Abstract
Background
Several management options exist for colonic decompression in the setting of malignant large bowel obstruction, including oncologic resection, surgical diversion, and SEMS as a bridge-to-surgery. Consensus has yet to be reached on optimal treatment pathways. The aim of the present study was to perform a network meta-analysis comparing short-term postoperative morbidity and long-term oncologic outcomes between oncologic resection, surgical diversion, and self-expanding metal stents (SEMS) in left-sided malignant colorectal obstruction with curative intent.
Methods
Medline, Embase, and CENTRAL were systematically searched. Articles were included if they compared two or more of the following in patients presenting with curative left-sided malignant colorectal obstruction: (1) emergent oncologic resection; (2) surgical diversion; and/or (3) SEMS. The primary outcome was overall 90-day postoperative morbidity. Pairwise meta-analyses were performed with inverse variance random effects. Random-effect Bayesian network meta-analysis was performed.
Results
From 1277 citations, 53 studies with 9493 patients undergoing urgent oncologic resection, 1273 patients undergoing surgical diversion, and 2548 patients undergoing SEMS were included. Network meta-analysis demonstrated a significant improvement in 90-day postoperative morbidity in patients undergoing SEMS compared to urgent oncologic resection (OR0.34, 95%CrI0.01–0.98). Insufficient RCT data pertaining to overall survival (OS) precluded network meta-analysis. Pairwise meta-analysis demonstrated decreased five-year OS for patients undergoing urgent oncologic resection compared to surgical diversion (OR0.44, 95%CI0.28–0.71, p < 0.01).
Conclusions
Bridge-to-surgery interventions may offer short- and long-term benefits compared to urgent oncologic resection for malignant colorectal obstruction and should be increasingly considered in this patient population. Further prospective study comparing surgical diversion and SEMS is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is the second most common cause of cancer-related mortality worldwide and initially presents as an urgent large bowel obstruction (LBO) in 10–30% of cases [1]. There are several causes of a mechanical LBO, including diverticular stricture, volvulus, and inflammatory bowel disease, however colorectal malignancy accounts for approximately 50% of cases [1]. The management of any LBO involves urgent decompression to prevent intestinal ischemia and perforation, however choosing the most appropriate decompression method can be challenging given the need to consider underlying etiology, as well as short- and long-term outcomes. Consensus has not been reached on how to optimally care for these patients [2].
Surgical management of a malignant LBO that doesn’t require emergency surgery due to perforation or ischemia is complex and must initially consider location and potential for resection. Various options for intestinal decompression of left-sided LBOs are available and include oncologic resection with or without anastomosis, proximal diversion, and endoluminal stenting as a bridge-to-surgery. Prior to the introduction of self-expanding metallic stents (SEMS), urgent oncologic resection and surgical diversion were the most common treatment modalities for malignant LBO [3, 4]. However, over the past two decades SEMS has gained popularity as a minimally invasive method of achieving intestinal decompression [5]. Initially, SEMS were exclusively used for palliation in the setting of unresectable disease [6]. More recently they have been utilized as bridge to definitive surgery. [7,8,9,10,11]
Several randomized controlled trials (RCTs) have compared urgent oncologic resection and SEMS; some suggesting no significant difference, and others suggesting superior short-term outcomes with SEMS [7, 9, 12, 13]. Two RCTs comparing urgent oncologic resection and surgical diversion have been performed, neither of which definitively concluded superiority of either intervention [4, 14]. To our knowledge, no RCTs comparing bridge-to-surgery interventions have been conducted [15, 16]. Multiple systematic reviews and meta-analyses have also been performed with both RCT and observational data, all of which suggest SEMS and surgical diversion are safe and effective alternatives to urgent oncologic resection [17]. No study to date has compared all three treatment options in a comprehensive analysis. Therefore, the aim of the present study was to perform a network meta-analysis comparing short-term postoperative morbidity and long-term oncologic outcomes between urgent oncologic resection, proximal surgical diversion, and SEMS in left-sided malignant colorectal obstruction with curative intent.
Methods
Search strategy
The following databases covering the period from database inception through March 2021 were searched: Medline, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL). The search was designed and conducted by a medical research librarian with input from study investigators. Search terms included “malignant colonic obstruction”, “SEMS”, “colostomy”, “colectomy” and more (Table 7). The references of published studies and grey literature were searched manually to ensure that all relevant articles were included. Trial registries (e.g., ClinicalTrials.gov, EU Clinical Trials Register, etc.) were searched. This systematic review and meta-analysis was reported in accordance with the Preferred Reporting items for Systematic Reviews and Meta-Analyses (PRISMA) and the Confidence in Network Meta-Analysis (CINeMA) guidelines. [18, 19]
Study selection
Articles were eligible for inclusion if they compared short-term morbidity or long-term oncologic outcomes in patients presenting with left-sided malignant LBOs undergoing two or more of the following interventions: (1) urgent oncologic resection; (2) proximal surgical diversion (i.e., loop ileostomy or loop colostomy); and/or (3) SEMS. Randomized controlled trials, as well as prospective and retrospective observational studies were eligible for inclusion. Studies including both right- and left-sided malignant obstruction were eligible for inclusion if less than 33.3% of included patients had right-sided obstruction. Studies including both left-sided colonic malignant obstruction and rectal malignant obstruction were eligible for inclusion if less than 20% of included patients had rectal obstruction. Studies including patients managed with palliative intent were excluded unless outcomes were reported separately from patients managed with curative intent. Studies in which more than 50% of included patients had metastatic disease at the time of presentation were excluded due to a disproportionate decrease in expected long-term survival. Studies including patients undergoing interventions for benign colorectal obstruction were not eligible for analysis.
