Abstract
Objective
Investigate the effect of passive, active or no intra-operative work breaks on static, median and peak muscular activity, muscular fatigue, upper body postures, heart rate, and heart rate variability.
Background
Although laparoscopic surgery is preferred over open surgery for the benefit of the patient, it puts the surgeons at higher risk for developing musculoskeletal disorders especially due to the less dynamic and awkward working posture. The organizational intervention intraoperative work break is a workplace strategy that has previously demonstrated positive effects in small-scale intervention studies.
Methods
Twenty-one surgeons were exposed to three 90-min conditions: no breaks, 2.5-min passive (standing rest) or active (targeted stretching and mobilization exercises) breaks after 30-min work blocks. Muscular activity and fatigue of back, shoulder and forearm muscles were assessed by surface electromyography; upper body posture, i.e., spinal curvature, by inclination sensors; and heart rate and variability (HRV) by electrocardiography. Generalized estimating equations were used for statistical analyses. This study (NCT03715816) was conducted from March 2019 to October 2020.
Results
The HRV-metric SDNN tended to be higher, but not statistically significantly, in the intervention conditions compared to the control condition. No statistically significant effects of both interventions were detected for muscular activity, joint angles or heart rate.
Conclusion
Intraoperative work breaks, whether passive or active, may counteract shoulder muscular fatigue and increase heart rate variability. This tendency may play a role in a reduced risk for developing work-related musculoskeletal disorders and acute physical stress responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Work-related musculoskeletal disorders (WRMSD) are well recognized among the general working population. Also, among all surgeons, the prevalence of WRMSD is reported to be 19% [1]. In different studies, about 55% [2], 74% [3], 87% [4], or up to 88% [5] of the laparoscopic surgeons reported experiencing musculoskeletal symptoms or discomfort due to their work. Because of patient benefits laparoscopy is generally preferred over open surgery (e.g., lower infection rates, shorter recovery times) [6,7,8], particularly as a result of this also during the COVID-19-Pandemic [9]. However, laparoscopic procedures also carry risks for the surgeon in developing WRMSD [10], particularly due to table height, monitor position, and poor handling of instruments [11]. These risks embrace less dynamic working postures [12, 13], awkward body postures [13, 14], and higher activation levels of several upper extremity muscles [15].
Occupational ergonomics may counteract these risk factors [16]; however, applying such ergonomics in the operating theater is challenging, because of the safety of the patient that should not be endangered. Nevertheless, several studies have implemented and evaluated different interventions, including workplace interventions [17, 18] and work-organizational interventions [19,20,21,22]. Both types of interventions have been evaluated in both lab and field studies and provide promising results, namely decreased discomfort in back and neck [20, 21] and shoulders [19, 21, 22], decreased lower leg muscle loading [23], decreased stress-related cortisol in saliva [22], improved wrist posture [17, 18], and showed no significant influence on surgery duration [19, 22].
The work-organizational intervention of intraoperative work breaks is of particular interest, because it does not only target the laparoscopic surgeon, but all other medical staff as part of the surgical team as well [24]. The benefit of including both a principal and assisting surgeon in laparoscopic surgeries to encounter long periods of static posture was also concluded by Al-Hakim, Xiao [25]: “Having experienced assistant surgeons to accompany surgeons in long procedures help surgeons to take sometimes to exercise, leaving the assistant surgeon to perform surgical operations”. Various types and durations of intraoperative work breaks in laparoscopic surgery have been evaluated. For type, both passive [22] and active breaks [19,20,21, 26,27,28] have been studied compared to no breaks, but never all together in one study. Passive breaks are breaks within which workers rest; active breaks are breaks within which workers perform a non-work-related physical or cognitive activity [29]. For duration, implementation of 20-s [21], 1.5-min [19, 20], and 5-min breaks [22] every 20 to 40 min work have been investigated.
The objective of the present study is to examine whether muscular fatigue, static, median and peak muscular activity, upper body postures, heart rate, and heart rate variability are changed when comparing passive and active with no work breaks and active with passive work breaks. These are secondary outcomes of the study; the primary outcome subjective discomfort together with the remaining secondary outcomes [30] will be reported in another manuscript.
Materials and methods
Study design
This study is registered at ClinicalTrials.gov (No. NCT03715816) [31], received ethical approval by the local ethical committee of the Medical Faculty of the University and University Hospital Tübingen (no. 618/2018BO2), and followed the ethical standards of the Declaration of Helsinki [32]. This laboratory study is a controlled, randomized cross-over trial that was conducted from March 2019 to October 2020. The study included a control condition (without breaks) and two intervention conditions (passive and active breaks) and was not blinded. The order of the three conditions was assigned to the participants by drawing lots.
Study sample
We used the equation of Kadam and Bhalerao [33] to calculate the required sample size. Maintaining a 5% level of significance and 80% study power and using the converted results of the primary outcome perceived discomfort from Dorion and Darveau [21] (i.e., 1.6857 effect size and 1.9337 pooled SD), a sample size of 21 was determined, excluding potential drop-outs.
The subjects, i.e., laparoscopic surgeons, were recruited by direct advertisement and internal email announcements at the Department of Women’s Health at the University Hospital Tübingen. Eligibility criteria were: minimum age 18, proficiency in German, experience with (simulated) laparoscopy, and ability to perform the PEG-transfer task within 3 min [34]. We recruited 25 surgeons; after drop-out of four surgeons (one: too little time; two: acute injury on back and clavicula; one: employment contract ended), the final study sample consisted of 21 surgeons, whose demographics and musculoskeletal status, collected during the first laboratory visit (see Experimental Procedure for details), are summarized in Table 1.
