Abstract
Background
Mobile applications can facilitate or improve gastrointestinal surgical care by benefiting patients, healthcare providers, or both. The extent to which applications are currently in use in gastrointestinal surgical care is largely unknown, as reported in literature. This systematic review was conducted to provide an overview of the available gastrointestinal surgical applications and evaluate their prospects for surgical care provision.
Methods
The PubMed, EMBASE and Cochrane databases were searched for articles up to October 6th 2022. Articles were considered eligible if they assessed or described mobile applications used in a gastrointestinal surgery setting for healthcare purposes. Two authors independently evaluated selected studies and extracted data for analysis. Descriptive data analysis was conducted. The revised Cochrane risk of bias (RoB-2) tool and ROBINS-I assessment tool were used to determine the methodological quality of studies.
Results
Thirty-eight articles describing twenty-nine applications were included. The applications were classified into seven categories: monitoring, weight loss, postoperative recovery, education, communication, prognosis, and clinical decision-making. Most applications were reported for colorectal surgery, half of which focused on monitoring. Overall, a low-quality evidence was found. Most applications have only been evaluated on their usability or feasibility but not on the proposed clinical benefits. Studies with high quality evidence were identified in the areas of colorectal (2), hepatopancreatobiliary (1) and bariatric surgery (1), reporting significantly positive outcomes in terms of postoperative recovery, complications and weight loss.
Conclusions
The interest for applications and their use in gastrointestinal surgery is increasing. From our study, it appears that most studies using applications fail to report adequate clinical evaluation, and do not provide evidence on the effectiveness or safety of applications. Clinical evaluation of objective outcomes is much needed to evaluate the efficacy, quality and safety of applications being used as a medical device across user groups and settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The use of smartphones and mobile application software (apps) is deeply integrated into society and their potential is being increasingly recognized in healthcare. In the past decade, the development of healthcare apps has rapidly increased, with the intention of providing medical solutions to some extent. At present, over 400.000 healthcare apps are available for download in mobile app stores worldwide [1].
To date, the number of apps used in gastrointestinal surgical care is limited compared with that in other surgical disciplines [2]. This may change rapidly. Apps are believed to offer great possibilities to support or improve gastrointestinal surgical care, and overall healthcare is on the lookout of the smart use of digital solutions in times of limited resources. Apps may facilitate patients, healthcare providers (HCP), or both. Apps have the potential to improve information provision, communication between patients and HCP, clinical decision-making, perioperative guidance and monitoring, and education/training. In addition, apps may be used to register clinically relevant variables as apps can be developed to connect with sensors or other measurement devices such as a camera, an activity tracker, a biosensor, or a blood pressure monitoring device [3,4,5].
The use of apps in healthcare is not without controversy or debate [6, 7]. As apps may influence patient-reported or clinical outcomes, they must be properly developed and validated. Apps or software in general to be used as a medical device must comply with standards as described by the European Medical Device Regulation (MDR) or the American Food and Drug Administration (FDA), safeguarding the quality and safety of the app [8, 9]. However, the distribution of apps is limitedly regulated by the app stores, with minimum supervision on whether these specific legislations are indeed met. Even if they are met, it is not guaranteed that the use of the app will lead to valid and reliable results across situations and user settings [7, 10]. For that, scientific research validating apps with well-designed research protocols is required. To date, a clear overview of properly validated gastrointestinal surgical apps is lacking. Therefore, this systematic review focuses on the following research questions: (1) Which apps that are used in gastrointestinal surgical care have been described in literature? (2) Are these apps clinically evaluated on objective outcomes and able to improve gastrointestinal surgical care?
Methods
This systematic review was conducted in line with the Cochrane Handbook for Systematic Reviews of Interventions version 6.0 and reported according to PRISMA 2020 [11]. This study was registered in Open Science Framework (https://doi.org/10.17605/OSF.IO/X56RA. Studies were considered eligible if they assessed or described mobile apps used in a gastrointestinal surgery setting and were published in 2010 or later. The search was last updated October 6th 2022. A mobile app is defined as a software program which operates only on a smartphone or tablet (and thus, not web-based software). Keywords related to mobile apps and gastrointestinal surgery were incorporated into the search strategy. The search string is presented in the appendix. The included articles were cross-referenced to identify any additional relevant studies. Studies were excluded if (1) the described mobile app was only used to register study outcomes (e.g. number of complications and operation time), (2) the articles were conference proceedings or study abstracts, as they do not provide adequate insights into the app or its evaluation, (3) reviews, and (4) the results were published in a language other than English. Two reviewers (SvdS and MB) independently assessed all titles and abstracts according to the inclusion and exclusion criteria in the software tool “Rayyan”. Studies were included in the full-text evaluation when both reviewers agreed on inclusion. Disagreements were resolved through appraisal by a third reviewer (EB).
The methodological quality of the randomized controlled trials was assessed using the Revised Cochrane risk of bias tool for randomized trials (RoB-2) [12]. This tool determines the overall risk of bias that is based on the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes and selection of reported results. The ROBINS-I tool was used to determine the methodological quality of non-randomized studies, in which the overall risk of bias is based confounding, participant selection, intervention classification, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results [13].
