Abstract
Background
In the advancement of transanal local excision, robot-assisted transanal minimal invasive surgery is the newest development. In the confined area of the rectum, robot-assisted surgery should, theoretically, be superior due to articulated utensils, video enhancement, and tremor reduction, however, this has not yet been investigated. The aim of this study was to review the evidence reported to-date on experience of using robot-assisted transanal minimal invasive surgery for treatment of rectal neoplasms.
Methods
A comprehensive literature search of Embase and PubMed from May to August 2021were performed. Studies including patients diagnosed with rectal neoplasia or benign polyps who underwent robot-assisted transanal minimal invasive surgery were included. All studies were assessed for risk of bias through assessment tools. Main outcome measures were feasibility, excision quality, and complications.
Results
Twenty-five studies with a total of 322 local excisions were included. The studies included were all retrospective, primarily case-reports, -series, and cohort studies. The median distance from the anal verge ranged from 3.5 to 10 cm and the median size was between 2.5 and 5.3 cm. Overall, 4.6% of the resections had a positive resection margin. The overall complication rate was at 9.5% with severe complications (Clavien–Dindo score III) at 0.9%.
Conclusion
Based on limited, retrospective data, with a high risk of bias, robot-assisted transanal minimal invasive surgery seems feasible and safe for local excisions in the rectum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the evolution of local excision for polyps and early-stage rectal cancers, Prof. Gerhard Buess pioneered the field in the 1980s with the introduction of transanal endoscopic microsurgery (TEM) [1]. Compared to conventional transanal excision (TAE) using anoscopic instrumentation, TEM improved the oncologic specimen quality with respect to negative resection margins (R0), decreased number of tumor fragmentation, and recurrences [2], thus limiting the need for extensive surgery such as abdominoperineal resections. Furthermore, TEM made the proximal end of the rectum accessible, a limitation to TAE [2, 3]. Regardless of the superiority, the implementation of TEM is challenged by its steep acquisition costs, the long learning curve, and its availability often limited to specialized centers [4].
The next landmark came in 2009 with the introduction of transanal minimal invasive surgery (TAMIS), a technique similar to TEM with respect to minimal invasiveness, however, using a laparoscopic platform through the anus [5]. Utilizing omnipresent standard laparoscopic instruments and transferability of operating skills already known by surgeons, TAMIS quickly gained widespread use as a valuable alternative to TEM [6]. Moreover, TAMIS does not require lesion-depending positioning and thus increased setup-time, but can be performed in lithotomy position where, if needed, abdominal access can be obtained should the peritoneum be breached unintentionally.
Yet TAMIS has several limitations. First and foremost, the rigid nature of laparoscopic instruments, a severe obstacle in the confined lumen of the rectum. Additional shortcomings include the contested space outside the anal verge where a second surgeon is needed for the camera, as well as the rigid vision provided by the laparoscope. A potential solution to the abovementioned, and the next leap forward for TAMIS, could be the introduction of robot-assisted transanal surgery. Theoretical benefits of adding a robotic platform to TAMIS should be superior oncologic excisions through fine motion scaling, increased dexterity via articulated instruments, ease of working in small spaces, and increased ergonomics for the surgeon.
Robot-assisted TAMIS (R-TAMIS) is still novel, but its feasibility has been demonstrated in cadaveric models and lately case reports from single- and multicenter studies have been published with promising results [7,8,9].
The objective of this study was to conduct a systematic review of the literature to assess the evidence reported to-date on experience using R-TAMIS for treatment of rectal neoplasms.
Materials and methods
This systematic review was reported according to the Preferred Reporting Items for systematic reviews and meta-analyses protocol (PRISMA 2020) guidelines [10]. No evidence synthesis was undertaken. Our intention was to register the current review in the PROSPERO database, however, due to a focus on COVID-19, new registrations were not allowed during the synthesis of the review. The protocol is added as supplementary data. The data extraction template and extracted data from the included studies can be made available upon request to the corresponding author.
Definition of TAMIS and R-TAMIS
TAMIS is defined as the use of conventional laparoscopic utensils, i.e., laparoscopic camera, -graspers, and -electrocautery, together with a single transanal port. Several different ports have been used to date with the SILS Port (Coviden, Mansfield, MA, USA) and the GelPOINT Path Transanal Access Platform (Applied Medical, Rancho Santa Margarita, CA, USA) the most common. TAMIS is an intentionally wide term comprising a wide range of procedures beyond local excision.
R-TAMIS is defined as a procedure with a robotic system docked through a single port transanally.