Intervention
The simulation of laparoscopic work lasted 90 min, which approximates the average duration of laparoscopic surgeries of 75 min [35]. In a highly controlled environment, 2.5-min breaks were provided after 30- and 60-min work blocks. This duration, frequency, and timing are based on the positive findings reported by previous studies that investigated intraoperative breaks [19, 20, 22]. Two experimental conditions were performed including passive or active breaks, and one control condition was performed not including breaks. In line with its definition [29], participants were instructed to just rest while standing during the passive breaks. In this study, the participant was allowed to lay down the laparoscopic instruments. The active work breaks contained a standardized audio recording (transcribed protocol, see Supplemental Digital Content 1) with instructions to perform a set of exercises focusing on posture correction, normalization of tissue tension, mobilization of soft tissue, and relaxation [26]. The exercise protocol was designed by the authors (TL, BS) in collaboration with a physical therapist, and aimed to target body regions that are particularly affected by discomfort or complaints, i.e., the neck, shoulders, and lower back [25, 36, 37]. We did not control for the number of repetitions and range of motion of each exercise.
Experimental procedure
Prior to the experiment, the participant visited the lab for a familiarization to the experiment. During this first visit, the participant was explained about the study and its goals, filled out all necessary forms for participation and informed consent, practiced all tasks as simulated during the three conditions, and was asked about demographics (see Table 1) and musculoskeletal status according to the German version [38] of the standardized Nordic Musculoskeletal Questionnaire [39]. The experimental set-up was individually adjusted [40]: the table height provided a ~ 105° elbow angle while holding the instrument in a ~ 60° downward angle in the simulation device relative to the lower arm; the monitor height provided a ~ 10° downward view to avoid neck extension [41]. After adjustment, individual floor-table and floor-monitor heights were note, which equaled 71.5 cm (SD 4.9) and 105.7 cm (SD 6.9), respectively. The familiarization trial and the three experimental and control conditions were performed on separate days.
The 90-min simulation consisted of several tasks performed in a Pelvic Trainer (Szabo, ID Trust Medical, Belgium). Each task required optics (Karl Storz 26003AA HOPKINS II Optik 0°, Karl Storz SE & Co. KG, Tuttlingen, Germany) and the bimanual handling of one or two out of three tools: 34-cm long laparoscopic Maryland bipolar forceps (Model No. 20 195-225, ERBE Elektromedizin GmbH, Tübingen, Germany); 33-cm long forceps with 1:2 teeth (RS225-595, RUDOLF Medical GmbH & Co. KG, Fridingen, Germany); 36-cm long forceps without teeth (33,321 KW, KARL STORZ SE & Co. KG, Tuttlingen, Germany). The laboratory’s illuminance and room temperature were regulated to be 5–9 lx and 24–26 °C, respectively.
The 90-min simulation included three 30-min blocks, of which each block contained five tasks in a set order: peg-transfer, pick-and-place, pick-and-tighten, pick-and-thread, pull-and-stick. For a visualization of these task, see Fig. 1. Directly before and after the simulation, a hot-wire task was performed. A schematic overview of the tasks including breaks is provided in Fig. 2. During the pick-and-place and pick-and-thread tasks, a foot pedal was integrated with which a box was opened in order to pick the parts (i.e., beads). A comprehensive explanation of all six simulated tasks including the used instruments is provided in the study protocol [30].
The time course of an experimental condition; in the control condition, the blue blocks (breaks) will vanish. Each block and letter is related to a task, including the hot-wire (A), peg-transfer (B), pick-and-place (C), pick-and-tighten (D), pick-and-thread (E) and pull-and-stick task (F). The arrows indicate the time points of recordings of muscle activity, upper body posture, and heart rate (B1-9) during the peg-transfer task (B)
Data collection and data analysis
Data collection and analysis of the primary outcome perceived rating of discomfort and the secondary outcomes performance, workload, and subjective evaluation will be reported in another manuscript.
Muscular activity and localized muscular fatigue
Electrical activity of seven muscles was recorded by surface electromyography (EMG) by placing two pre-gelled Ag/AgCl electrodes (42 × 24, Kendall™ H93SG ECG Electrodes, Covidien, Zaltbommel, Netherlands) in bipolar configuration (IED 25 mm) over the muscle belly [42, 43]. The ground electrode was placed over vertebra C7. The following muscles were recorded: erector spinae longissimus lumbalis (ES at vertebra L3, bilateral), trapezius descendens (TD, bilateral), deltoideus acromialis (DA, right), extensor digitorum (ED, right), flexor carpi radialis (FCR, right). EMG signals were collected using a data analyzer with data logger (PS11-UD, THUMEDI® GmbH & Co. KG, Thum, Germany; CMMR > 96 dB; overall effective sum of noise < 0.8 μV RMS; linearity ± 0.15 dB at 25–1100 Hz). EMG signals were differential amplified, analog filtered (high-pass filter, 4th order, − 3 dB at 4 Hz; low-pass filter, 11th order, − 3 dB at 1300 Hz), and sampled (4096 Hz). Synchronous to data storage, EMG signals were real-time transformed into the frequency domain (1024-point Fast Fourier Transformation, Bartlett-window, 50% overlap), digitally high-pass filtered (11th order, − 3 dB at 16 Hz), and digitally average-filtered to remove powerline interferences (11th order, 50 Hz and first seven harmonics) by replacing it by the spectral values of a 4-Hz wide band around its center frequency by means of both spectral neighbors. Root-mean-square (RMS [μV]) and median power frequency (MPF [Hz]) were real-time calculated from the power spectrum and stored synchronously to the raw data by the PS11.
EMG was recorded continuously during 5-s maximal voluntary contractions (MVCs; see Supplemental Digital Content 2 for details about the MVCs) [44] and simulated laparoscopy. Two MVCs per muscle were performed with 1-min break in between prior to each condition. The maximal RMS of both MVCs per muscle was used to normalize the RMS of the experiment and expressed as percentage (%MVE). The 10th (static), 50th (median), and 90th percentiles (peak) of the RMS during B1, B3, B4, B6, B7, and B9 (cf. Figure 2) were calculated [45]. For localized muscular fatigue, we calculated the slope expressed as change per minute of the median RMS and MPF and plotted them against each other in joint analyses of the EMG spectrum and amplitude (JASA) [46]. In JASA plots, the lower right quadrant indicates muscular fatigue, reflected by an increased RMS and a decreased MPF.