Data were extracted independently by two reviewers (SvdS and MB) in a standardized form that included: year of publication, country, study design, number of participants, characteristics of included participants, type of surgery, name of the app, platform of the app, functionalities of the app, and study outcomes. All study outcomes on usability, satisfaction and clinical outcomes were included because apps may have heterogeneous aims and functionalities. Conflicts among reviewers were resolved by consensus. The results of studies were summarized according to the apps described. The apps were categorized based on their functionalities to provide a structured overview of available apps. The apps were described within these categories and were assessed on their outcome evaluations.
Results
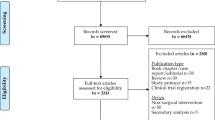
In total, 477 studies were screened for eligibility based on their title and abstract. After a full-text assessment, 38 studies were included of which 29 apps were described (Fig. 1). Patients were targeted as users in all apps except in three apps which were used by surgeons [45, 48, 53]. The apps were classified into seven categories: monitoring, weight loss, postoperative recovery, education, communication, prognosis, and clinical decision-making. The majority of the studies focused on colorectal surgery and monitoring (Fig. 2). An overview of the study’s characteristics is presented in Table 1. Due to the heterogeneity of the study designs and apps, a meta-analysis was impeded. In total, seven randomized control trials and seven comparative cohort studies were included. Only four studies had an overall low risk of bias as summarized in Tables 2, 3 [33, 38, 42, 53].
Monitoring
Almost half of the identified apps were used to monitor the clinical condition of patients who underwent gastrointestinal surgery [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. In general, the monitoring apps provided information about the operation, postoperative care, and self-management, contained daily assessments of the surgical wound (image uploading), symptoms and recovery progress, and some apps shared this information with the HCP.
Six apps monitored patients after colorectal surgery. These apps had a completion rate of the daily assessments between 21 and 84%, and had good patient satisfaction. [14,15,16,17,18,19,20,21,22,23,24]. The app of Keng et al. had a 30-day readmission rate of 6% in comparison with a reported rate of 18% prior to the start of the cohort study [14]. However, postoperative outcomes were not improved in a randomized controlled trial (RCT); only patient-reported outcomes did improve [15]. In another RCT, it will be evaluated whether the app could prevent unplanned hospital visits [16]. The app “Caresense” also had a communication feature. The app was evaluated in combination with the same-day discharge (SDD) protocol. The postoperative outcomes of patients using the app were comparable to patient without the app[17, 18]. The app was also evaluated in a retrospective study, in which the patient did not follow the SSD protocol. The app significantly decreased the rate of preventable emergency department visits [19]. The app is available in the app stores, but not freely accessible. The app “Maela” was successfully tested on it feasibility and all post-discharge complications were detected by the app [20]. The app is available in the app stores, but not freely accessible. The app of Symer et al. generated alerts for 26,7% of the patients and one patient within this group was readmitted [21]. The app “MobiMD” was initially developed for several gastrointestinal procedures but its feasibility was successfully tested on mainly colorectal patients [22]. The effect of the app on hospital readmissions will be evaluated in a RCT [23]. The app “how2trak” is focused on surgical wound and symptom surveillance and its feasibility evaluation has not yet been completed [24].
Two apps monitored patients after undergoing hepatopancreatobiliary surgery and both had a high reporting adherence [25,26,27,28]. The “Interaktor” app was evaluated in a cohort, in which patients using the app reported significantly less symptoms and higher self-care activity rates compared to a historical control group[25,26,27]. The app is available in the app stores. The already available “MyPlate” app monitored postoperative dietary intake and was used by the dietitian to guide patients during counseling visits. Caloric goals were achieved by 82.4% of the patients [28].
Two apps monitored patients after upper gastrointestinal surgery and both were globally tested on their feasibility [29,30,31]. The app “SurgeryDiary” had a high overall daily submission rate [29]. The app “UDD” (Upper Digestive Disease) was indicated as a helpful tool for reporting and identifying problems, and enhanced communication with HCP [30]. However, the scoring of dumping-related symptoms and pain which was used in the app was not yet adequate [31].
One app monitored bariatric patients and provided advice on whether the patients were on track or to seek symptom management by reviewing the educational materials or contacting a HCP [32]. The app was evaluated in a cohort in which clinical outcomes such as hospital stay or readmission did not differ between app users and the control group. Although adherence was relatively low, most patients were satisfied with the app.
Weight loss
Two apps mainly focused on a healthy diet, provided nutritional information and allowed bariatric patients to monitor their intake and weight [33, 34]. The already available app “MyfitnessPal” also allowed patients to make a diet program. The app was clinically evaluated in a RCT in which the control group was not allowed to use the app and only received self-monitoring journals [33]. The percentage of weight loss after two years was significantly higher for patients using the app (71,5%) than for those who did not use the app (59,1%). The other app, developed by Dolan et al., had high adherence, but a relatively low patient satisfaction [34].