Literature search strategy
A comprehensive search in PubMed and EMBASE were performed in August 2021. The key terms used were “robot assisted OR robotic TAMIS,” “robot assisted OR robotic transanal surgery,” “robot assisted OR robotic NOTES,” and “robot assisted OR robotic transanal local excision.” The literature search included papers published from 2013 to 2021.
Selection criteria
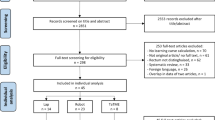
Studies including patients diagnosed with rectal neoplasia or benign polyps who underwent R-TAMIS surgery were included for screening (Fig. 1). Case-reports, patient series, clinical trials, and abstracts written in English language were included. There were no exclusion criteria.
If institutions had more publications including the same study population, the latest or most complete paper was chosen. The studies were independently screened, sought for retrieval, and reviewed by first author CJ. Extraction of data was carried out by both CJ and ANC. Disagreements with regards to data extraction were resolved by a third author. All studies were assessed for the risk of bias according to the Joanna Briggs Institutes (JBI) assessment tools for different study designs by CJ and ANC [11]. The screening, full text review, and data extraction were performed in Covidence (www.covidence.org).
Study characteristics
The following data were registered from the included studies: author, enrollment date, year of publication, country, number of patients, robot system, transanal port applied, age, BMI, gender, ASA score, distance from the anal verge, location of tumor, estimated blood loss, the duration of surgery, docking time, number of re-dockings, tumor size, lesion size, tumor pathology, depth of excision, closure of defect, positive resection margins (R1), neoadjuvant treatment, specimen fragmentation, cost, length of stay, lesion recurrence, follow-up duration, number of conversions and complications. The certainty of the evidence of each included study was assessed according to the “The GRADE working group” [12].
To enable comparison across studies, we presented continuous variables from all studies as median (range). Studies presenting insufficient data, or data in other metrics were either calculated via tables or authors were contacted per email in attempt to retrieve the missing data. Due to incomplete data presented in the studies, calculation of a median across the studies was not possible. Hence data are presented as a range of the presented medians.
Results
The literature search revealed 25 studies on R-TAMIS for local excision of rectal neoplasms, which were all included in the review. The studies comprised case- reports and series, cohort studies, and conference abstracts with a “certainty of evidence” level ranging from “very low” to “low” (Table 1). A total of 322 robot-assisted TAMIS procedures were performed across ten countries from 2013 to 2021.
Operative setup
Four different robotic systems were used, i.e., the Flex® Colorectal Drive Robotic System (10 cases) by Medrobotics corporation, Raynham, MA, USA, and four robotic systems from Intuitive Surgical, Inc., Sunnyvale, CA.; the da Vinci S, the da Vinci Si, the da Vinci Xi, and the da Vinci SP.
The primary interface was the GelPOINT™ Path Transanal Access Platform used in 20 of the 25 studies (80%). The Covidien SILS™ port was used in two studies from Arnott et al. [21] and Paull et al. [28]. A glove port was utilized in three studies, in one study together with a circular anal dilator [14].
The duration of surgery varied between 15 and 357 min, with one outlier of 552 minutes due to a conversion into a low anterior resection [34] (Table 2). Docking time was reported sporadically between 3 and 300 min [18, 34], however, the study reporting an upper range of 300 min did not provide a reason for the potential outliers in the study [18]. The second highest upper range reported was 75 min [15].
Patient demographics
The youngest patient was 22 years old [33] and the oldest 88 years [16]. BMI was between 17.8 [28] and 43.4 kg/m2 [25]. The female:male ratio was 122:125 among the included studies. The ASA score was rarely described, but for the studies that listed this, the ratio was 10:99:45:2 for ASA score I, II, III, and IV, respectively.
Specimen
Local excisions were carried out in rectum throughout its length with Marks et al. [34] reporting excisions on the edge of the anal verge and as far as 30 cm above the verge. The latter was a polypectomy located in the sigmoid colon, intussuscepted via a colonoscope to the rectum, and excised. The median distance from the anal verge was 3.5–10 cm. The position of the excisions spread among anterior (33) lateral (43) and posterior (33).
Full thickness excision was the primary depth exerted with a reported total of 264 full thickness excisions (Table 3). There was six submucosal excisions and six studies not stating resection thickness. The size of the excision was given either as cm or cm2. This ranged from 0.5 to 8.2 cm or 0.5 up to 48 cm2. The median was between 2.5 and 5.3 cm or 5.3–17.00 cm2. For the tumors removed the dimensions ranged between 1 and 6 cm, with a reported median between 2.5 and 2.8 cm. The blood loss ranged from 0 up to 185 ml [34]. Generally, the defect was closed with sutures, however, four studies describing 17 cases with defects left open [8, 15, 16, 26].
