Abstract
Background
To investigate factors associated with risk for rebleeding and 30-day mortality following prophylactic transarterial embolization in patients with high-risk peptic ulcer bleeding.
Methods
We retrospectively reviewed medical records and included all patients who had undergone prophylactic embolization of the gastroduodenal artery at Rigshospitalet, Denmark, following an endoscopy-verified and treated peptic Sulcer bleeding, from 2016 to 2021. Data were collected from electronic health records and imaging from the embolization procedures. Primary outcomes were rebleeding and 30-day mortality. We performed logistical regression analyses for both outcomes with possible risk factors. Risk factors included: active bleeding; visible hemoclips; Rockall-score; anatomical variants; standardized embolization procedure; and number of endoscopies prior to embolization.
Results
We included 176 patients. Rebleeding occurred in 25% following embolization and 30-day mortality was 15%. Not undergoing a standardized embolization procedure increased the odds of both rebleeding (odds ratio 3.029, 95% confidence interval (CI) 1.395–6.579) and 30-day overall mortality by 3.262 (1.252–8.497). More than one endoscopy was associated with increased odds of rebleeding (odds ratio 2.369, 95% CI 1.088–5.158). High Rockall-score increased the odds of 30-day mortality (odds ratio 2.587, 95% CI 1.243–5.386). Active bleeding, visible hemoclips, and anatomical variants did not affect risk of rebleeding or 30-day mortality. Reasons for deviation from standard embolization procedure were anatomical variations, targeted treatment without embolizing the gastroduodenal artery, and technical failure.
Conclusions
Deviation from the standard embolization procedure increased the risk of rebleeding and 30-day mortality, more than one endoscopy prior to embolization was associated with higher odds of rebleeding, and a high Rockall-score increased the risk of 30-day mortality. We suggest that patients with these risk factors are monitored closely following embolization. Early detection of rebleeding may allow for proper and early re-intervention.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute upper gastrointestinal bleeding caused by a peptic ulcer is a frequent and life-threatening condition [1]. The gold standard treatment is endoscopic intervention [2]. Rebleeding following treatment for a peptic ulcer bleeding increases mortality significantly [2]. Thus, it is of utmost importance to reduce the risk of rebleeding.
Despite achieving hemostasis endoscopically, 12–25% of patients experience rebleeding, depending on several factors (e.g. ulcer type, patient comorbidities, etc.) [3, 4]. Following successful endoscopic hemostasis, surgeons can consider prophylactic transarterial embolization to reduce the risk of rebleeding. Transarterial embolization decreases both the rate of rebleeding, the need for reintervention, and mortality [5]. Further, transarterial embolization has a high technical success rate, a low complication rate, and does not prolong in-hospital stay significantly [5].
In Denmark, patients do not undergo prophylactic embolization routinely. Thus, it is the surgeon performing the endoscopy, who assesses the need for prophylactic embolization. Among other factors, this evaluation is based on the Rockall score of the patient, and the Forrest classification of the ulcer (Table 1) [6, 7]. Two studies have investigated the effect of prophylactic transarterial embolization following peptic ulcer bleeding in a randomized setup [8, 9]. However, both studies performed the prophylactic arterial embolization after just one endoscopy. In our population, patients were evaluated by surgeons based on the Rockall-score and might have undergone more than one endoscopy [10].
The aim of this study was to retrospectively analyze patients who have undergone prophylactic transarterial embolization at Rigshospitalet, Denmark, following a peptic ulcer bleeding. We aimed to evaluate the risk of rebleeding and 30-day mortality following embolization, and identify risk factors for rebleeding and mortality.
Materials and methods
The study was a retrospective, single-center cohort study. The study was approved by the local ethics committee of the Capital Region of Denmark (R-22007493). The study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies [11].
The study was conducted at Rigshospitalet, Copenhagen, Denmark. Rigshospitalet is a referral center for Zealand, with a population of approximately 2.6 million inhabitants. Patients with peptic ulcer bleeding are typically treated endoscopically at the local and regional hospitals, and referred to Rigshospitalet for embolization. Data collection was performed between September 21 and December 28, 2022. We included all patients who had undergone attempted prophylactic transarterial embolization at Rigshospitalet between January 1, 2016, and December 31, 2021. Patients were followed up until December 28, 2022. For patients to be included into the study, they had to have undergone attempted transarterial embolization following a peptic ulcer bleeding, within the same hospital admission. Patients were excluded if they had incomplete medical records. Patients were identified through a local registry at the Department of Radiology, Rigshospitalet. Data were collected through electronic health records as well as radiological imaging software. Follow-up was also performed through the electronic health records.