The other three apps were aimed at engagement and stimulation of physical activity and a healthy diet of bariatric patients [35,36,37]. The extensive app of Sysko et al. was provided in combination with eight weekly virtual check-ins to review weight loss and the overall process before bariatric surgery [35]. The app was evaluated in a pilot RCT. On average, patients opened the app five times per week and entered their weight twice per week. Patients using the app showed a significant moderate decrease in stress and anxiety, whereas the effect on the caloric intake, weight loss and quality of life did not improve. The app of Mundi et al. provided automatic text messages stimulating a healthy lifestyle, and patients using this app had an average postoperative weight loss of 7.3 kg [36]. The app “PromMera” monitors and stimulates physical activity and self-registered vitamin intake, but its clinical evaluation in a RCT has not yet been completed [37].
Postoperative recovery
Four apps intended to improve postoperative recovery, providing perioperative information and feedback on the postoperative recovery process [34,35,36,37,38,39,40]. The app “IkHerstel” (I recover) was initially developed for gynecological patients and adapted to fit a general gastrointestinal surgical population [38]. The app was evaluated in a RCT, in which the control group received access to a placebo website containing standard general information [39]. The time until postoperative return to normal daily activities significantly was shortened of four days in the intervention group (21 vs 25 days), whereas other postoperative complications did not differ. Patients were satisfied with the app and had relatively high involvement with the app and the activity tracker [40]. The app is available in the app stores, but not freely accessible.
The other three apps were more focused on improving compliance to the recovery protocol after colorectal surgery, providing daily recovery milestones, and questionnaires to track patient compliance and assess patient-reported outcomes [37,38,39,40]. The app of Pecorelli et al. had a high usability score and patient satisfaction [41]. Subsequently, the app was evaluated in a RCT in which overall adherence to the postoperative recovery protocol and other postoperative outcomes did not improve [42]. The app “ERAS APPtimisation” specifically targets patient related elements of the Enhanced Recovery After Surgery (ERAS) protocol, and daily activity was monitored and simulated using an activity tracker [43]. The clinical evaluation in a RCT has not yet been completed. The comparable “IColon” app which incorporated slightly different ERAS elements, will be clinically evaluated in an observational study [44].
Educational apps
The “Touch Surgery” app facilitated three modules for laparoscopy to practice surgical procedures and cognitive tasks. Although the app was successfully validated based on its construct, face and content, training with the app did not improve students’ performance on a VR trainer [45]. The app is freely available in the app stores.
The app “Iprocto” provided a 3D model of various structures in the lower abdomen to improve the information provision to patients during the preoperative consult [46]. The intervention group used this app during consultations, whereas the control group did not use the app. The intervention group reported significantly higher scores of the clarity on the doctor and satisfaction regarding the proctologic visit than the control group.
The “Stoma-M” app provided educational information and contact details of stoma care units and associations in Turkey [47]. The app was evaluated in a quasi-experimental study, in which the intervention group received the app on a provided Android phone, while the control group received a booklet containing the same content as provided in the app. The app did not improve psychosocial adaptation and stoma-related problems.
Communication
The commonly known app “WhatsApp” was evaluated as a communication tool among surgeons [48]. In this study, surgeons treated patients in two cohorts:1) surgeons who communicated using traditional procedures, such as e-mail, phone calls, and collegial meetings, or 2) surgeons who used the “WhatsApp Surgery Group”, in which surgeons could communicate with each other. No differences in surgical clinical outcomes were reported between the two groups.
The app of Doğan et al. enabled bariatric patients to have a live consultation with researchers and contained educational materials [49]. The app did not improve self-care, quality of life and the self-body image. Although significant differences in BMI were reported between the intervention and the control group, the weight loss towards the preoperative weight was not analyzed.
Moon et al. developed a peer support app for patients with low anterior resection syndrome [50]. The app consisted of information modules and a peer support forum in which patients could communicate with mentors monitored by a team of HCP’s. The app will be evaluated in a RCT on its impact on patients-reported outcomes.
Prognosis
The app of Gabriel et al. contained a prediction model of the 5 years overall survival of postoperative patients with stage II or III colon cancer which was based on a large retrospective cohort study [51]. However, the app itself has not been tested on its usability, effectiveness and reliability in clinical care.
The already available “AWARE” app collected behavioral data of patients after pancreatic surgery, which was used in combination with an activity tracker to predict postoperative symptoms with a 73.5% accuracy [52]. However, the prediction was calculated afterwards and was not included in the app. Thus, the clinical relevance of the app has not been evaluated.
Clinical decision-making
The app “Pancreatic Surgery” contained a multimodal algorithm for early recognition and minimally invasive management of postoperative complications after pancreatic surgery, in which the HCP were instructed to enter data daily. The app was evaluated in a RTC, and patients who were treated in accordance with the algorithm in the app had significantly less postoperative complications than those who received usual care [53]. The app is freely available in the app stores.