Upper body postures
Six two-dimensional gravimetric inclination sensors (PS11; sample rate 8 Hz; resolution 0.1° and 125 ms in time; maximum static error 0.5°) were placed on the forehead, vertebrae T1, T10, L1, and L5, measuring flexion and lateral flexion angles with respect to the absolute perpendicular. The difference in flexion angles was used to calculate cervical lordosis or neck flexion (forehead–T1), thoracic kyphosis (T1–T10), and lumbar lordosis (L1–L5). The difference in lateral flexion angles was used to calculate neck lateral flexion (forehead–T1). The sensor on T10 was used to determine trunk flexion. The average angles during B1, B3, B4, B6, B7, and B9 (cf. Figure 2) were calculated.
Heart rate and variability
The electrical activity of the heart was recorded using electrocardiography (ECG) by two pre-gelled Ag/AgCl electrodes placed ~ 5 cm cranial and ~ 3 cm left-lateral from the distal end of the sternum and over the anterior to mid-axillary line at the fifth left rib. ECG signals were continuously recorded (sample rate 1000 Hz) and processed in real-time to calculate heart rate (HR [bpm]) and interbeat intervals (IBI [ms]). The IBI timeseries were checked for artifacts and erroneous intervals were excluded and replaced by polynomial interpolation (2nd order) using MATLAB R2020a (The MathWorks Inc., Natwick, MA, USA). The corrected IBI timeseries were processed with Kubios HRV (Standard V3.3.1, Biosignal Analysis and Medical Imaging Group, Department of Applied Physics, University of Eastern Finland, Kuopio, Finland) to calculate the following heart rate variability parameters in the time domain [47]: SD of IBIs (SDNN [ms]) and root mean squared successive differences between IBIs (RMSSD [ms]). The mean HR, IBI, SDNN, and RMSSD were calculated during B1, B3, B4, B6, B7, and B9 (one-minute duration each) (cf. Figure 2).
Statistical analysis
We checked normal distributions of all parameters by Shapiro–Wilk tests [48] and visually inspected histograms, skewness, and kurtosis. The fatigue parameters RMSSLOPE and MPFSLOPE were normally distributed, all other parameters showed a light to strong positive skew. Descriptive and muscular fatigue data are presented as means with standard deviations and muscular activity, upper body postures and heart rate and variability are presented as medians with interquartile ranges and as boxplots. Statistical analyses were performed using IBM SPSS Statistics for Windows (V28.0.0.0, IBM Corp., Armonk, NY, USA) and statistical significance was accepted when p < 0.05.
We performed generalized estimating equations (GEE) with exchangeable correlation matrices and linear scale responses to test the within-subject effects of condition (three levels: no, passive, active breaks) on RMSSLOPE and MPFSLOPE. We performed generalized estimating equations (GEE) with exchangeable correlation matrices and inverse Gaussian scale responses to test the within-subject effects of condition (three levels: no, passive, active breaks) and time (six levels: B1, B3, B4, B6, B7, B9) on the following parameters: RMSSTATIC, RMSMEDIAN, RMSPEAK, angleMEDIAN, HR, IBI, SDNN, RMSSD. In case of significant main or interaction effects, we performed Šidák for post hoc pairwise comparisons.
We calculated effect sizes for main or interaction effects using Cohen’s index w and for pairwise comparisons using Cohen’s d (average SD of both comparators as standardizer) [49] and interpreted them as small (w ≥ 0.1; d ≥ 0.2), medium (w ≥ 0.3; d ≥ 0.5), or large (w ≥ 0.5; d ≥ 0.8) [50].
Results
Results for muscular fatigue are presented in Table 2, for muscular activity in Table 3, and for posture, heart rate, and heart rate variability in Table 4. Medians and interquartile ranges for muscular activity, posture, heart rate, and heart rate variability are provided as Supplemental Digital Content 3.
Muscular fatigue
There were a few individual tendencies pointing to localized muscular fatigue (Fig. 3). In particular, the RMS of the TDR showed to statistically significant more signs of muscular fatigue (p < 0.05; d = − 0.598) in the condition without breaks (slope 0.023) compared to active breaks (0.001). However, this significantly increased RMS was not accompanied by a significantly decreased MPF of the TDR. Overall, among most subjects and conditions, slopes of the RMS and MPF were spread throughout the JASA plots. This means that neither the control nor the intervention conditions led to clear signs of localized muscular fatigue, with an exception of the right shoulder muscle that tended to show fewer signs of muscular fatigue in the active break condition compared to the control.
Muscular activity
None of the muscular activity levels showed a statistically significant effect of Condition.
Statistically significant main effects of Time were found for RMSSTATIC (p < 0.001; w = 0.253), RMSMEDIAN (p < 0.01; w = 0.218), and RMSPEAK (p < 0.05; w = 0.176) of ESR. Both RMSSTATIC (MD = − 0.449%MVE; p = 0.045; d = − 0.199) and RMSMEDIAN (MD = − 0.493%MVE; p = 0.033; d = − 0.137) decreased from B3 to B7. RMSPEAK of the ESR showed a statistically significant interaction effect of Condition × Time (p < 0.05; w = 0.231) without significant post hoc pairwise comparisons.
Statistically significant main effects of Time were found for RMSSTATIC (p < 0.001; w = 0.257), RMSMEDIAN (p < 0.01; w = 0.237), and RMSPEAK (p < 0.05; w = 0.204) of ESL. For all three levels, muscular activity decreased on average from B1 to B7 with − 0.777 (p < 0.001; d = − 0.267), − 0.753 (p = 0.001; d = − 0.225), and − 0.694%MVE (p = 0.009; d = − 0.178), respectively.