Data extraction
Two reviewers independently evaluated the systematically searched titles and abstracts using a standardized, pilot-tested form. Discrepancies that occurred at the title and abstract screening phases were resolved by inclusion of the study. At the full-text screening stage, discrepancies were resolved by consensus between the two reviewers. If the disagreement persisted, a third reviewer was consulted. Three reviewers independently conducted data extraction into a data collection form designed a priori. The extracted data included study characteristics, patient demographics, disease characteristics, treatment characteristics, short-term postoperative outcomes, and long-term oncologic outcomes.
Outcomes assessed
The primary outcome was overall postoperative morbidity. Postoperative morbidity was defined as any reported deviation from the expected postoperative course within 90 days of definitive oncologic resection. For studies including urgent oncologic resection, postoperative morbidity was reported from the index intervention, whereas for studies including bridge-to-surgery interventions, postoperative morbidity only included deviation from the expected postoperative course following the second planned intervention (i.e., definitive oncologic resection). As such, the present compared morbidity associated with oncologic resection for patients treated in the emergent setting with either oncologic resection or a bridge-to-surgery intervention (i.e., SEMS or surgical diversion). The aim, thus, was to evaluate whether emergent bridge-to-surgery interventions, were actually associated with improved postoperative outcomes or whether they were potentially unnecessary interventions.
Secondary outcomes included: (1) frequency of specific postoperative complications following definitive oncologic resection (i.e., anastomotic leak, postoperative ileus, intraabdominal abscess, sepsis, etc.); (2) overall postoperative mortality within 90 days of definitive oncologic resection; (3) number of patients requiring permanent ostomy following definitive oncologic resection; (4) long-term oncologic outcomes. Long-term oncologic outcomes included overall survival (OS), disease-free survival (DFS), local recurrence (LR), and distant recurrence (DR).
Risk of bias assessment
Risk of bias for each included RCT was assessed using the Revised Cochrane Risk of Bias Tool for RCTs [20]. The included RCTs were deemed as high, low, or unclear risk with respect to each category specified within the tool and an overall risk of bias was assigned according to a predetermined algorithm. Risk of bias for each included observational study was assessed with the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) assessment tool [21]. Similarly to RCTs, all included observational studies were judged as high, low, or unclear risk. Two reviewers assessed the studies according to these tools independently and discrepancies were discussed until consensus was reached. Only studies written in the English language were analyzed according to the aforementioned risk of bias tools.
Statistical analysis
All statistical analysis and meta-analysis were performed on R 4.0.2 (Auckland, New Zealand) and Cochrane Review Manager 5.3 (London, United Kingdom). The threshold for statistical significance was set a priori at a p of < 0.05. A pairwise meta-analyses was performed using an inverse variance random effects model for all meta-analyzed outcomes. Pooled effect estimates were obtained by calculating the mean difference (MD) in outcomes for continuous variables and odds ratios (OR) for dichotomous variables along with their respective 95% confidence intervals (CI) to confirm the effect size estimation. Mean and standard deviation (SD) was estimated for studies and studies that only reported median and interquartile range (IQR) using the estimation method described by Wan et al. [22] Assessment of heterogeneity was completed using the inconsistency (I [2]) statistic. [23]
A random-effect Bayesian network meta-analysis was performed for the primary outcome. All analyses were performed in R 4.0.2 (Auckland, New Zealand) using the “gemtc”, “rjags”, and “dmetar” packages. Estimates were obtained using the Markov Chains Monte Carlo (MCMC) method with non-informative priors. In total, 5,000 initial iterations were used as adaptation, followed by 100,000 iterations for estimations. Convergence was assessed via the Brooks-Gelman-Rubin statistic [24]. Consistency of results from direct and indirect evidence was analyzed using the node-splitting analysis of inconsistency. A rank order of treatments was derived using the mean rank and 95% credible interval (CrI) based on the estimated effect size distributions in MCMC simulations for statistically significant results and presented as a rankogram. A probability below 90% for ranking first was considered inadequate to confidently report a treatment option as the best for the given outcome [25]. Treatments were also ranked using the surface under the curve cumulative ranking probabilities (SUCRA). Within-study bias and indirectness were assessed according to CINeMA guidelines [19]. A network sensitivity analysis was performed for studies not reporting interval from bridge to surgery procedure to definitive oncologic resection. The network meta-analysis data analysis plan was designed in consultation with an independent statistician.
Results
Study characteristics
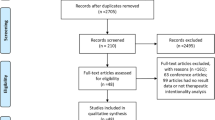
Of the 1,277 relevant citations identified, 53 studies (12 RCTs, four prospective cohorts, 37 retrospective cohorts) met inclusion criteria. Eleven studies compared urgent oncologic resection and surgical diversion, 34 studies compared urgent oncologic resection and SEMS, six studies compared surgical diversion and SEMS, and two studies compared all three interventions. A PRISMA flow diagram of the study selection process is illustrated in Supplemental Fig. 1. Across all included studies, a total of 9,493 patients underwent emergency oncologic resection (71.3%), 1273 patients underwent surgical diversion with colostomy (9.6%), and 2548 patients underwent endoluminal stenting (19.1%). Eight of the included studies, none of which were RCTs, contained patients with colon cancers proximal to the splenic flexure (1.1% of the pooled population). Six of the included studies, none of which were RCTs, contained patients with proximal rectal cancers (0.5% of the pooled population). Detailed study characteristics for included RCTs and observational studies are reported in Table 1 and Supplemental Table 1, respectively.