Discussion
Healthcare apps may offer great possibilities to support or improve gastrointestinal surgical care, provided that the development and validation process are properly conducted and the app itself complies with professional standards and medical device regulations [8, 9]. This systematic review showed that most the gastrointestinal apps, which have been described in literature, at best had a low-quality evidence and were limited in their evaluation methodology. Small sample sizes, lack of comparison with a control group and subjective outcomes defined were common limitations. Most of the identified apps were only assessed on their usage, usability, satisfaction and feasibility, which was rarely measured with a valid and reusable questionnaire. Studies of higher-level evidence in the area of colorectal [38, 42]. Hepatopancreatobiliary [53] and bariatric surgery [33] reported mostly positive outcomes on postoperative recovery, complications and weight loss.
In total, the review retrieved 29 apps developed for use by patients, surgeons, or both. In the selected studies, there was a predominant focus on monitoring the patient’s postoperative condition and symptoms in the area of colorectal surgery. Apps that fall within the same category share many similar functionalities, with minimum variance in functionality. It is fair to state that apps that fall into different categories are not mutually exclusive in their functionalities regarding their category inclusion. Across all app categories, studies have indicated a potential benefit of apps, except for the categories of communication and prognosis. Users of apps generally seemed to be satisfied with the apps, while reported patient engagement was highly variable across the categories and domains. Patient engagement with the app is, of course, a driver of the potential clinical effect of apps aimed at patient care. Patient engagement not only depends on the specific features that the app offers but also relates to the context and phase of care the patient is receiving, the patients’ digital literacy, and the apps’ overall usability and stability. Most studies did not report participants’ digital literacy, although it can be assumed that participants had sufficient proficiency, as patients with insufficient proficiency probably did not participate. It is important to acknowledge digital literacy and to compensate for digital literacy as well as possible, as the effectiveness of apps may be substantially less.
Although over 150 gastrointestinal surgical apps for use on a smartphone or tablet are available in the app stores, only a limited amount (29) is reflected in studies as could be retrieved from scientific literature by this systematic review [54,55,56] Non-validated or poorly validated apps are potentially harmful, especially if they may have a direct effect on clinical outcomes such as diagnosis or decision support tools. This underlines the need for high quality clinical research to safeguard the effectiveness and safety of apps, and to provide HCP's a better understanding of the potential impact of an app on surgical care. It is important to realize that apps can be published in the app stores claiming to be effective or reliable without presenting a snippet of evidence to support clinical safety or efficacy. There are no specific rules or regulations in the submission guidelines for the app stores, which is an important issue [57, 58]. When scientific evidence is needed to safeguard the efficacy, quality and safety of apps to be in clinical settings, and with the medical device regulations in place, the public should at least be able to discern apps that are built and proofed reliably from those that are not before they are downloaded and granted permission from the user. App stores are encouraged to change their submission guidelines for apps that act as a medical device.
Healthcare apps which are used to monitor, guide, diagnose, or treat patients must be regarded as a medical device and thereby have to comply to medical device regulations (FDA or MDR).[8, 9]. The regulations have strict requirements for the (technical) development, validation and quality surveillance of the app, and the manufacture itself. Even with legislation in place, HCP’s or manufacturers may be unaware of the importance of such legislation, which may impede the quality and safety of apps. Although apps evaluated in a clinical study do not have to fully comply to the regulations, it is worthwhile to note that only one author has mentioned the regulations [39]. It is unclear if other apps would be allowed under the medical device regulations. However, it is not guaranteed that the app will lead to valid outcomes if they have met the regulations [7, 10]. Therefore, well-designed scientific research validating apps are needed. As with researching medical devices or drugs, conducting research with healthcare apps is time- and cost-consuming. The role of app manufacturers with commercial interests and eagerness of the public to use apps are potential hazards. It is essential that an expert HCP is involved in the development and validation of healthcare apps. Not only to safeguard content, but also to ensure that apps are well researched and vetted before they become accepted in clinical practice. Although the development process of the apps identified in this review has been rarely or obscurely described, the involvement of HCP is presumed. HCP’s are mostly not involved in unvalidated apps which are available in the app stores, resulting in a potential higher risk [51]. Moreover, apps that collect and/or process medical data must comply with data privacy regulations [59, 60] Specific standards needs to be followed, but not all app manufacturers are familiar with them [61]. Most of the included apps collect or process patient data (25/29), however, only three have mentioned privacy measures [30, 48, 50]. This does not have to imply that these apps do not comply with data privacy regulations as the development process was generally obscurely described.
Since the use of apps in healthcare has grown rapidly, hospitals and health insurers are increasingly demanding that apps are adequately validated before deployment in clinical care. However, they struggle with the minimum required proof of evidence. Conventionally, a RCT is the golden standard, and is especially applicable for high-risk apps which are classified as medical devices. But there are also other methods to validate apps of which mixed methods studies are an excellent example [62]. It is important that all evaluations are published, to shape the proof of evidence of apps. It is recommended that medical apps used in research or clinical practice comply with the suggestions summarised in Table 4.
