robotic minimally invasive surgery. In: 2015 IEEE international conference on robotics and automation (ICRA), pp 2894–2900. https://doi.org/10.1109/ICRA.2015.7139594
Liu T, Cavusoglu MC (2016) Needle grasp and entry port selection for automatic execution of suturing tasks in <? Pub _newline ? > robotic minimally invasive surgery. IEEE Trans Autom Sci Eng 13(2):552–563. https://doi.org/10.1109/TASE.2016.2515161
Fontanelli GA, Yang G-Z, Siciliano B (2018) A comparison of assistive methods for suturing in MIRS. In: 2018 IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 4389–4395. https://doi.org/10.1109/IROS.2018.8593607
D’Ettorre C, Dwyer G, Du X, Chadebecq F, Vasconcelos F, De Momi E, Stoyanov D (2018) Automated pick-up of suturing needles for robotic surgical assistance. arXiv:1804.03141
Colan J, Nakanishi J, Aoyama T, Hasegawa Y (2021) Optimization-based constrained trajectory generation for robot-assisted stitching in endonasal surgery. Robotics 10(1):27. https://doi.org/10.3390/robotics10010027
Lu B, Chen W, Jin Y-M, Zhang D, Dou Q, Chu HK, Heng P-A, Liu Y-H (2020) A learning-driven framework with spatial optimization for surgical suture thread reconstruction and autonomous grasping under multiple topologies and environmental noises. arXiv:2007.00920
Pedram SA, Shin C, Ferguson PW, Ma J, Dutson EP, Rosen J (2021) Autonomous suturing framework and quantification using a cable-driven surgical robot. IEEE Trans Robot 37(2):404–417. https://doi.org/10.1109/TRO.2020.3031236
Minamoto M, Tanaka S, Hori S, Sogabe M, Miyazaki T, Kawashima K (2022) Future needle position estimation of suturing operation using deep learning. In: 2022 IEEE international conference on mechatronics and automation (ICMA), pp 624–628. https://doi.org/10.1109/ICMA54519.2022.9855970
Chiu Z-Y, Richter F, Funk EK, Orosco RK, Yip MC (2020) Bimanual regrasping for suture needles using reinforcement learning for rapid motion planning. arXiv:2011.04813
Dehghani H, Sun Y, Cubrich L, Oleynikov D, Farritor S, Terry B (2021) An optimization-based algorithm for trajectory planning of an under-actuated robotic arm to perform autonomous suturing. IEEE Trans Biomed Eng 68(4):1262–1272. https://doi.org/10.1109/TBME.2020.3024632
Wu B, Wang L, Liu X, Wang L, Xu K (2021) Closed-loop pose control and automated suturing of continuum surgical manipulators with customized wrist markers under stereo vision. IEEE Robot Autom Lett 6(4):7137–7144. https://doi.org/10.1109/LRA.2021.3097260
Iyer S, Looi T, Drake J (2013) A single arm, single camera system for automated suturing. In: 2013 IEEE international conference on robotics and automation, pp 239–244. https://doi.org/10.1109/ICRA.2013.6630582
Leonard S, Wu KL, Kim Y, Krieger A, Kim PCW (2014) Smart tissue anastomosis robot (Star): a vision-guided robotics system for laparoscopic suturing. IEEE Trans Biomed Eng 61(4):1305–1317. https://doi.org/10.1109/TBME.2014.2302385
Saeidi H, Le HND, Opfermann JD, Leonard S, Kim A, Hsieh MH, Kang JU, Krieger A (2019) Autonomous laparoscopic robotic suturing with a novel actuated suturing tool and 3d endoscope. In: 2019 international conference on robotics and automation (ICRA), pp 1541–1547. https://doi.org/10.1109/ICRA.2019.8794306
Kam M, Saeidi H, Hsieh MH, Kang JU, Krieger A (2021) A confidence-based supervised-autonomous control strategy for robotic vaginal cuff closure. In: 2021 IEEE international conference on robotics and automation (ICRA), pp 12261–12267. https://doi.org/10.1109/ICRA48506.2021.9561685