Statistically significant main effects of Time were found for RMSSTATIC (p < 0.01; w = 0.216), RMSMEDIAN (p < 0.01; w = 0.232) and RMSPEAK (p < 0.001; w = 0.260) of TDR. RMSSTATIC (Fig. 4) increased from B1 to B6 (MD = 1.178%MVE; p = 0.037; d = 0.356) and B9 (MD = 1.288%MVE; p = 0.009; d = 0.391); RMSMEDIAN increased from B1 (MD = 1.526%MVE; p = 0.008; d = 0.349) and B7 (MD = 0.667%MVE; p = 0.006; d = 0.138) to B9; RMSPEAK increased from B1 to B4 (MD = 1.445%MVE; p = 0.043; d = 0.249). RMSSTATIC showed a statistically significant interaction effect of Condition × Time (p < 0.05; w = 0.232), where activity at B9 was higher than at B1 (MD = 2.025%MVE; p = 0.016; d = 0.617), B4 (MD = 1.516%MVE; p = 0.003; d = 0.414), and B7 (MD = 1.095%MVE; p = 0.004; d = 0.281) in the condition without breaks.
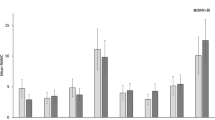
Boxplots displaying minimum, 1st quantile, median, 3rd quantile and maximum of the RMSSTATIC of the right trapezius descendens (TDR, left upper corner), RMSPEAK of the left trapezius descendens (TDL, right upper corner), RMSMEDIAN of the extensor digitorum (ED, left lower corner), and SDNN (right lower corner). The three conditions are displayed with black (without breaks), dark gray (passive breaks), or light gray filling (active breaks)
Statistically significant main effects of Time were found for RMSSTATIC (p < 0.001; w = 0.298), RMSMEDIAN (p < 0.001; w = 0.269), and RMSPEAK (p < 0.001; w = 0.296) of TDL. RMSSTATIC increased from B1 to B4 (MD = 0.875%MVE; p = 0.011; d = 0.266), B6 (MD = 0.824%MVE; p = 0.014; d = 0.271) and B9 (MD = 1.240%MVE; p < 0.001; d = 0.355) and from B3 to B9 (MD = 0.901%MVE; p = 0.002; d = 0.256); RMSMEDIAN increased from B1 (MD = 1.435%MVE; p = 0.001; d = 0.300) and B3 (MD = 1.064%MVE; p < 0.001; d = 0.220) to B9; RMSPEAK (Fig. 4) increased from B1 (MD = 1.662%MVE; p = 0.017; d = 0.264) and B3 (MD = 1.227%MVE; p < 0.001; d = 0.192) to B6 and from B3 to B9 (MD = 1.357%MVE; p < 0.001; d = 0.208). RMSPEAK showed a statistically significant interaction effect of Condition × Time (p < 0.001; w = 0.295), without significant post hoc pairwise comparisons.
DA showed statistically significant interaction effects of Condition × Time for RMSSTATIC (p < 0.01; w = 0.262), RMSMEDIAN (p < 0.05; w = 0.242), and RMSPEAK (p < 0.001; w = 0.332), without significant post hoc pairwise comparisons.
Statistically significant main effects of Time were found for RMSSTATIC (p < 0.001; w = 0.285) and RMSMEDIAN (p < 0.001; w = 0.248) of ED. RMSSTATIC decreased from B1 to B3 (MD = − 1.662%MVE; p < 0.001; d = − 0.448), B4 (MD = − 0.858%MVE; p = 0.015; d = − 0.218), B6 (MD = − 1.500%MVE; p < 0.001; d = − 0.384) and B7 (MD = − 0.964%MVE; p = 0.002; d = − 0.247) and increased from B3 o B4 (MD = 0.804%MVE; p = 0.016; d = 0.233); RMSMEDIAN (Fig. 4) decreased from B1 to B3 (MD = − 0.836%MVE; p = 0.002; d = − 0.211). Statistically significant interaction effects of Condition × Time were found for RMSSTATIC (p < 0.05; w = 0.246), RMSMEDIAN (p < 0.01; w = 0.263), and RMSPEAK (p < 0.01; w = 0.285). RMSMEDIAN increased from B3 to B4 (MD = 1.370%MVE; p = 0.022; d = 0.221) for the condition with active breaks; RMSPEAK decreased from B1 to B4 (MD = − 3.394%MVE; p = 0.031; d = − 0.429) for the condition with passive breaks.
For FCR, a statistically significant main effect of Time was found for RMSPEAK (p < 0.05; w = 0.195) and statistically significant interaction effects of Condition × Time were found for RMSSTATIC (p < 0.05; w = 0.222) and RMSPEAK (p < 0.05; w = 0.238). None of the post hoc pairwise comparisons were statistically significant.
Upper body postures
None of the joint angles showed a statistically significant effect of Condition
NF showed a statistically significant main effect of Time (p < 0.01; w = 0.241); NF increased from B3 to B7 (MD = 0.359°; p = 0.045; d = 1.710). TK showed a statistically significant main effect of Time (p < 0.01; w = 0.207) and interaction effect of Condition × Time (p < 0.001; w = 0.376), but post hoc pairwise comparisons were not statistically significant.
Heart rate and variability
HR showed no statistically significant main or interaction effects. The HRV parameter SDNN (Fig. 4) showed a statistically significant main effect of Condition (p < 0.05; w = 0.150), but without significant post hoc pairwise comparisons. SDNN also showed a statistically significant main effect of Time (p < 0.001; w = 0.280), which increased from B1 to B4 (MD = 7.536 ms; p = 0.007; d = 0.471), B6 (MD = 10.126 ms; p < 0.001; d = 0.565), B7 (MD = 7.452 ms; p = 0.035; d = 0.458), and B9 (MD = 7.399 ms; p = 0.027; d = 0.453). SDNN also showed a statistically significant interaction effect of Condition × Time (p < 001; w = 0.308); B4 in the condition with passive breaks was higher than B1 in the conditions without breaks (MD = 13.767 ms; p = 0.004; d = 0.818) and with active breaks (MD = 13.561 ms; p = 0.015; d = 0.777). The HRV parameter RMSSD showed a statistically significant interaction effect of Condition × Time (p < 001; w = 0.305), but without statistically significant post hoc pairwise comparisons.