The following variables were extracted from the patient history in the electronic health record: age, sex, comorbidities, smoking and alcohol history, plasma hemoglobin prior to upper endoscopy, weight, height, use of non-steroidal anti-inflammatory drugs and anti-coagulants, blood pressure and pulse prior to upper endoscopy (pulse < 100, systolic blood pressure > 100; pulse > 100, systolic blood pressure > 100; systolic blood pressure < 100), American Society of Anaesthesiologists score [12], time of last upper endoscopy prior to embolization, placement of ulcer, size of ulcer, endoscopic method of bleeding control, if bleed control was achieved (yes/no), Forrest classification [7], diagnosis as per the Rockall-score [6], Helicobacter pylori status, number of upper endoscopies prior to embolization, rebleed after embolization, date of rebleed, need for reintervention, type of reintervention, death at time of follow-up, date of death, cause of death, discharge date from hospital, readmission related to bleeding ulcer, and date of readmission. The following variables were extracted from the digital subtraction angiography (DSA) by a trained interventional radiologist: time of embolization, active bleeding on digital subtraction angiography, visible hemoclips during digital subtraction angiography, type of embolization material, standard embolization (yes/no), any anatomic variants on digital subtraction angiography, visualization of cystic artery (yes/no), embolization of cystic artery, non-target embolization (yes/no). We defined a standard embolization as coiling of the gastroduodenal artery and side branches, as well as any actively bleeding sites (Fig. 1), as this is the standard embolization technique employed at our institution. Consequently, non-standard embolization includes all embolizations not following the above, e.g., attempted embolizations with technical failure, anatomical variants making a standard approach impossible, different embolization materials. All data were entered into a Research Electronic Data Capture (REDCap) database.
Overview of arteries involved in prophylactic transarterial embolization with embolization of the gastroduodenal artery and side branches. AA abdominal aorta, CeT celiac trunk, LGA left gastric artery, SA splenic artery, CHA common hepatic artery, RGA right gastric artery, PHA proper hepatic artery, LHA left hepatic artery, RHA right hepatic artery, CA cystic artery, GDA gastroduodenal artery, SPDA superior pancreaticoduodenal artery, RGEA right gastroepiploic artery, SMA superior mesenteric artery, IPDA inferior pancreacitoduodenal artery
Normally distributed data were reported as mean (SD) and non-normally distributed data were reported as median (range). The Rockall-score was grouped (≤ 3, 4–5, ≥ 6) based on the Danish Society of Gastroenterology and Hepatology guidelines [10]. Both primary outcomes (rebleeding and 30-day overall mortality) were dichotomous outcomes. To investigate the association between rebleeding and 30-day overall mortality, we performed a χ2-test. If there was an association, we then calculated the odds ratio to quantify the association. Finally, we performed a binomial logistical regression, evaluating the effect of several variables on the likelihood of rebleeding and 30-day mortality. The variables included in the model were: active bleeding during transarterial angiography, visible hemoclips following endoscopy during transarterial angiography, Rockall score (grouped), normal anatomy, standard embolization procedure, and number of endoscopies performed prior to embolization (grouped into either one endoscopy or more than one endoscopies). Results with p < 0.05 were considered statistically significant.
Results
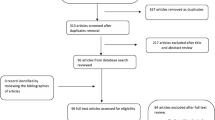
We identified a total of 302 transarterial embolizations performed at Rigshospitalet from 2016 to 2021. Of these, 277 were unique patients. We excluded 101 patients for the following reasons: 12 were missing medical records, 85 had other diagnoses than peptic ulcer bleeding, and 4 had not had an upper endoscopy prior to transarterial embolization. In total, 176 patients were included in the study, see Fig. 2. All embolizations were performed by one of seven experienced interventional radiologists at Rigshospitalet. For descriptive and outcome data, see Table 2.
Of the 26 patients who died within 30 days of embolization, 15 (58%) had experienced a rebleeding following embolization. The χ2-test of independence showed an increased likelihood of 30-day mortality in patients who had suffered a rebleeding following transarterial embolization (p < 0.001), and the odds ratio of 30-day mortality in patients who had suffered a rebleeding following transarterial embolization was 5.6897 (95% confidence interval 2.3665–13.6795).
None of the included patients had significant complications directly related to the transarterial embolization, i.e., none suffered from ischemia or perforation, and no complications arose in the patients who had non-target embolization or coiling of the cystic artery.