Mosafer Khoorjestan S, Rouhi G (2019) An automatic suturing machine for intestinal anastomosis: advantages compared with hand-suturing technique. Surg Innov 26(2):209–218. https://doi.org/10.1177/1553350618808007
Chow D-L, Newman W (2015) Trajectory optimization of robotic suturing. In: 2015 IEEE international conference on technologies for practical robot applications (TePRA), pp 1–6. https://doi.org/10.1109/TePRA.2015.7219672
Sen S, Garg A, Gealy DV, McKinley S, Jen Y, Goldberg K (2016) Automating multi-throw multilateral surgical suturing with a mechanical needle guide and sequential convex optimization. In: 2016 IEEE international conference on robotics and automation (ICRA), pp 4178–4185. https://doi.org/10.1109/ICRA.2016.7487611
Matsunaga T, Tomizuka D, Yu K, Mizoguchi T, Ohnishi K (2016) Construction of motion reproduction system using haptic forceps robots for needle insertion. In: IECON 2016—42nd annual conference of the IEEE industrial electronics society, pp 722–727. https://doi.org/10.1109/IECON.2016.7793920
Pedram SA, Ferguson P, Ma J, Dutson E, Rosen J (2017) Autonomous suturing via surgical robot: an algorithm for optimal selection of needle diameter, shape, and path. In: 2017 IEEE international conference on robotics and automation (ICRA), pp 2391–2398. https://doi.org/10.1109/ICRA.2017.7989278
Lu B, Yu XB, Lai JW, Huang KC, Chan KCC, Chu HK (2020) A learning approach for suture thread detection with feature enhancement and segmentation for 3-d shape reconstruction. IEEE Trans Autom Sci Eng 17(2):858–870. https://doi.org/10.1109/TASE.2019.2950005
Schwaner KL, Iturrate I, Andersen JKH, Jensen PT, Savarimuthu TR (2021) Autonomous bi-manual surgical suturing based on skills learned from demonstration. In: 2021 IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 4017–4024. https://doi.org/10.1109/IROS51168.2021.9636432
Özgüner O, Shkurti T, Lu S, Newman W, Çavuşoğlu MC (2021) Visually guided needle driving and pull for autonomous suturing. In: 2021 IEEE 17th international conference on automation science and engineering (CASE), pp 242–248. https://doi.org/10.1109/CASE49439.2021.9551453
Amirshirzad N, Sunal B, Bebek O, Oztop E (2021) Learning medical suturing primitives for autonomous suturing. In: 2021 IEEE 17th international conference on automation science and engineering (CASE), pp 256–261. https://doi.org/10.1109/CASE49439.2021.9551415
Feng X, Zhang X, Shi X, Li L (2022) Research on intraoperative cornea-instrument-robot interaction for autonomous cornea suturing. In: 2022 12th international conference on CYBER technology in automation, control, and intelligent systems (CYBER), pp 253–258. https://doi.org/10.1109/CYBER55403.2022.9907641
Shin HG, Park I, Kim K, Kim HK, Chung WK (2021) Corneal suturing robot capable of producing sutures with desired shape for corneal transplantation surgery. IEEE Trans Robot 37(1):304–312. https://doi.org/10.1109/TRO.2020.3031885
Jackson RC, Yuan R, Chow D-L, Newman W, Çavuşoğlu MC (2015) Automatic initialization and dynamic tracking of surgical suture threads. In: 2015 IEEE international conference on robotics and automation (ICRA), pp 4710–4716. https://doi.org/10.1109/ICRA.2015.7139853
Hu Y, Gu Y, Yang J, Yang G-Z (2018) Multi-stage suture detection for robot assisted anastomosis based on deep learning. In: 2018 IEEE international conference on robotics and automation (ICRA), pp 4826–4833. https://doi.org/10.1109/ICRA.2018.8461131