Conclusion
Healthcare providers and patients must be aware of the level of evidence of apps that they prescribe or use. Although apps may offer great potential to improve gastrointestinal surgical care, only a limited number of available gastrointestinal surgical apps have been researched and described in peer-reviewed literature to date. It is of great concern that most studies evaluating gastrointestinal surgical apps fail to generate a high level of scientific evidence, needed to guarantee the efficacy, quality and safety of apps. To fully utilize the potential of gastrointestinal surgical apps in standard surgical care, more and higher quality of research is needed.
References
Georgiou M (2022) Developing a healthcare app in 2023: what do patients really want? Available from: https://imaginovation.net/blog/developing-a-mobile-health-app-what-patients-really-want/. Accessed 31 Jan Mar 2023
Mobasheri MH, Johnston M, Syed U, King D, Darzi A (2015) The uses of smartphones and tablet devices in surgery: a systematic review of the literature. Surgery 158(5):1352–1371. https://doi.org/10.1016/j.surg.2015.03.029
Ventola CL (2014) Mobile devices and apps for health care professionals: uses and benefits. Pharm Ther 39(5):356–364
Purohit B, Kumar A, Mahato K, Chandra P (2020) Smartphone-assisted personalized diagnostic devices and wearable sensors. Curr Opin Biomedl Eng 13:42–50. https://doi.org/10.1016/j.cobme.2019.08.015
Barros Almeida I, Garcez Barretto Teixeira L, Oliveira de Carvalho F et al (2021) Smart dressings for wound healing: a review. Adv Skin Wound Care 34(2):1–8. https://doi.org/10.1097/01.ASW.0000725188.95109.68
Charani E, Castro-Sánchez E, Moore LS, Holmes A (2014) Do smartphone applications in healthcare require a governance and legal framework? It depends on the application! BMC Med 12:1–3. https://doi.org/10.1186/1741-7015-12-29
Magrabi F, Habli I, Sujan M, Wong D, Thimbleby H, Baker M, Coiera E (2019) Why is it so difficult to govern mobile apps in healthcare? BMJ Health Care Inform 26(1):e100006. https://doi.org/10.1136/bmjhci-2019-100006
European Union (2017) Regulation (EU) 2017/745 of the European Parliament and of the Council
Shuren J, Patel B, Gottlieb S (2018) FDA regulation of mobile medical apps. JAMA 320(4):337–338. https://doi.org/10.1001/jama.2018.8832
Gordon WJ, Landman A, Zhang H, Bates DW (2020) Beyond validation: getting health apps into clinical practice. Digit Med 3(1):14. https://doi.org/10.1038/s41746-019-0212-z
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. https://doi.org/10.1136/bmj.n71
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Keng CJS, Goriawala A, Rashid S, Goldstein R, Schmocker S, Easson A, Kennedy E (2020) Home to stay: an integrated monitoring system using a mobile app to support patients at home following colorectal surgery. J Patient Exp 7(6):1241–1246. https://doi.org/10.1177/2374373520904194
Pooni A, Brar MS, Anpalagan T, Schmocker S, Rashid S, Goldstein R, Goriawala A, Easson A, Kennedy ED (2022) Home to stay: a randomized controlled trial evaluating the effect of a post-discharge mobile app to reduce 30-day re-admission following elective colorectal surgery. Ann Surg. https://doi.org/10.1097/SLA.0000000000005527
Anpalagan T, Schmocker S, Raval M, Baxter NN, Brar MS, Easson A, Feldman LS, Lee L, Liberman AS, Scales DC, Kennedy ED (2022) Home to stay: a randomized controlled trial protocol to assess use of a mobile app to reduce readmissions following colorectal surgery. Colorectal Dis. https://doi.org/10.1111/codi.16312
Lee L, Eustache J, Baldini G, Liberman AS, Charlebois P, Stein B, Fiore JF Jr, Feldman LS (2022) Enhanced recovery 2.0—same day discharge with mobile app follow-up after minimally invasive colorectal surgery. Ann Surg 276(6):e812–e818. https://doi.org/10.1097/SLA.0000000000004962
Lee L, Eustache J, Tran-McCaslin M, Basam M, Baldini G, Rudikoff AG, Liberman S, Feldman LS, McLemore EC (2022) North American multicentre evaluation of a same-day discharge protocol for minimally invasive colorectal surgery using mHealth or telephone remote post-discharge monitoring. Surg Endosc. https://doi.org/10.1007/s00464-022-09208-8
Eustache J, Latimer EA, Liberman S, Charlebois P, Stein B, Fiore JF Jr, Feldman LS, Lee L (2021) A mobile phone app improves patient-physician communication and reduces emergency department visits after colorectal surgery. Dis Colon Rectum. https://doi.org/10.1097/DCR.0000000000002187