Discussion
Performing surgeries laparoscopically instead of open is beneficial for the patient’s well-being [6,7,8]; however, surgeons are put at higher risk for developing musculoskeletal complaints and disorders due to the constrained and static working postures associated with laparoscopy [2,3,4,5]. This study evaluated the organizational measure of passive and active intraoperative work breaks compared to no breaks during simulated laparoscopy with respect to muscular fatigue, muscular activity, upper body posture, heart rate, and heart rate variability. Muscular fatigue tended to be less in the intervention conditions compared to the control condition. Also, the heart rate variability metric SDNN tended to be lower in the control condition without breaks compared to the intervention conditions with breaks. Both tendencies reflects to an effect in favor of intraoperative (passive/active) work breaks, because less muscular fatigue [51] and more heart rate variability [47] may reduce the risk associated with work-related the physical stress response. No (tendencies of) statistically significant effects of work break intervention were detected for muscular activity, joint angles or heart rate. The factor time had a statistically significant effect mainly on the muscular activity parameters, which generally tended to increase over time irrespective of the experimental condition (Fig. 4).
Muscular fatigue
A “less-is-better”-principle applies to muscular fatigue, which is an acknowledged precursor of developing musculoskeletal disorders [51]. In previous studies, especially the dominant trapezius showed to be vulnerable to localized muscular fatigue, since the trapezius is often the main affected muscle in the lead up to musculoskeletal complaints [17, 52]. Although the right (dominant) trapezius muscle tended to fatigue more in the control condition compared to the intervention conditions, this was based on its RMS only. Therefore, only the dominant shoulder muscles might benefit from intraoperative work breaks, whereas for all other muscles we could not detect comparable tendencies in the 1.5-h laparoscopy as simulated in the current study (Fig. 3; Table 2).
Muscular activity
Related to muscular fatigue is the risk that prolonged activity of small, single muscle fibers may cause degenerative muscular changes, even with very low levels of static muscular activity [53]. However, over time, only the bilateral trapezius muscle increased its static, median and peak level over time. Note that the left (i.e., non-dominant) and right (i.e., dominant) body sides for the forearm extensors showed muscular activity fluctuation over time of < 1%MVC. In line with the tendency for muscular fatigue, muscular activity on the dominant right side also appears to increase slightly more over time compared to the non-dominant left side, suggesting that the dominant shoulder may be more susceptible to developing future musculoskeletal disorders [54]. However, the trapezius muscles as well as the other back and forearm muscles remained below the 30%MVC-threshold-limit-value for laparoscopic work (estimated hand activity level of 6) [55]. This is in line with a recent study that documented traditional laparoscopic work, where the peak muscular activity level did not exceed 15%MVC [56]. Since the current study could not identify significant effects of both intervention conditions on any of the muscular activity levels, a potential protective effect of passive or active work breaks with respect to the risk of developing musculoskeletal disorders could not be identified [57]. Although upper threshold limit values for hand activities are set, future research remains challenged for providing lower threshold limit values for hand activities, including the duration of static work periods with respect to recovery in light of the Cinderella hypothesis and muscular fatigue [58, 59]. Some argue that a lower threshold limit value of 2 to 5%MVC should not be exceeded [60]; if this limit value is to be validated, laparoscopic work has to be considered risky considering that static muscular activity levels exceeded 2%MVC in several muscles [56].
Upper body postures
Neck flexion ≥ 20° [61], and more thoracic kyphosis [62] and limited lumbar lordosis [63] are identified as potential risk factors for developing neck-related musculoskeletal disorders or low back pain, respectively. The current study found a statistically significant increase of the neck flexion angle over time; however, median neck flexion angles were in the range of 4.4 to 7.2° and would not be considered risky in terms of developing musculoskeletal disorders. None of the joint angles investigated were influenced by the implementation of work breaks to a significant extent.
Heart rate and variability
An increased heart rate and reduced heart rate variability have been associated with work-related physical and mental stress, particularly reflecting the dominant role of the sympathetic nervous system during work [47, 64]. The HRV parameter SDNN, a parameter recommended for physical stress, tended to be lower in the condition without work breaks (38 ms) than in the conditions with passive (40 ms) and active work breaks (39 ms). However, this tendency is too minimal to detect a statistically significant or relevant influence of the implementation of work breaks on the sympathetic activity response to laparoscopic surgery.
Study implications and limitations
The reason for investigating work break interventions comes from promising results identified among office workers, who predominantly perform computer work. Recent studies showed that especially back and neck pain are reduced when providing active work breaks [65, 66]. Also for intensive care unit nurses, active work breaks are being recommended [67]. Since several field pilot studies have been performed among laparoscopic surgeons with the main outcome of perceived musculoskeletal discomfort, the current exploratory study aimed to provide insights into the acute effects of implementing passive and active work breaks in a simulated environment on physical strain and stress parameters of gynecological surgeons. We could not identify significant effects of both types of work breaks, only tendencies in favor of a reduced risk for work-related musculoskeletal disorders and physical stress response. Considering previous and the current study results, a future field feasibility study should provide insights into the acceptability and practicability of intraoperative active work breaks. When promising, a follow-up cluster randomized controlled trial should reveal the true effectiveness with respect to different outcome measures of intraoperative active work breaks.
Although the required sample size was reached (note that the calculation was based on rating of perceived discomfort data), the current study has some limitations that should be mentioned. First, we focused on the principal surgeon only, without considering potential effects work breaks may have on the remaining surgical team including assistant surgeons, anesthesiologists, and nurses [25]. Second, we focused on physical strain and stress parameters only, whereas outcomes such as discomfort, performance, and mental status may be interesting to investigate as well. Another manuscript will report on some of these outcomes. Third, due to the laboratory setting and the simulated nature of the study set-up, we are not able to provide direct associations with work-related musculoskeletal disorders. Fourth, the analysis of HRV was based on only one minute in each case, i.e., shorter than recommended in the current German guideline [47]. Finally, the way we have analyzed and reported outcomes may be improved in future studies by providing insights in the accumulated time surgeons spent working in particular muscular loads and static postures (i.e., joint angles). Configuring such an extensive profile is possible by applying the Exposure Variation Analysis [68].