Gu Y, Hu Y, Zhang L, Yang J, Yang G-Z (2018) Cross-scene suture thread parsing for robot assisted anastomosis based on joint feature learning. In: 2018 IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 769–776. https://doi.org/10.1109/IROS.2018.8593622
Gao S, Ji S, Feng M, Lu X, Tong W (2021) A study on autonomous suturing task assignment in robot-assisted minimally invasive surgery. Int J Med Robot Comput Assist Surg 17(1):1–10. https://doi.org/10.1002/rcs.2180
Jackson RC, Desai V, Castillo JP, Çavuşoğlu MC (2016) Needle-tissue interaction force state estimation for robotic surgical suturing. In: 2016 IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 3659–3664. https://doi.org/10.1109/IROS.2016.7759539
Dehghani H, Farritor S, Oleynikov D, Terry B (2018) Automation of suturing path generation for da Vinci-like surgical robotic systems. In: 2018 design of medical devices conference, V001T07A008. https://doi.org/10.1115/DMD2018-6871
Zhong F, Wang Y, Wang Z, Liu Y-H (2019) Dual-arm robotic needle insertion with active tissue deformation for autonomous suturing. IEEE Robot Autom Lett 4(3):2669–2676. https://doi.org/10.1109/LRA.2019.2913082
Tian Y, Draelos M, Tang G, Qian R, Kuo A, Izatt J, Hauser K (2020) Toward autonomous robotic micro-suturing using optical coherence tomography calibration and path planning. In: 2020 IEEE international conference on robotics and automation (ICRA), pp 5516–5522. https://doi.org/10.1109/ICRA40945.2020.9196834
Seo H, Badiei Khuzani M, Vasudevan V, Huang C, Ren H, Xiao R, Jia X, Xing L (2020) Machine learning techniques for biomedical image segmentation: an overview of technical aspects and introduction to state-of-art applications. Med Phys 47:e148–e167. https://doi.org/10.1002/mp.13649
Ghosh S, Das N, Das I, Maulik U (2019) Understanding deep learning techniques for image segmentation. ACM Comput Surv 52(4):1–35. https://doi.org/10.1145/3329784
Smith TO, Sexton D, Mann C, Donell S (2010) Sutures versus staples for skin closure in orthopaedic surgery: meta-analysis. BMJ 340:c1199. https://doi.org/10.1136/bmj.c1199
Clay FSH, Walsh CA, Walsh SR (2011) Staples vs subcuticular sutures for skin closure at cesarean delivery: a metaanalysis of randomized controlled trials. Am J Obstet Gynecol 204(5):378–383
Cochetti G et al (2020) Surgical wound closure by staples or sutures? Systematic review. Medicine 99(25):e20573
Ahmed K, Ibrahim A, Wang TT, Khan N, Challacombe B, Khan MS, Dasgupta P (2012) Assessing the cost effectiveness of robotics in urological surgery—a systematic review. BJU Int 110(10):1544–1556. https://doi.org/10.1111/j.1464-410X.2012.11015.x
Lotan Y (2012) Is robotic surgery cost-effective: no. Curr Opin Urol 22(1):66–69. https://doi.org/10.1097/MOU.0b013e32834d4d76
Acknowledgements
The authors would like to thank the University of California San Diego librarians who assisted with formulating a systematic search strategy for this review.
Funding
No funding was used to support this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Benjamin T. Ostrander, Daniel Massillon, Leo Meller, Zih-Yun Chiu, Michael Yip and Ryan K. Orosco have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ostrander, B.T., Massillon, D., Meller, L. et al. The current state of autonomous suturing: a systematic review. Surg Endosc 38, 2383–2397 (2024). https://doi.org/10.1007/s00464-024-10788-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-024-10788-w