Agri F, Hahnloser D, Demartines N, Hübner M (2020) Gains and limitations of a connected tracking solution in the perioperative follow-up of colorectal surgery patients. Colorectal Dis 22(8):959–966. https://doi.org/10.1111/codi.14998
Symer MM, Abelson JS, Milsom J, McClure B, Yeo HL (2017) A mobile health application to track patients after gastrointestinal surgery: results from a pilot study. J Gastrointest Surg 21(9):1500–1505. https://doi.org/10.1007/s11605-017-3482-2
Diehl TM, Barrett JR, Van Doorn R et al (2022) Promoting patient engagement during care transitions after surgery using mobile technology: lessons learned from the MobiMD pilot study. Surgery S0039–6060(21):01241–01251. https://doi.org/10.1016/j.surg.2021.12.018
Diehl TM, Barrett JR, Van Doorn R, Cherney Stafford LM, Hanlon BM, Weber SM, Voils CI, Abbott DE (2022) Promoting patient engagement during care transitions after surgery using mobile technology: lessons learned from the MobiMD pilot study. Surgery 172(1):219–225. https://doi.org/10.1016/j.surg.2021.12.018
Valk HA, Garcia-Ochoa C, Fontaine Calder J, Miller T, Rashidi B, McIsaac C, Musselman R (2022) A Mobile app for wound and symptom surveillance after colorectal surgery: protocol for a feasibility randomized controlled trial. JMIR Res Protoc 11(1):e26717. https://doi.org/10.2196/26717
Gustavell T, Langius-Eklöf A, Wengström Y, Segersvärd R, Sundberg K (2019) Development and feasibility of an interactive smartphone app for early assessment and management of symptoms following pancreaticoduodenectomy. Cancer Nurs 42(3):E1–E10. https://doi.org/10.1097/NCC.0000000000000584
Gustavell T, Sundberg K, Langius-Eklöf A (2020) Using an interactive app for symptom reporting and management following pancreatic cancer surgery to facilitate person-centered care: descriptive study. JMIR Mhealth Uhealth 8(6):e17855. https://doi.org/10.2196/17855
Gustavell T, Sundberg K, Segersvärd R, Wengström Y, Langius-Eklöf A (2019) Decreased symptom burden following surgery due to support from an interactive app for symptom management for patients with pancreatic and periampullary cancer. Acta Oncol 58(9):1307–1314. https://doi.org/10.1080/0284186X.2019.1633473
Allenson K, Turner K, Gonzalez BD, Gurd E, Zhu S, Misner N, Chin A, Adams M, Cooper L, Nguyen D, Naffouje S, Castillo DL, Kocab M, James B, Denbo J, Pimiento JM, Malafa M, Powers BD, Fleming JB, Anaya DA, Hodul PJ (2021) Pilot trial of remote monitoring to prevent malnutrition after hepatopancreatobiliary surgery. BMC Nutr 7(1):82. https://doi.org/10.1186/s40795-021-00487-3
Wu JM, Ho TW, Chang YT, Hsu C, Tsai CJ, Lai F, Lin MT (2019) Wearable-based mobile health app in gastric cancer patients for postoperative physical activity monitoring: focus group study. JMIR Mhealth Uhealth 7(4):e11989. https://doi.org/10.2196/11989
Chlan LL, Wzientek C, Pierson KE, Ruddy KJ, Schrandt A, Burnette D, Lee MK, Yost KJ, Blackmon SH (2022) Upper digestive disease app for electronic patient-reported outcomes: a mixed methods pilot study. Ann Thorac Surg 114(4):1142–1151. https://doi.org/10.1016/j.athoracsur.2022.02.054
Traynor MD, Chlan LL, Wzientek C, Yost KJ, Pierson KE, Lee MK, Blackmon SH (2022) Agreement between UDD app and provider evaluation of esophagectomy symptoms in a mobile app tool. Ann Thorac Surg. https://doi.org/10.1016/j.athoracsur.2022.06.060
Heuser J, Maeda A, Yang L, Masino C, Duggal S, Jackson T, Okrainec A (2021) Impact of a mobile app to support home recovery of patients undergoing bariatric surgery. J Surg Res 261:179–184. https://doi.org/10.1016/j.jss.2020.12.005
Mangieri CW, Johnson RJ, Sweeney LB, Choi YU, Wood JC (2019) Mobile health applications enhance weight loss efficacy following bariatric surgery. Obes Res Clin Pract 13(2):176–179. https://doi.org/10.1016/j.orcp.2019.01.004
Dolan PT, Afaneh C, Dakin G, Pomp A, Yeo HL (2019) Lessons learned from developing a mobile app to assist in patient recovery after weight loss surgery. J Surg Res 244:402–408. https://doi.org/10.1016/j.jss.2019.06.063
Sysko R, Michaelides A, Costello K, Herron DM, Hildebrandt T (2022) An initial test of the efficacy of a digital health intervention for bariatric surgery candidates. Obes Surg. https://doi.org/10.1007/s11695-022-06258-8
Mundi MS, Lorentz PA, Grothe K, Kellogg TA, Collazo-Clavell ML (2015) Feasibility of smartphone-based education modules and ecological momentary assessment/intervention in pre-bariatric surgery patients. Obes Surg 25(10):1875–1881. https://doi.org/10.1007/s11695-015-1617-7