Conclusion
This randomized, controlled cross-over laboratory trial demonstrated tendencies in favor of 2.5-min intraoperative work breaks presented after 30-min work blocks, including the tendencies of decreased shoulder muscular fatigue and increased heart rate variability (SDNN). The tentative results may point toward a potential relieving effect on the risk for developing musculoskeletal disorders and physical stress responses. Note that the tendencies for potential positive effects of intraoperative work breaks as detected in the here investigated simulated laparoscopy are, in a real laparoscopic surgery, highly dependent on the complexity and duration of the surgery, composition of the surgical team (enough skilled surgeons present), sequence of the surgery (first surgery or after several surgeries), and physical condition of the surgeon. The effect of passive and active breaks on perceived musculoskeletal discomfort and performance is to be evaluated in a follow-up manuscript. Following the outcomes of this study and taking in consideration the context of a real surgical laparoscopy, a field feasibility study should validate the acceptability and practicability of intraoperative active work breaks during laparoscopic procedures, after which a cluster randomized controlled trial can assess its effectiveness.
References
Epstein S, Sparer EH, Tran BN, Ruan QZ, Dennerlein JT, Singhal D, Lee BT (2018) Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists—A systematic review and meta-analysis. JAMA Surg 153:e174947
Plerhoples TA, Hernandez-Boussard T, Wren SM (2012) The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robotic Surg 6:65–72
Alleblas CCJ, de Man AM, van den Haak L, Vierhout ME, Jansen FW, Nieboer TE (2017) Prevalence of musculoskeletal disorders among surgeons performing minimally invasive surgery. Ann Surg 266:905–920
Park A, Lee G, Seagull FJ, Meenaghan N, Dexter D (2010) Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg 210:306–313
Dabholkar T, Yardi S, Dabholkar Y (2016) Prevalence of work-related musculoskeletal symptoms in surgeons performing minimally invasive surgery: a review of literature. Int Surg J. https://doi.org/10.18203/2349-2902.isj20161437
Li H, Zheng J, Cai JY, Li SH, Zhang JB, Wang XM, Chen GH, Yang Y, Wang G (2017) Laparoscopic vs open hepatectomy for hepatolithiasis: an updated systematic review and meta-analysis. World J Gastroenterol 23:7791–7806
Yi X, Chen S, Wang W, Zou L, Diao D, Zheng Y, He Y, Li H, Luo L, Xiong W, Wan J (2017) A systematic review and meta-analysis of laparoscopic and open distal pancreatectomy of nonductal adenocarcinomatous pancreatic tumor (NDACPT) in the pancreatic body and tail. Surg Laparosc Endosc Percutan Tech 27:206–219
Tan S, Wu G, Zhuang Q, Xi Q, Meng Q, Jiang Y, Han Y, Yu C, Yu Z, Li N (2016) Laparoscopic versus open repair for perforated peptic ulcer: a meta analysis of randomized controlled trials. Int J Surg 33:124–132
Vigneswaran Y, Prachand VN, Posner MC, Matthews JB, Hussain M (2020) What is the appropriate use of laparoscopy over open procedures in the current COVID-19 climate? J Gastrointest Surg 24:1686–1691
Stucky C-CH, Cromwell KD, Voss RK, Chiang Y-J, Woodman K, Lee JE, Cormier JN (2018) Surgeon symptoms, strain, and selections: systematic review and meta-analysis of surgical ergonomics. Ann Med Surg 27:1–18
Catanzarite T, Tan-Kim J, Whitcomb EL, Menefee S (2018) Ergonomics in surgery: A review. Female Pelvic Med Reconstr Surg 24:1–12
Berguer R, Rab GT, Abu-Ghaida H, Alarcon A, Chung J (1997) A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc 11:139–142
Nguyen NT, Ho HS, Smith WD, Philipps C, Lewis C, De Vera RM, Berguer R (2001) An ergonomic evaluation of surgeons’ axial skeletal and upper extremity movements during laparoscopic and open surgery. Am J Surg 182:720–724
Arrighi-Allisan AE, Garvey KL, Wong A, Filip P, Shah J, Spock T, Del Signore A, Cosetti MK, Govindaraj S, Iloreta AM (2021) Ergonomic analysis of functional endoscopic sinus surgery using novel inertial sensors. Laryngoscope. https://doi.org/10.1002/lary.29796
Berguer R, Chen J, Smith WD (2003) A comparison of the physical effort required for laparoscopic and open surgical techniques. Arch Surg 138:967–970
Alaqeel M, Tanzer M (2020) Improving ergonomics in the operating room for orthopaedic surgeons in order to reduce work-related musculoskeletal injuries. Ann Med Surg 56:133–138
Krämer B, Seibt R, Stoffels A-K, Rothmund R, Brucker SY, Rieger MA, Steinhilber B (2018) An ergonomic field study to evaluate the effects of a rotatable handle piece on muscular stress and fatigue as well as subjective ratings of usability, wrist posture and precision during laparoscopic surgery: an explorative pilot study. Int Arch Occup Environ Health 91:1021–1029
Steinhilber B, Seibt R, Reiff F, Rieger MA, Krämer B, Rothmund R (2016) Effect of a laparoscopic instrument with rotatable handle piece on biomechanical stress during laparoscopic procedures. Surg Endosc 30:78–88
Hallbeck MS, Lowndes BR, Bingener J, Abdelrahman AM, Yu D, Bartley A, Park AE (2017) The impact of intraoperative microbreaks with exercises on surgeons: A multi-center cohort study. Appl Ergon 60:334–341
Park AE, Zahiri HR, Hallbeck MS, Augenstein V, Sutton E, Yu D, Lowndes BR, Bingener J (2017) Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: A multicenter cohort study. Ann Surg 265:340–346