Treatment characteristics
All patients included in the quantitative analysis eventually underwent definitive oncologic resection. In the urgent oncologic resection group, the most commonly reported procedures were unspecified segmental colectomy (n = 6,498; 65.4%) and Hartmann’s procedure (n = 600; 6.0%). Twenty percent of urgent oncologic resections were performed laparoscopically. In the surgical diversion group, the most commonly reported interval oncologic procedures were anterior resection (n = 223; 17.5%) and left hemicolectomy (n = 208; 16.4%). Following surgical diversion as a bridge-to-surgery, 32.8% of resections were performed laparoscopically. In the SEMS group, the most commonly reported interval oncologic procedures were anterior resection (n = 472; 18.5%) and left hemicolectomy (n = 255; 10.0%). Following SEMS as a bridge-to-surgery, 48.2% of resections were performed laparoscopically. Detailed treatment characteristics for included RCTs and observational studies are reported in Table 2 and Supplemental Table 2, respectively.
Of the 42 studies that examined the use of SEMS, 34 reported stent-associated complications. The pooled rate of stent associated complications was 11.0%. The most common complication was perforation (n = 62), followed by stent migration (n = 38) and recurrent obstruction (n = 23). Twenty studies reported technical success rate. Technical success was defined as SEMS correctly placed across the malignant obstruction with fluoroscopic confirmation. The technical success rate ranged from 70.0% to 100%. Stent associated complications as reported by individual studies are presented in Table 3.
Postoperative morbidity
In total, 43 studies reported postoperative morbidity. The overall rate of postoperative morbidity was 27.8%; 26.0% in the urgent oncologic resection group, 44.8% in the surgical diversion group, and 27.2% in the SEMS group. Upon removing the study by Mabardy et al., which accounted for 75.8% of the urgent oncologic resection group, the rate of postoperative morbidity in this group was 37.2% [26]. Pairwise meta-analysis demonstrated a significantly increased rate of postoperative morbidity in patients undergoing urgent oncologic resection compared to SEMS (OR 2.14, 95%CI 1.56–2.94, p < 0.01, I2 = 69%) (Table 4). Moreover, SEMS significantly reduced the rate of postoperative morbidity compared to surgical diversion on pairwise meta-analysis (OR 0.61, 95%CI 0.39–0.94, p = 0.03, I2 = 29%). There was no significant difference in postoperative morbidity between patients undergoing urgent oncologic resection and surgical diversion as a bridge to surgery (OR 1.11, 95%CI 0.54–2.29, p = 0.78, I2 = 76%).
Bayesian network meta-analysis (Fig. 1) only including RCTs demonstrated a significant improvement in rate of postoperative morbidity in patients undergoing SEMS compared to urgent oncologic resection (OR 0.34, 95%CrI 0.01–0.98). There was no significant difference in the rate of postoperative morbidity between SEMS and surgical diversion (OR 0.52, 95%CrI 0.06–4.1) (Fig. 2). [4, 7, 9,10,11,12,13,14, 27,28,29]
Rankogram suggested a 65.3% likelihood that SEMS was the best treatment option in terms of postoperative morbidity (Fig. 3). SUCRAs were 0.82, 0.56, and 0.13 for SEMS, surgical diversion, and urgent oncologic resection, respectively (Fig. 4). A network meta-regression accounting for the high risk of bias did not significantly impact the results. Sensitivity analysis accounting for studies that failed to report the interval from bridge-to-surgery procedure to definitive oncologic resection did not alter results. Interval from bridge-to-surgery procedure to definitive oncologic resection ranged from five to 19 days.
Short-term outcomes
Detailed short-term outcomes are reported in Table 5 and Supplemental Table 3 for RCTs and observational studies, respectively. The overall rate of postoperative mortality was 4.4%; 4.2% in the urgent oncologic resection group, 6.1% in the surgical diversion group, and 4.4% in the SEMS group. Pairwise meta-analysis failed to demonstrate a significant difference between the three approaches in postoperative mortality. Similarly, there was no significant difference in anastomotic leak rate following definitive oncologic resection between the three approaches (Table 4).
Permanent stoma
Patients receiving urgent oncologic resection were significantly more likely to be left with a permanent stoma compared to patients undergoing definitive oncologic resection following SEMS (OR 2.91, 95%CI 2.10–4.04, p < 0.01, I2 = 24%). There was no significant difference in rates of permanent stoma between patients undergoing urgent oncologic resection and surgical diversion (OR 2.18, 95%CI 0.94–5.07, p = 0.07, I2 = 67%), nor between patients undergoing SEMS and surgical diversion (OR 0.64, 95%CI 0.25–1.65, p = 0.35, I2 = 51%).
Long-term oncologic outcomes
A pairwise meta-analysis demonstrated a significantly decreased five-year OS for patients undergoing urgent oncologic resection compared to patients undergoing surgical diversion (OR 0.44, 95%CI 0.28–0.71, p < 0.01, I2 = 0%). There were no statistically significant differences between urgent oncologic resection and SEMS, nor between SEMS and surgical diversion. Detailed long-term outcomes (i.e., OS, DFS, LR, DR) are reported in Table 6 and Supplemental Table 4 for RCTs and observational studies, respectively. Insufficient RCT data comparing these three treatment options in terms of OS, DFS, LR, and DR precluded a Bayesian network meta-analysis of long-term oncologic data.