The rebleeding binomial logistic regression model was statistically significant, χ2 (6) = 19.207, p = 0.004. The model explained 15.3% (Nagelkerke R2) of the variance in rebleeding and correctly classified 76.7% of cases. Sensitivity was 18.2%, specificity was 96.2%, positive predictive value was 61.5% and negative predictive value was 77.9%. Of the six predictor variables (active bleeding, visible hemoclips, Rockall score, normal anatomy, standard embolization procedure, and number of endoscopies prior to embolization) two were statistically significant, standard embolization procedure and number of endoscopies prior to embolization, see Table 3. Patients who did not undergo a standard embolization procedure had 3.298 (1.484–7.329) higher odds (95% confidence interval) of rebleeding compared with patients who underwent a standard embolization procedure. Patients who had more than one endoscopy prior to embolization had 2.369 (1.088–5.158) higher odds (95% confidence interval) of rebleeding compared with patients who only had one endoscopy.
The 30-day overall mortality binomial logistic regression model was not statistically significant, χ2 (6) = 12.082, p = 0.06. However, the Hosmer and Lemeshow goodness of fit test was not significant (p = 0.379), indicating that the model is not a poor fit, for predicting categorical outcomes. The model explained 11.7% (Nagelkerke R2) of the variance in 30-day overall mortality and correctly classified 85.8% of cases. Sensitivity was 3.8%, specificity was 100%, positive predictive value was 100% and negative predictive value was 85.7%. Of the six predictor variables (active bleeding, visible hemoclips, Rockall score, normal anatomy, standard embolization procedure, and number of endoscopies prior to embolization) two were statistically significant, Rockall score and standard embolization procedure, see Table 4. Patients who underwent a non-standard embolization procedure had 3.340 (1.273–8.765) higher odds (95% confidence interval) of 30-day mortality compared with patients who underwent a standard embolization procedure. Further, a higher Rockall score was associated with an increased likelihood of 30-day mortality.
Finally, we performed a qualitative analysis of embolizations defined as non-standard. In Table 5, reasons for them being defined as non-standard are listed, and the procedures were divided according to if the patient experienced rebleeding following the non-standard embolization or not.
Discussion
This retrospective cohort study included 178 patients who underwent prophylactic transarterial embolization following endoscopic treatment for peptic ulcer bleeding. Following transarterial embolization, 25% of patients suffered from rebleeding and overall 30-day mortality was 14%. The risk of 30-day mortality was significantly increased in patients who suffered from rebleeding. Not undergoing a standard treatment, embolization of the gastroduodenal artery and side branches as well as actively bleeding sites (if any), increased the odds of rebleeding by 3.298, and the odds of dying within 30 days of the procedure by 3.340. Having undergone more than one endoscopy prior to embolization increased the odds of rebleeding by 2.369, and a high Rockall-score at the time of the last endoscopy prior to transarterial embolization was associated with a high likelihood of overall 30-day mortality.
A strength of this study is the near complete follow-up of all patients due to electronic health records and the unique Danish patient identification number system. Furthermore, the authors had access to both surgical reports and digital subtraction angiographies, so procedures could be reevaluated. This study was limited by the nature of a retrospective design, by having no control group, and possible selection bias in the patients who were lost to follow-up due to incomplete medical records.
To our knowledge, there is no definitive consensus on which vessels to embolize, and we found no firm guidelines dictating if the gastroduodenal artery always should be embolized, even when a visible actively bleeding vessel is identified during the digital subtraction angiography [5, 13, 14].
Compared with previous studies, we had a high rate of rebleeding, similar mortality rate, and similar adverse event rate [5, 8, 9, 14, 15]. Most patients in our study had undergone more than one endoscopy prior to embolization, which was not the case in most comparable studies. The Rockall scores of our patients were comparable with another retrospective study [15], but our rate of rebleeding was higher. Compared with the randomized clinical trial, also performed in Denmark, most patients in the current study had undergone more than one endoscopy, their clinical condition was worse, and they had more comorbidities [8]. Overall, we attribute the higher rate of rebleeding in our cohort to the worse overall clinical condition of our patients, possibly due to delay of embolization as patients had undergone more endoscopies prior to embolization. As in other studies, we found no adverse events specifically related to the transarterial embolization procedure in our study.
In general, characeristics of patients, who undergo prophylactic transarterial embolization following peptic ulcer bleeding, might vary extensively between different countries. This to a high extent depends on the accessibility of endovascular treatment. Naturally, this limits the external validity of our study.
In conclusion, our study in patients with high-risk peptic ulcer showed a high risk of rebleeding (25%) and mortality (15%) following prophylactic transarterial embolization, with higher likelihood of mortality in patients with rebleeding. We found that patients who had undergone a non-standard prophylactic transarterial embolization had a higher risk of rebleeding and 30-day mortality, patients who had undergone more than one endoscopy prior to embolization had higher odds of rebleeding, and patients with a high Rockall-score had a higher risk of 30-day mortality. However, comparison between our study population and the populations of previous trials was difficult, warranting further research to identify patients who benefit from prophylactic transarterial embolization, and to identify patients at high risk of rebleeding and mortality.