Dorion D, Darveau S (2013) Do micropauses prevent surgeon’s fatigue and loss of accuracy associated with prolonged surgery? An experimental prospective study. Ann Surg 257:256–259
Engelmann C, Schneider M, Kirschbaum C, Grote G, Dingemann J, Schoof S, Ure BM (2011) Effects of intraoperative breaks on mental and somatic operator fatigue: a randomized clinical trial. Surg Endosc 25:1245–1250
Nishimoto W, Kawahira H, Shimomura Y, Nishizawa Y, Ito M (2019) A standing posture support device that reduces laparoscopic surgeons’ occupational lower limb stress. Minim Invasiv Ther 28:151–156
Gilbreth FB (1916) Motion study in surgery. Can J Med Surg 40:22–31
Al-Hakim L, Xiao J, Sengupta S (2017) Ergonomics perspective for identifying and reducing internal operative flow disruption for laparoscopic urological surgery. Surg Endosc 31:5043–5056
Coleman Wood KA, Lowndes BR, Buus RJ, Hallbeck MS (2018) Evidence-based intraoperative microbreak activities for reducing musculoskeletal injuries in the operating room. Work 60:649–659
Abdelall ES, Lowndes BR, Abdelrahman AM, Hawthorne HJ, Hallbeck MS (2018) Mini breaks, many benefits: development and pilot testing of an intraoperative microbreak stretch web-application for surgeons. Proc Hum F Ergonomics Soc Annu Meet 62:1042–1046
Giagio S, Volpe G, Pillastrini P, Gasparre G, Frizziero A, Squizzato F (2019) A preventive program for work-related musculoskeletal disorders among surgeons: Outcomes of a randomized controlled clinical trial. Ann Surg 270:969–975
Luger T, Maher CG, Rieger MA, Steinhilber B (2019) Work-break schedules for preventing musculoskeletal symptoms and disorders in healthy workers. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012886.pub2
Luger T, Rieger MA, Bonsch R, Krämer B, Seibt R, Steinhilber B (2020) Active and passive work breaks during simulated laparoscopy among laparoscopic surgeons: Study protocol for a controlled, randomised cross-over laboratory trial. BMJ Open 10:e038952
ClinicalTrials.gov [Internet] (2018) Work breaks during simulated minimally invasive surgery. ClinicalTrials.gov Identifier NCT03715816, Bethesda (MD): National Library of Medicine (US)
World Medical Association (2013) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Kadam P, Bhalerao S (2010) Sample size calculation. Int J Ayurveda Res 1:55–57
Wenger L, Richardson C, Tsuda S (2015) Retention of fundamentals of laparoscopic surgery (FLS) proficiency with a biannual mandatory training session. Surg Endosc 29:810–814
Shushan A, Mohamed H, Magos AL (1999) How long does laparoscopic surgery really take? Lessons learned from 1000 operative laparoscopies. Hum Reprod 14:39–43
Schmidt J, Rothmund R, Michaelis M, Rieger MA, Steinhilber B (2017) Welche muskuloskelettalen Beschwerden und arbeitsorganisatorische Maßnahmen zu ihrer Reduktion berichtet das chirurgische Personal in der Gynäkologie? Studiendesign einer standardisierten Befragung mit Fokus auf laparoskopische Eingriffe [What musculoskeletal complaints and work organisation measures to reduce them are reported by surgical staff in gynaecology? Study design of a standardized survey with focus on laparoscopic interventions]. 63. Frühjahrskongress 2017 der Gesellschaft für Arbeitswissenschaft e.V., Dortmund, Germany
Steinhilber B, Karle E, Schmidt J, Rothmund R, Michaelis M, Rieger MA, Krämer B (2019) Prevalence of musculoskeletal complaints in minimal invasive surgery. 10th International Conference on the Prevention of Work-Related Musculoskeletal Disorders (PREMUS), Bologna, Italy
Caffier G, Steinberg U, Liebers F (1999) Praxisorientiertes Methodeninventar zur Belastungs- und Beanspruchungsbeurteilung im Zusammenhang mit arbeitsbedingten Muskel-Skelett-Erkrankunen, Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAUA). Dortmund / Berlin, Germany
Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sørensen F, Andersson G, Jørgensen K (1987) Standardized Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 18:233–237
Steinhilber B, Reiff F, Seibt R, Rieger MA, Martus P, Krämer B, Rothmund R (2017) Ergonomic benefits from a laparoscopic instrument with rotatable handle piece depend on the area of the operating field and working height. Hum Factors 59:1048–1065
van Det MJ, Meijerink WJ, Hoff C, Totte ER, Pierie JP (2009) Optimal ergonomics for laparoscopic surgery in minimally invasive surgery suites: a review and guidelines. Surg Endosc 23:1279–1285
Criswell E (2010) Cram’s Introduction to Surface Electromyography, 2nd, Edition. Jones & Bartlett Learning Publishers, Sudbury, Massachusetss, USA
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development and recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374
Biering-Sørensen F (1984) Physical measurements as risk indicators for low-back trouble over a one-year period. Spine 9:106–119
Jonsson B (1982) Measurement and evaluation of local muscular strain in the shoulder during contrained work. J Human Ergol 11:73–88
Luttmann A, Jäger M, Sokeland J, Laurig W (1996) Electromyographical study on surgeons in urology. II Determination of muscular fatigue Ergonomics 39:298–313
Sammito S, Thielmann B, Klussmann A, Deußen A, Braumann K-M, Böckelmann I (2021) Nutzung der Herzschlagfrequenz und der Herzfrequenzvariabilität in der Arbeits-medizin und der Arbeitswissenschaft - S2k-Leitlinie [Use of heart rate and heart rate variability in occupational medicine and occupational science - S2k-guideline].