Eighteen studies (four RCTs, 14 observational) compared urgent oncologic resection and SEMS, two observational studies compared urgent oncologic resection and surgical diversion, and four observational studies compared SEMS and surgical diversion in terms of the number of patients receiving adjuvant chemotherapy. Pairwise meta-analysis demonstrated a significant increase in the proportion of patients receiving adjuvant chemotherapy in the urgent oncologic resection group compared to the surgical diversion group (two studies; OR 5.39, 95%CI 1.66–17.51, p < 0.01, I2 = 0%). There were no other differences between groups on pairwise analyses and there were insufficient RCT data to complete a network meta-analysis.
Risk of bias
Within-study bias and indirectness for studies included in the network meta-analysis are reported in Figs. 5 and 6, respectively. Supplemental Fig. 2 presents the pooled risk of bias assessment for the included RCTs according to the Revised Cochrane Risk of Bias Tool for RCTs. Overall, 10 of the 12 included RCTs (83.3%) were found to be at a low risk of bias. The two studies found to be at high risk of bias were RCTs that compared the efficacy of surgical diversion and SEMS as bridges to surgery for malignant colonic obstruction (Supplemental Fig. 3).
Supplementary Figs. 4 and 5 present the risk of bias assessment of the included observational studies according to the ROBINS-I tool. Overall, 41.7% of the included observational studies were found to be at low risk of bias. The majority of studies that were found to be at high risk of bias were due to the possibility of confounding.
Discussion
The COVID-19 pandemic has caused significant and sustained interruptions in regular colorectal screening and early diagnosis of colorectal cancer; therefore, the prevalence of malignant LBOs is likely to increase [35, 36]. Several management options exist for colonic decompression and include oncologic resection with or without anastomosis, proximal diversion, and SEMs as a bridge-to-surgery. Studies have compared two approaches; however, no large-scale study has ever directly evaluated all three approaches simultaneously. This network meta-analysis pooled data from 53 studies, including 12 RCTs, comparing urgent oncologic resection, surgical diversion as a bridge-to-surgery, and/or SEMS as a bridge-to-surgery for left-sided malignant colorectal obstruction. Short-term data suggest SEMS significantly reduces 90-day postoperative morbidity as compared to urgent oncologic resection. Whereas long-term data indicate a reduction in permanent stoma rate in the SEMS group compared to urgent oncologic resection. Moreover, long-term data also demonstrate a significant improvement in five-year OS for patients managed with surgical diversion as compared to urgent oncologic resection.
Clinical management of left-sided malignant colorectal obstruction is often individualized on the basis of patient age and comorbidities, surgical intent, and available resources and subspecialty expertise [37]. Guideline recommendations vary and have evolved over recent years. The European Society of Gastrointestinal Endoscopy (ESGE) 2014 guidelines made a strong recommendation against the use of SEMS as a bridge-to-surgery [38]. However, the updated 2020 ESGE guidelines now recommend that SEMS be discussed with patients presenting with potentially curable left-sided malignant colorectal obstruction as part of a shared decision-making process [39]. The American Society of Colon and Rectal Surgeons (ASCRS) currently recommends either urgent oncologic resection or SEMS as a bridge-to-surgery on the basis of moderate quality evidence [37]. Whereas, the World Society of Emergency Surgery (WSES) guidelines state SEMS cannot be considered as part of the routine management of left-sided malignant obstruction outside of select cases and tertiary care centres. [2]
Initial study of SEMS in the setting of colorectal malignancy raised concerns regarding the risk of perforation [28]. A large European RCT comparing palliative resection and endoluminal stenting in the setting of stage IV obstructing colorectal cancer prematurely closed following preliminary analysis which demonstrated endoluminal stent associated perforation in nearly half of the enrolled patients [6]. Moreover, two of the RCTs included in the present review were discontinued prior to completion due to high rates of stent failure (i.e., technical failure or ongoing clinical obstruction following stent deployment) [9, 28]. However, since this time, numerous RCTs and large observational studies assessing bridge-to-surgery interventions have presented reassuring rates of stent-related morbidity and successful stent placement [7, 10, 13]. A 2011 Cochrane Review including five RCTs found a perforation rate of 6% as well as technically and clinically successful stent placements in approximately 80% of cases [40]. As such, rates of stent-related morbidity are likely acceptable.
Surgical diversion is a common approach for left-sided malignant colorectal obstruction in clinical practice, however current guidelines infrequently address its use [37]. Surgical diversion was more commonly used and studied prior to the advent of SEMS, but recent cohort studies have re-demonstrated their potential utility [15, 41, 42]. The present study included 11 studies comparing urgent oncologic resection and surgical diversion, and six studies comparing SEMS and surgical diversion. Network meta-analysis failed to demonstrate a difference in postoperative morbidity between surgical diversion and the other two approaches, and pairwise meta-analysis demonstrated an improvement in five-year OS as compared to urgent oncologic resection. The interval between urgent presentation and definitive oncologic resection afforded by surgical diversion allows for complete and thorough staging investigations as well as multidisciplinary evaluation and medical optimization, which can contribute to improved oncologic outcomes [41]. While these advantages are shared between surgical diversion and SEMS, surgical diversion has the added benefit of avoiding stent related complications [42]. Unfortunately, lack of prospective, randomized data comparing surgical diversion and SEMS makes it difficult to distinguish between the two approaches.