Bonn SE, Hult M, Spetz K, Löf M, Andersson E, Wiren M, Trolle Lagerros Y (2020) App technology to support physical activity and intake of vitamins and minerals after bariatric surgery (the PromMera Study): protocol of a randomized controlled clinical trial. JMIR Res Protoc 9(8):e19624. https://doi.org/10.2196/19624
den Bakker CM, Schaafsma FG, van der Meij E, Meijerink WJ, van den Heuvel B, Baan AH, Davids PH, Scholten PC, van der Meij S, van Baal WM, van Dalsen AD, Lips DJ, van der Steeg JW, Leclercq WK, Geomini PM, Consten EC, Schraffordt Koops SE, de Castro SM, van Kesteren PJ, Cense HA, Stockmann HB, Ten Cate AD, Bonjer HJ, Huirne JA, Anema JR (2019) Electronic health program to empower patients in returning to normal activities after general surgical and gynecological procedures: intervention mapping as a useful method for further development. J Med Internet Res 21(2):e9938. https://doi.org/10.2196/jmir.9938
van der Meij E, Anema JR, Leclercq WKG, Bongers MY, Consten ECJ, Schraffordt Koops SE, van de Ven PM, Terwee CB, van Dongen JM, Schaafsma FG, Meijerink WJHJ, Bonjer HJ, Huirne JAF (2018) Personalised perioperative care by e-health after intermediate-grade abdominal surgery: a multicentre, single-blind, randomised, placebo-controlled trial. Lancet 392(10141):51–59. https://doi.org/10.1016/S0140-6736(18)31113-9
den Bakker CM, Huirne JA, Schaafsma FG, de Geus C, Bonjer HJ, Anema JR (2019) Electronic health program to empower patients in returning to normal activities after colorectal surgical procedures: mixed-methods process evaluation alongside a randomized controlled trial. J Med Internet Res 21(1):e10674. https://doi.org/10.2196/10674
Pecorelli N, Fiore JF Jr, Kaneva P, Somasundram A, Charlebois P, Liberman AS, Stein BL, Carli F, Feldman LS (2018) An app for patient education and self-audit within an enhanced recovery program for bowel surgery: a pilot study assessing validity and usability. Surg Endosc 32(5):2263–2273. https://doi.org/10.1007/s00464-017-5920-3
Mata J, Pecorelli N, Kaneva P, Moldoveanu D, Gosselin-Tardiff A, Alhashemi M, Robitaille S, Balvardi S, Lee L, Stein BL, Liberman S, Charlebois P, Fiore JF Jr, Feldman LS (2020) A mobile device application (app) to improve adherence to an enhanced recovery program for colorectal surgery: a randomized controlled trial. Surg Endosc 34(2):742–751. https://doi.org/10.1007/s00464-019-06823-w
Rauwerdink A, Jansen M, de Borgie CAJM, Bemelman WA, Daams F, Schijven MP, Buskens CJ (2019) Improving enhanced recovery after surgery (ERAS): ERAS APPtimize study protocol, a randomized controlled trial investigating the effect of a patient-centred mobile application on patient participation in colorectal surgery. BMC Surg 19(1):125. https://doi.org/10.1186/s12893-019-0588-3
Bertocchi E, Barugola G, Gentile I, Zuppini T, Zamperini M, Guerriero M, Avesani R, Bonadiman S, Anselmi C, Ruffo G (2021) iColon a patient-focused mobile application for perioperative care in colorectal surgery: an observational, real-world study protocol. BMJ Open 11(11):e045526. https://doi.org/10.1136/bmjopen-2020-045526
Kowalewski KF, Hendrie JD, Schmidt MW, Proctor T, Paul S, Garrow CR, Kenngott HG, Müller-Stich BP, Nickel F (2017) Validation of the mobile serious game application Touch Surgery™ for cognitive training and assessment of laparoscopic cholecystectomy. Surg Endosc 31(10):4058–4066. https://doi.org/10.1007/s00464-017-5452-x
Gaj F, Bellucci M, Biviano I (2017) iProcto: new digital technology in Proctology. A randomized study. Clin Ter 168(3):e186-191
Yiğitoğlu ET, Şendir M (2021) Effect of a mobile patient education application on adjustment to stoma and development of peristomal skin lesions: a quasi-experimental study. Wound Manag Prev 67(12):30–40
Nardo B, Cannistrà M, Diaco V, Naso A, Novello M, Zullo A, Ruggiero M, Grande R, Sacco R (2016) Optimizing patient surgical management using whatsapp application in the italian healthcare system. Telemed e-health. https://doi.org/10.1089/tmj.2015.0219
Deniz Doğan S, Arslan S (2022) The effects of e-mobile training and consultancy services on bariatric surgery patients: a randomized clinical trial. Obes Surg 1:1–8. https://doi.org/10.1007/s11695-022-06255-x