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol 4:863
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Earlbaum Associates, Hillsdale
Straker L, Mathiassen SE (2009) Increased physical work loads in modern work–A necessity for better health and performance? Ergonomics 52:1215–1225
Nordander C, Hansson GA, Ohlsson K, Arvidsson I, Balogh I, Stromberg U, Rittner R, Skerfving S (2016) Exposure-response relationships for work-related neck and shoulder musculoskeletal disorders–Analyses of pooled uniform data sets. Appl Ergon 55:70–84
Sjøgaard G, Lundberg U, Kadefors R (2000) The role of muscle activity and mental load in the development of pain and degenerative processes at the muscle cell level during computer work. Eur J Appl Physiol 83:99–105
Diederichsen LP, Norregaard J, Dyhre-Poulsen P, Winther A, Tufekovic G, Bandholm T, Rasmussen LR, Krogsgaard M (2007) The effect of handedness on electromyographic activity of human shoulder muscles during movement. J Electromyogr Kinesiol 17:410–419
American Conference of Governmental Industrial Hygienists (2001) Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. ACGIH, Cincinnati
Sjøgaard G, Mann S, Jensen JSD, Oestergaard AS, Dalager T (2021) The elixir of muscle activity and kinesiology in a health perspective: Evidence of worksite tailored exercise training alleviating muscle disorders. J Electromyogr Kinesiol 61:102600
Nakphet N, Chaikumarn M, Janwantanakul P (2014) Effect of different types of rest-break interventions on neck and shoulder muscle activity, perceived discomfort and productivity in symptomatic VDU operators: A randomized controlled trial. Int J Occup Saf Ergon 20:339–353
Takala EP (2002) Static muscular load, an increasing hazard in modern information technology. Scand J Work Environ Health 28:211–213
Østensvik T, Veiersted KB, Nilsen P (2009) A method to quantify frequency and duration of sustained low-level muscle activity as a risk factor for musculoskeletal discomfort. J Electromyogr Kinesiol 19:283–294
Jonsson B (1978) Kinesiology. With special reference to electromyographic kinesiology. In: Cobb WA, Van Duijn H (eds) Contemporary clinical neurophysiology. Elsevier Scientific Publishing Company, Amsterdam, pp 417–428
Norasi H, Tetteh E, Sarker P, Mirka GA, Hallbeck MS (2021) Exploring the relationship between neck flexion and neck problems in occupational populations: A systematic review of the literature. Ergonomics. https://doi.org/10.1080/00140139.2021.1976847
Tatsumi M, Mkoba EM, Suzuki Y, Kajiwara Y, Zeidan H, Harada K, Bitoh T, Nishida Y, Nakai K, Shimoura K, Aoyama T (2019) Risk factors of low back pain and the relationship with sagittal vertebral alignment in Tanzania. BMC Musculoskelet Disord 20:584
Sadler SG, Spink MJ, Ho A, De Jonge XJ, Chuter VH (2017) Restriction in lateral bending range of motion, lumbar lordosis, and hamstring flexibility predicts the development of low back pain: A systematic review of prospective cohort studies. BMC Musculoskelet Disord 18:179
Sato TO, Hallman DM, Kristiansen J, Skotte JH, Holtermann A (2018) Different autonomic responses to occupational and leisure time physical activities among blue-collar workers. Int Arch Occup Environ Health 91:293–304
Waongenngarm P, van der Beek AJ, Akkarakittichoke N, Janwantanakul P (2021) Effects of an active break and postural shift intervention on preventing neck and low-back pain among high-risk office workers: A 3-arm cluster-randomized controlled trial. Scand J Work Environ Health 47:306–317
Akkarakittichokea N, Waongenngarm P, Janwantanaku P (2021) The effects of active break and postural shift interventions on recovery from and recurrence of neck and low back pain in office workers: A 3-arm cluster-randomized controlled trial. Musculoskelet Sci Pract 56:102451
Armas M, Aronowitz D, Gaona R, Coppa G, Barrera R (2021) Active breaks initiative during hospital rounds in the surgical ICU to improve wellness of healthcare providers: an observational descriptive study. World J Surg 45:1026–1030
Mathiassen SE, Winkel J (1991) Quantifying variation in physical load using exposure-vs-time data. Ergonomics 34:1455–1468
Acknowledgements
The authors would like to thank physiotherapist Georg Haupt for his support in developing the exercise protocol for the active work breaks and Nadine Badie, Julia Gabriel and Xaver Thalhofer for their support in data collection.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work of Tessy Luger was partially supported by a 2-year personal intramural grant from the Junior Academy of the Faculty of Medicine of the University of Tübingen, Germany (fortüne; grant no. 2480–1-0). Rosina Bonsch received a 1-year scholarship for her medical dissertation from the Liselotte and Dr. Karl Otto Winkler-Foundation for Occupational Medicine, Germany (grant no. T0226/33768/2019). The remainder of the work of the Institute of Occupational and Social Medicine and Health Services Research was financially supported by an unrestricted grant of the employers’ association of the metal and electrical industry Baden-Württemberg, Germany (Südwestmetall; grant no. N/A). Fortüne: Faculty of Medicine of the University of Tübingen, Germany, 2480-1-0, Tessy Luger, Liselotte and Dr. Karl Otto Winkler-Foundation for Occupational Medicine, Germany, T0226/33768/2019, Rosina Bonsch, Südwestmetall, Germany
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The work of Tessy Luger was partially supported by a 2-year personal intramural grant from the Junior Academy of the Faculty of Medicine of the University of Tübingen, Germany (fortüne; Grant No. 2480-1-0). Rosina Bonsch received a 1-year scholarship for her medical dissertation from the Liselotte and Dr. Karl Otto Winkler-Foundation for Occupational Medicine, Germany (Grant No. T0226/33768/2019). The remainder of the work of the Institute of Occupational and Social Medicine and Health Services Research was financially supported by an unrestricted grant of the employers’ association of the metal and electrical industry Baden-Württemberg, Germany (Südwestmetall; Grant No. N/A). Doctors of philosophy Tessy Luger and Benjamin Steinhilber, medical doctor Rosina Bonsch, engineer Robert Seibt, and Professors Bernhard Krämer and Monika A. Rieger have no conflicts of interest or financial ties to disclose.
Amendment to study protocol
We did not include order of the experimental condition as a confounding factor, because it was randomized across subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luger, T., Bonsch, R., Seibt, R. et al. Intraoperative active and passive breaks during minimally invasive surgery influence upper extremity physical strain and physical stress response—A controlled, randomized cross-over, laboratory trial. Surg Endosc 37, 5975–5988 (2023). https://doi.org/10.1007/s00464-023-10042-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10042-9