In addition to allowing for patient recovery prior to oncologic resection, bridge-to-surgery techniques convert an urgent operation into a controlled semi-elective resection. As a result, higher quality oncologic resections are performed using minimally invasive approaches and without the need for permanent stoma. The present review identified that 20% of urgent oncologic resections were performed laparoscopically, whereas 32.8% and 48.2% of oncologic resections following surgical diversion and SEMS, respectively, were performed laparoscopically. Laparoscopic resection following SEMS has been shown to be safe and feasible. [43,44,45]
Similarly, higher quality oncologic resections in a more controlled environment may improve long-term oncologic outcomes [67]. In the present study, while the data were limited and heterogeneous, there was a significant improvement in five-year OS for patients undergoing surgical diversion followed by definitive oncologic resection, as compared to the patients undergoing urgent oncologic resection. It is also possible that patients undergoing surgical diversion at the time of index presentation went on to receive neoadjuvant therapy prior to their definitive oncologic resection, which is associated with improved long-term survival in these patients [58, 61]. Given the lack of local versus distant recurrence data in the included studies, it is difficult to determine whether the quality of the oncologic resection or neoadjuvant therapies were more impactful. There were no differences between any of the other interventional comparisons. Further studies are required to confirm these findings.
The strengths of the present systematic review and network meta-analysis include the comprehensive search strategy, rigorous methodology, thorough risk of bias assessment, quality of the included evidence, number of included studies and number of patients within the included studies, and adherence to the transitivity principle [34]. Moreover, this is the first network meta-analysis comparing three treatment strategies for left-sided malignant colorectal obstruction. The study limitations include the lack of RCT evidence pertaining to long-term oncologic outcomes precluding network meta-analysis, lack of consistently reported AJCC T-stage and overall stage, variable bridge-to-surgery interval periods, variable postoperative follow-up periods, a paucity of RCTs comparing SEMS and surgical diversion, and heterogeneity amongst the included studies. The lack of AJCC stage reporting significantly impacts the interpretation of the long-term oncologic data, as this is the most significant predictor of long-term outcomes in these patients [1]. The heterogeneity amongst studies is highlighted by the high I2 statistics computed during the pairwise analyses. A number of factors likely contribute, such as different bridge-to-surgery periods, variability in reported outcomes, variable postoperative time intervals during which morbidity and mortality were recorded, variation in skill level of the treating physicians, and heterogenous follow-up. Ultimately, the network meta-analysis of included RCTs follows the transivity principle and gives us confidence that our primary outcome from the network meta-analysis can be trusted. Yet, this clinical question can still benefit from further high-quality prospective studies that have similar protocols and outcome variables. Included observational data were frequently deemed to be at high risk of bias as a result of uncontrolled confounding. For example, patients in the urgent oncologic resection group were consistently older and thus at increased risk of postoperative morbidity as compared to patients in the surgical diversion or endoluminal stenting group [3, 27, 31]. The pairwise meta-analysis pertaining to prevalence of permanent stoma in particular was likely impacted from this selection bias as older patients are less likely to undergo stoma reversal. Nonetheless, the network meta-analysis was limited to RCT data and sensitivity analyses were performed to limit any potential confounding effect on the primary outcome. Moreover, the large number of included observational studies do place our data at risk of being impacted by missing data in the primary studies. Lastly, there are limitations to the SEMS-related data that should be accounted for when interpreting the results of this study. Namely, there was variability in the types of stents used across studies, temporality may impact the results as stent-related technology has improved over the past several years, endoscopist prior experience with SEMS was seldomly reported, and technical details of SEMS placement were variably reported. As the number of patients presenting with malignant colorectal obstruction continues to grow, high quality data highlighting the strengths and weaknesses of current treatment strategies are needed.
Conclusions
This systematic review and network meta-analysis suggests that there may be short-term benefits with the use of SEMS as compared to urgent oncologic resection, as well as potential long-term benefits with the use of SEMS or surgical diversion as compared to urgent oncologic resection. Network meta-analysis did not demonstrate significant difference between either of the bridge-to-surgery approaches. Ultimately, bridge-to-surgery interventions (i.e., surgical diversion, SEMS) may offer short- and long-term benefits, with acceptable safety profiles, compared to urgent oncologic resection for malignant colorectal obstruction and thus should be increasingly considered in this patient population. Further prospective study comparing surgical diversion and SEMS as a bridge-to-surgery is needed, in addition to high quality data pertaining to long-term oncologic outcomes.
Author Contributions
TMK, JES, ZC, VA, KA, AD, DH, CE: Conception and design of the study. Generation, collection, assembly, analysis and/or interpretation of data. Drafting and revision of the manuscript. Approval of the final version of the manuscript. Agree to be accountable for all aspects of the work.