Moon J, Monton O, Smith A, Garfinkle R, Zhao K, Zelkowitz P, Loiselle CG, Fiore JF Jr, Sender Liberman A, Morin N, Faria J, Ghitulescu G, Vasilevsky CA, Bhatnagar SR, Boutros M (2021) Interactive online informational and peer support application for patients with low anterior resection syndrome: patient survey and protocol for a multicentre randomized controlled trial. Colorectal Dis 23(5):1248–1257. https://doi.org/10.1111/codi.15602
Gabriel E, Attwood K, Thirunavukarasu P, Al-Sukhni E, Boland P, Nurkin S (2016) Predicting individualized postoperative survival for stage ii/iii colon cancer using a mobile application derived from the national cancer data base. J Am Coll Surg 222(3):232–244. https://doi.org/10.1016/j.jamcollsurg.2015.12.019
Low CA, Li M, Vega J, Durica KC, Ferreira D, Tam V, Hogg M, Zeh Iii H, Doryab A, Dey AK (2021) Digital biomarkers of symptom burden self-reported by perioperative patients undergoing pancreatic surgery: prospective longitudinal study. JMIR Cancer 7(2):e27975. https://doi.org/10.2196/27975
Smits FJ, Henry AC, Besselink MG, Busch OR, van Eijck CH, Arntz M, Bollen TL, van Delden OM, van den Heuvel D, van der Leij C, van Lienden KP, Moelker A, Bonsing BA, Borel Rinkes IH, Bosscha K, van Dam RM, Derksen WJM, den Dulk M, Festen S, Groot Koerkamp B, de Haas RJ, Hagendoorn J, van der Harst E, de Hingh IH, Kazemier G, van der Kolk M, Liem M, Lips DJ, Luyer MD, de Meijer VE, Mieog JS, Nieuwenhuijs VB, Patijn GA, Te Riele WW, Roos D, Schreinemakers JM, Stommel MWJ, Wit F, Zonderhuis BA, Daamen LA, van Werkhoven CH, Molenaar IQ, van Santvoort HC, Dutch Pancreatic Cancer Group (2022) Algorithm-based care versus usual care for the early recognition and management of complications after pancreatic resection in the Netherlands: an open-label, nationwide, stepped-wedge cluster-randomised trial. Lancet 399(10338):1867–1875. https://doi.org/10.1016/S0140-6736(22)00182-9
Stevens DJ, Jackson JA, Howes N, Morgan J (2014) Obesity surgery smartphone apps: a review. Obes Surg 24(1):32–36. https://doi.org/10.1007/s11695-013-1010-3
O’Neill S, Brady RR (2012) Colorectal smartphone apps: opportunities and risks. Colorectal Dis 14(9):e530–e534. https://doi.org/10.1111/j.1463-1318.2012.03088.x
Kulendran M, Lim M, Laws G, Chow A, Nehme J, Darzi A, Purkayastha S (2014) Surgical smartphone applications across different platforms: their evolution, uses, and users. Surg Innov 21(4):427–440. https://doi.org/10.1177/1553350614525670
Apple. App Store review guidelines. Available from: https://developer.apple.com/app-store/review/guidelines/ Accessed 31 Jan 2023
Google. Developer Content policy. Available from: https://play.google.com/about/developer-content-policy/ Accessed 31 Jan 2023
European Parliament (2016) Regulation (EU) 2016/679 of the European Parliament and of the Council. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02016R0679-20160504&qid=1532348683434
Tariq RA, Hackert PB (2023) Patient confidentiality. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 30137825.
Jansen M, Meijer HAW, van der Storm SL, Barsom EZ, Schijven MP. Can I safely prescribe an app? Responsibilities and legislation every healthcare provider should know about. Submitted 2023
Bonten TN, Rauwerdink A, Wyatt JC, Kasteleyn MJ, Witkamp L, Riper H, van Gemert-Pijnen LJ, Cresswell K, Sheikh A, Schijven MP, Chavannes NH, EHealth Evaluation Research Group (2020) Online guide for electronic health evaluation approaches: systematic scoping review and concept mapping study. J Med Internet Res 22(8):e17774. https://doi.org/10.2196/1777
Funding
None of the authors have any funding sources for this research.
Author information
Authors and Affiliations
Contributions
SvdS, MB, EB and MP were involved in the conceptualization, study design, and search strategy. SvdS and MB all database searching, the article screening, data extraction, and critical appraisal. EZ contributed to conflict resolution during screening. SvdS and MB contributed to data curation, analysis and interpretation, and wrote the original draft. All authors contributed to reviewing and editing of the final manuscript.
Corresponding author
Ethics declarations
Disclosures
The authors Sebastiaan van der Storm, Mustufa Bektaş, Esther Barsom and Marlies Schijven have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Storm, S.L., Bektaş, M., Barsom, E.Z. et al. Mobile applications in gastrointestinal surgery: a systematic review. Surg Endosc 37, 4224–4248 (2023). https://doi.org/10.1007/s00464-023-10007-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10007-y