References
Hreinsson JP, Kalaitzakis E, Gudmundsson S, Björnsson ES (2013) Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol 48:439–447. https://doi.org/10.3109/00365521.2012.763174
Kaminskis A, Kratovska A, Ponomarjova S, Tolstova A, Mukans M, Stabiņa S, Gailums R, Bernšteins A, Ivanova P, Boka V, Pupelis G (2017) Preventive transarterial embolization in upper nonvariceal gastrointestinal bleeding. World J Emerg Surg 12:3. https://doi.org/10.1186/s13017-016-0114-1
Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L (2008) Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol 103:2625–2632. https://doi.org/10.1111/j.1572-0241.2008.02070.x
Wong SK, Yu LM, Lau JY, Lam YH, Chan AC, Ng EK, Sung JJ, Chung SC (2002) Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut 50:322–325. https://doi.org/10.1136/gut.50.3.322
Chang JHE, Lye TJY, Zhu HZ, Syn NL, Tang SS, Gogna A, Chan WH, Ong HS, Tan JTH, Lim CH (2021) Systematic review and meta-analysis of prophylactic transarterial embolization for high-risk bleeding peptic ulcer disease. J Vasc Interv Radiol 32:576-584.e5. https://doi.org/10.1016/j.jvir.2020.12.005
Rockall TA, Logan RF, Devlin HB, Northfield TC (1996) Risk assessment after acute upper gastrointestinal haemorrhage. Gut 38:316–321. https://doi.org/10.1136/gut.38.3.316
Forrest JA, Finlayson ND, Shearman DJ (1994) Endoscopy in gastrointestinal bleeding. Lancet 2:394–397. https://doi.org/10.1016/s0140-6736(74)91770-x
Laursen SB, Hansen JM, Andersen PE, Schaffalitzky de Muckadell OB (2014) Supplementary arteriel embolization an option in high-risk ulcer bleeding–a randomized study. Scand J Gastroenterol 49:75–83. https://doi.org/10.3109/00365521.2013.854829
Lau JYW, Pittayanon R, Wong KT, Pinjaroen N, Chiu PWY, Rerknimitr R, Holster IL, Kuipers EJ, Wu KC, Au KWL, Chan FKL, Sung JJY (2019) Prophylactic angiographic embolisation after endoscopic control of bleeding to high-risk peptic ulcers: a randomised controlled trial. Gut 68:796–803. https://doi.org/10.1136/gutjnl-2018-316074
Danish Society for Gastroenterology and Hepatology (2018) Behandling af blødende gastroduodenale ulcera. https://dsgh.dk/wp-content/uploads/2022/06/bloedendeulcera.pdf
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577. https://doi.org/10.7326/0003-4819-147-8-200710160-00010
Dripps RD, Lamont A, Eckenhoff JE (1961) The role of anesthesia in surgical mortality. JAMA 178:261–266. https://doi.org/10.1001/jama.1961.03040420001001
Kuyumcu G, Latich I, Hardman RL, Fine GC, Oklu R, Quencer KB (2018) Gastrodoudenal embolization: indications, technical pearls, and outcomes. J Clin Med 7:101. https://doi.org/10.3390/jcm7050101
Ini’ C, Distefano G, Sanfilippo F, Castiglione DG, Falsaperla D, Giurazza F, Mosconi C, Tiralongo F, Foti PV, Palmucci S, Venturini M, Basile A (2023) Embolization for acute nonvariceal bleeding of upper and lower gastrointestinal tract: a systematic review. CVIR Endovasc 6:18. https://doi.org/10.1186/s42155-023-00360-3
Mille M, Huber J, Wlasak R, Engelhardt T, Hillner Y, Kriechling H, Aschenbach R, Ende K, Scharf JG, Puls R, Stier A (2015) Prophylactic transcatheter arterial embolization after successful endoscopic hemostasis in the management of bleeding duodenal ulcer. J Clin Gastroenterol 49:738–745. https://doi.org/10.1097/MCG.0000000000000259
Funding
Open access funding provided by Copenhagen University. This study was investigator initiated and received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Dennis Zetner, Ida Roost Rasmussen, Camilla Palmquist Frykman, Lasse Rehné Jensen, Ruben Juul Jensen, Emma Possfelt-Møller, Mikkel Taudorf and Luit Penninga have no conflicts of interest or financial ties to disclose. No funding was received for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zetner, D., Rasmussen, I.R., Frykman, C.P. et al. Risk factors for rebleeding and mortality following prophylactic transarterial embolization for patients with high-risk peptic ulcer bleeding: a single-center retrospective cohort study. Surg Endosc 38, 2010–2018 (2024). https://doi.org/10.1007/s00464-024-10709-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-024-10709-x