References
Yeo HL, Lee SW (2013) Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg 17(11):2007–2012. https://doi.org/10.1007/s11605-013-2343-x
Pisano M, Zorcolo L, Merli C et al (2017) WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. https://doi.org/10.1186/s13017-018-0192-3
Fielding ALP, Stewart-brown S, Blesovsky L, Fielding LP, Stewart-brown S, Blesovsky L (1979) Large-bowel obstruction caused by cancer: a prospective study. BMJ 2:515–517
Kronborg O (1995) Acute obstruction from tumour in the left colon without spread—a randomized trial of emergency colostomy versus resection. Int J Colorectal Dis 10(1):1–5. https://doi.org/10.1007/BF00337576
Kim EJ, Kim YJ (2016) Stents for colorectal obstruction: past, present, and future. World J Gastroenterol 22(2):842–852. https://doi.org/10.3748/wjg.v22.i2.842
van Hooft JE, Fockens P, Marinelli AW et al (2008) Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy 40(3):184–191. https://doi.org/10.1055/s-2007-995426
Arezzo A, Balague C, Targarona E et al (2017) Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc 31(8):3297–3305. https://doi.org/10.1007/s00464-016-5362-3
van Silfhout L, Smeekens EAJ, van Eekeren RRJP, Burger JPW (2020) Outcomes of stenting as a bridge to surgery in malignant colonic obstruction, with emphasis on perforation rate and clinical success. Surg Laparosc, Endosc Percutaneous Tech 30(4):332–338. https://doi.org/10.1097/SLE.0000000000000787
Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL (2011) Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25(6):1814–1821. https://doi.org/10.1007/s00464-010-1471-6
Ho KS, Quah HM, Lim JF, Tang CL, Eu KW (2012) Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis 27(3):355–362. https://doi.org/10.1007/s00384-011-1331-4
Tung KLM, Cheung HYS, Ng LWC, Chung CCC, Li MKW (2013) Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Sur 6(2):78–81. https://doi.org/10.1111/ases.12030
Arezzo A, Forcignanò E, Bonino MA et al (2020) Long-term oncologic results after stenting as a bridge to surgery versus emergency surgery for malignant left-sided colonic obstruction. Ann Surg 272(5):703–708. https://doi.org/10.1097/sla.0000000000004324
Ghazal AHA, El-Shazly WG, Bessa SS, El-Riwini MT, Hussein AM (2013) Colonic endolumenal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg 17(6):1123–1129. https://doi.org/10.1007/s11605-013-2152-2
Krstic S, Resanovic V, Alempijevic T et al (2014) Hartmann’s procedure vs loop colostomy in the treatment of obstructive rectosigmoid cancer. World J Emerg Surg 9(1):1–6. https://doi.org/10.1186/1749-7922-9-52
Awotar GK, Guan G, Sun W et al (2017) Reviewing the management of obstructive left colon cancer: assessing the feasibility of the one-stage resection and anastomosis after intraoperative colonic irrigation. Clin Colorectal Cancer 16(2):e89–e103. https://doi.org/10.1016/j.clcc.2016.12.001
Öistämö E, Hjern F, Blomqvist L, Falkén Y, Pekkari K, Abraham-Nordling M (2016) Emergency management with resection versus proximal stoma or stent treatment and planned resection in malignant left-sided colon obstruction. World J. Surg Oncol 14(1):1–7. https://doi.org/10.1186/s12957-016-0994-2
Amelung FJ, Mulder CLJ, Verheijen PM, Draaisma WA, Siersema PD, Consten ECJ (2015) Acute resection versus bridge to surgery with diverting colostomy for patients with acute malignant left sided colonic obstruction: systematic review and meta-analysis. Surg Oncol 24(4):313–321. https://doi.org/10.1016/j.suronc.2015.10.003
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Int Med 151(4):264–269. https://doi.org/10.1371/journal.pmed.1000097
Nikolakopoulou A, Higgins JT, Papakkonstantinou T et al (2020) CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med 17:1–19
Higgins JP, Savovic J, Page MJ, Sterne J (2019) Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane Handbook for Systematic Reviews of Interventions. Wiley, Hoboken
Sterne J, Hernan M, Reeves B, et al (2016) The Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-I) assessment tool. Cochrane Database Syst. Rev. 355:14919.
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):135. https://doi.org/10.1186/1471-2288-14-135
Higgins J, Green S. (2011) Identifying and measuring heterogeneity. In: Cochrane Handbook for Systematic Reviews of Interventions. 5.1. Wiley Inc.
Brooks S, Gelman A (1998) Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7:434–445
Dias S, Welton N, Sutton A, Ades A (2011) NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision Making. National Institute for Health and Clinical Excellence
Mabardy A, Miller P, Goldstein R, Coury J, Hackford A, Dao H (2015) Stenting for obstructing colon cancer: fewer complications and colostomies. J Soc Laparoendosc Surg 19(1):1–8. https://doi.org/10.4293/JSLS.2014.00254
Cheung HYS, Chung CC, Tsang WWC, Wong JCH, Yau KKK, Li MKW (2009) Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg 144(12):1127–1132. https://doi.org/10.1001/archsurg.2009.216
van Hooft JE, Bemelman WA, Oldenburg B et al (2011) Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 12(4):344–352. https://doi.org/10.1016/S1470-2045(11)70035-3
Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S (2011) Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 35(8):1904–1910. https://doi.org/10.1007/s00268-011-1139-y
Lee GJ, Kim HJ, Baek JH, Lee WS, Kwon KA (2013) Comparison of short-term outcomes after elective surgery following endoscopic stent insertion and emergency surgery for obstructive colorectal cancer. Int J Surg 11(6):442–446. https://doi.org/10.1016/j.ijsu.2013.04.010
White SI, Abdool SI, Frenkiel B, Braun WV (2011) Management of malignant left-sided large bowel obstruction: a comparison between colonic stents and surgery. ANZ J Surg 81(4):257–260. https://doi.org/10.1111/j.1445-2197.2010.05477.x
Rodrigues-Pinto E, Morais R, Coelho C, Pereira P, Repici A, Macedo G (2019) Bridge-to-surgery versus emergency surgery in the management of left-sided acute malignant colorectal obstruction—efficacy, safety and long-term outcomes. Dig Liver Dis 51(3):364–372. https://doi.org/10.1016/j.dld.2018.11.006
Jaffe T, Thompson W (2015) Large-bowel obstruction in the adult: classic radiographic and CT findings, etiology, and mimics. Radiology 275(3):651–663
Foote CJ, Chaudhry H, Bhandari M et al (2015) Network meta-analysis: users’ guide for surgeons: Part I—credibility. Clin Orthop Relat Res 473(7):2166–2171. https://doi.org/10.1007/s11999-015-4286-x
Bagus B, Bagus M, Ayu S, Kade M (2020) Increasing of emergency presentation on colorectal cancer patients during COVID-19 pandemic: A retrospective study on single-center academic hospital. Clin Cancer Res. https://doi.org/10.1158/1557-3265.COVID-19-PO-080
Morris EJA, Goldacre R, Spata E et al (2021) Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. https://doi.org/10.1016/S2468-1253(21)00005-4
Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR (2017) The American society of colon and rectal surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum 60(10):999–1017. https://doi.org/10.1097/DCR.0000000000000926
van Hooft JE, van Halsema EE, Vanbiervliet G et al (2014) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European society of gastrointestinal endoscopy (ESGE) clinical guideline. Gastrointest Endosc 80(5):747-761.e75. https://doi.org/10.1016/j.gie.2014.09.018
Van Hooft JE, Veld JV, Arnold D et al (2020) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European society of gastrointestinal endoscopy (ESGE) guideline—update 2020. Endoscopy 52(5):389–407. https://doi.org/10.1055/a-1140-3017
Sagar J (2011) Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Systematic Rev. https://doi.org/10.1002/14651858.cd007378.pub2
Chéreau N, Lefevre JH, Lefrancois M, Chafai N, Parc Y, Tiret E (2013) Management of malignant left colonic obstruction: is an initial temporary colostomy followed by surgical resection a better option? Colorectal Dis 15(11):646–653. https://doi.org/10.1111/codi.12335
Mege D, Sabbagh C, Manceau G et al (2019) What is the best option between primary diverting stoma or endoscopic stent as a bridge to surgery with a curative intent for obstructed left colon cancer? results from a propensity score analysis of the french surgical association multicenter cohort of 5. Ann Surg Oncol 26(3):756–764. https://doi.org/10.1245/s10434-018-07139-0
Rho SY, Bae SU, Baek SJ et al (2013) Feasibility and safety of laparoscopic resection following stent insertion for obstructing left-sided colon cancer. J Korean Surg Soc 85(6):290–295. https://doi.org/10.4174/jkss.2013.85.6.290
Fleshman J, Sargent DJ, Green E et al (2007) Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST study group trial. Ann Surg 246(4):655–662. https://doi.org/10.1097/SLA.0b013e318155a762
Tabibian N, Swehli E, Boyd A, Umbreen A, Tabibian JH (2017) Abdominal adhesions: a practical review of an often overlooked entity. Ann Med Surg 15:9–13. https://doi.org/10.1016/j.amsu.2017.01.021
Sloothaak DAM, van den Berg MW, Dijkgraaf MGW et al (2014) Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 101(13):1751–1757. https://doi.org/10.1002/bjs.9645
Huddy S, Shorthouse A, Marks C (1988) The surgical treatment of intestinal obstruction due to left sided carcinoma the colon. Ann R Coll Surg Engl 70:40–43
de Almeida A, Gracias C, dos Santos NM, Aldeia F (1991) Abordagem cirrgica da obstruo maligna, aguda, do colon esquerdo o declnio da colostomia. Acta Med Port 4(5):257–262
Tan SG, Nambiar R (1995) Resection and anastomosis of obstructed left colonic cancer: primary or staged? Aust N Z J Surg 65(10):728–731. https://doi.org/10.1111/j.1445-2197.1995.tb00546.x
Baqué P, Chevallier P, Karimdjee Solihi F et al (2004) Colostomie de décharge vs endoprothèse colique autoexpansive: comparaison des deux techniques dans l’occlusion colique gauche aig̈ue par obstacle tumoral. Ann Chir 129(6–7):353–358. https://doi.org/10.1016/j.anchir.2004.04.010
Baik S, Kim N, Cho H et al (2006) Clinical outcomes of metallic stent insertion for obstructive colorectal cancer. Hepatogastroenterology 53(68):183–187
Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JWC (2006) Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: a case-matched study. J Gastrointest Surg 10(6):798–803. https://doi.org/10.1016/j.gassur.2006.02.006
Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC (2008) Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 12(1):51–55. https://doi.org/10.1007/s10151-008-0399-5
Jiang JK, Lan YT, Lin TC et al (2008) Primary vs delayed resection for obstructive left-sided colorectal cancer: impact of surgery on patient outcome. Dis Colon Rectum 51(3):306–311. https://doi.org/10.1007/s10350-007-9173-4
Park S, Hur H, Soh Min B, Kyu KN (2016) Short-term outcomes of an extralevator abdominoperineal resection in the prone position compared with a conventional abdominoperineal resection for advanced low rectal cancer: the early experience at a single institution. Ann Coloproctol 32(1):12–19. https://doi.org/10.3393/ac.2016.32.1.12
Cennamo V, Luigiano C, Manes G et al (2012) Colorectal stenting as a bridge to surgery reduces morbidity and mortality in left-sided malignant obstruction: a predictive risk score-based comparative study. Dig Liver Dis 44(6):508–514. https://doi.org/10.1016/j.dld.2011.12.011
Guo MG, Feng Y, Zheng Q et al (2011) Comparison of self-expanding metal stents and urgent surgery for left-sided malignant colonic obstruction in elderly patients. Dig Dis Sci 56(9):2706–2710. https://doi.org/10.1007/s10620-011-1648-4
Gianotti L, Tamini N, Nespoli L et al (2013) A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc 27(3):832–842. https://doi.org/10.1007/s00464-012-2520-0
Kavanagh DO, Nolan B, Judge C et al (2013) A comparative study of short- and medium-term outcomes comparing emergent surgery and stenting as a bridge to surgery in patients with acute malignant colonic obstruction. Dis Colon Rectum 56(4):433–440. https://doi.org/10.1097/DCR.0b013e3182760506
Kim HH, Kim HK, Cho SH et al (2009) Usefulness of self-expandable metallic stents for malignant colon obstruction. J Korean Soc Coloproctol 25(2):113–116. https://doi.org/10.3393/jksc.2009.25.2.113
Sabbagh C, Browet F, Diouf M et al (2013) Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction?: a comparative study with a propensity score analysis. Ann Surg 258(1):107–115. https://doi.org/10.1097/SLA.0b013e31827e30ce
Choi JM, Lee C, Han YM et al (2014) Long-term oncologic outcomes of endoscopic stenting as a bridge to surgery for malignant colonic obstruction: comparison with emergency surgery. Surg Endosc 28(9):2649–2655. https://doi.org/10.1007/s00464-014-3517-7
Quereshy FA, Poon JTC, Law WL (2014) Long-term outcome of stenting as a bridge to surgery for acute left-sided malignant colonic obstruction. Colorectal Dis 16(10):788–793. https://doi.org/10.1111/codi.12666
van den Berg MW, Sloothaak DAM, Dijkgraaf MGW et al (2014) Bridge-to-surgery stent placement versus emergency surgery for acute malignant colonic obstruction. Br J Surg 101(7):867–873. https://doi.org/10.1002/bjs.9521
Amelung FJ, ter Borg F, Consten ECJ, Siersema PD, Draaisma WA (2016) Deviating colostomy construction versus stent placement as bridge to surgery for malignant left-sided colonic obstruction. Surg Endosc 30(12):5345–5355. https://doi.org/10.1007/s00464-016-4887-9
Consolo P, Giacobbe G, Cintolo M et al (2017) Colonic acute malignant obstructions: effectiveness of self-expanding metallic stent as bridge to surgery. Turkish J Gastroenterol 28(1):40–45. https://doi.org/10.5152/tjg.2016.0249
Ho KM, Chan KM, Kwok SY, Lau PYY (2017) Colonic self-expanding metal stent (SEMS) as a bridge to surgery in left-sided malignant colonic obstruction: an 8-year review. Surg Endosc 31(5):2255–2262. https://doi.org/10.1007/s00464-016-5227-9
Pomazkin VI (2016) Long-term results of obstructing colonic cancer. Khirurgia 9:51–56. https://doi.org/10.17116/hirurgia2016951-56
Flor-Lorente B, Báguena G, Frasson M et al (2017) Self-expanding metallic stent as a bridge to surgery in the treatment of left colon cancer obstruction: cost-benefit analysis and oncologic results. Cirugía Española (English Edition) 95(3):143–151. https://doi.org/10.1016/j.cireng.2017.03.013
Gibor U, Perry ZH, Tirosh D et al (2017) Comparison of the long-term oncological outcomes of stent as a bridge to surgery and surgery alone in malignant colonic obstruction. Israel Med Assoc J 19(12):736–740
Lim TZ, Chan DKH, Tan KK (2017) Endoscopic stenting does not worsen long term outcomes amongst patients presenting with obstruction from colorectal cancers. Ann Surg Oncol 24(6):1618–1625. https://doi.org/10.1245/s10434-016-5724-z
Shabunin A, Bagateliya Z, Dolidze D, Vardanyan A (2017) Managerial and methodological aspects of emergency surgery for malignant colonic obstruction. Khirurgiia 11:15–22
Kang SI, Oh HK, Yoo JS et al (2018) Oncologic outcomes of preoperative stent insertion first versus immediate surgery for obstructing left-sided colorectal cancer. Surg Oncol 27(2):216–224. https://doi.org/10.1016/j.suronc.2018.04.002
Morita S, Yamamoto K, Ogawa A et al (2019) Benefits of using a self-expandable metallic stent as a bridge to surgery for right- and left-sided obstructive colorectal cancers. Surg Today 49(1):32–37. https://doi.org/10.1007/s00595-018-1701-4
Jung WB, Shin JY, Park JK (2020) Comparison of short-term outcome between diverting colostomy and colonic stent as a bridge to surgery for left colonic malignant obstruction. Medicine (United States). https://doi.org/10.1097/MD.0000000000019557
Katsuki R, Jo T, Yasunaga H, Ishimaru M, Sakamoto T (2020) Outcomes of self-expandable metal stent as bridge to surgery versus emergency surgery for left-sided obstructing colon cancer: a retrospective cohort study. Am J Surg. https://doi.org/10.1016/j.amjsurg.2020.06.012
Veld JV, Amelung FJ, Borstlap WA et al (2020) Decompressing stoma a s bridge to elective surgery is an effective strategy for left-sided obstructive colon cancer. Ann Surg 272(5):738–743. https://doi.org/10.1097/sla.0000000000004173
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Drs. Tyler McKechnie, Jeremy Springer, Zacharie Cloutier, Victoria Archer, Karim Alavi, Aristithes Doumouras, Dennis Hong, and Cagla Eskicioglu have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tyler McKechnie and Jeremy E. Springer are co-first authors.
Electronic supplementary information
Below is the link to the electronic supplementary material.
Appendix 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McKechnie, T., Springer, J.E., Cloutier, Z. et al. Management of left-sided malignant colorectal obstructions with curative intent: a network meta-analysis. Surg Endosc 37, 4159–4178 (2023). https://doi.org/10.1007/s00464-023-09929-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-09929-4