A reported total of 145 benign adenomas were excised, while adenocarcinomas accounted for 111 excisions. Of those 64 were T1, 13 were T2, 10 were T3, 13 were cCR, and 11 were unknown. The patients with a stage T2 and T3 adenocarcinoma were either staged T1 preoperatively (n = 11), not interested in more radical surgery [25, 34] or palliative [8, 16, 18, 26]. Regarding the 11 patients with a wrong perioperative staging, they were offered more radical surgery (total mesorectal excision (TME) or LAR) and/or chemoradiation therapy [8, 15, 16, 26, 25, 33]. The rest were 22 carcinoid/NET tumors [8, 21, 22, 25, 30, 34], nine excisions with complete clinical-pathological response (ypT0) [27, 33, 34], six scar polypectomies [14, 15], four GIST-tumors [25, 27, 34], four non-neoplasias [33], two rectal ulcers [30], and one submucosal leiomyoma [19].
Among the excisions, 12 cases (3.7%) had positive resection margins [8, 9, 15, 16, 25,26,27] and 304 negatives. Twelve studies stated lesion recurrence, with only two studies, Tomassi et al. and Yao et al., noticing a total of four occurrences (4 of 97 = 4.1%). Five studies, Huang et al., Marks et al., Ngu et al., Tomassi et al., and Yao et al., included a total of 23 patients receiving neoadjuvant chemo-radiation and two patients with neoadjuvant radiation therapy.
Adverse events
The overall complication rate was at 10.5%. The Clavien–Dindo score from 1 to 5 was 9:9:13:0:0. Of the 322 local excisions, 14 (4.3%) required conversions [15, 16, 21, 28, 34]. No mortality was reported. The most frequent complication was peritoneal entry (n = 7, 2.1%) [14,15,16, 18, 21, 28], four (1.2%) of them needed conversion for suturing [18, 21, 28], another two cases had laparoscopy with no positive finding [14, 16]. Post-operative bleeding was reported in six cases (1.9%) [17, 20, 25, 26, 32], where three (0.9%) required endoscopic therapy [25, 26]. There were five (1.6%) incidences of urinary retention [9, 15, 17, 20] and three (0.9%) cases with wound separation [34]. Development of abscess was reported in three (0.9%) cases [33, 34]. Arnott et al., Paull et al. and Tomassi et al. described one case each with a specimen fragmentation. Temporary anal stenosis was seen twice [21, 28]. There were two (0.6%) respiratory infections [17, 20], one case with clostridium difficile [8], one case with tenesmus [25], and one case with stool incontinence for 2 weeks [33].
LOS and costs
The length of stay varied from zero and up to 11 days [33]. Thirteen of the 25 studies reported a follow-up period ranging between no follow-up and up to 39 months described by Ngu et al. Two studies stated the costs. Lee et al. reported the median direct cost of the procedure to be 4440.92 USD (IQR 740.13 USD), while Ruiz et al. reported the total expense in materials, i.e., robotic instruments and GelPOINT™ port, per procedure to be 1889 USD.
Discussion
Since its inception, transanal minimal invasive local excision surgery of rectal neoplasms has gained widespread use due to the low morbidity and mortality compared to more radical excision, i.e., LAR or abdominoperineal resection (APR) [35]. The latest advancement for local excision transanally is R-TAMIS adding the benefits from robotic surgery. We sought to identify and summarize the latest knowledge on R-TAMIS outcomes regarding safety and feasibility. Our systematic review rendered 25 retrospective studies or case reports with a total of 322 R-TAMIS procedures performed. No information on mortality was reported.
The National Comprehensive Cancer Network (NCCN) recommends local excision for early-stage tumors within 3 to 8 cm from the anal verge, and for which there is no evidence of nodal involvement [36]. Several studies in our review, describe excisions exceeding 8 cm and a median lesion size beyond 3 cm. The larger size and distance to the anal verge than recommended by the NCCN did not increase the complication rate compared to L-TAMIS. When evaluating the number of complications for patients undergoing R-TAMIS, 10.5% suffered from complications. A large L-TAMIS series of 200 cases by Lee et al. [37] found a complication rate of 7%, and a recent published review on L-TAMIS with more than 1200 cases reported a complication rate of 18.4% [38].
Excisions in the middle, upper third or even above the rectal border are more at risk of peritoneal entry, which we found to be the most frequent and often severe complication (Clavien–Dindo ≥ III). Even though seven studies report excisions performed ≥ 15 cm from the anal verge, they were not the cases reporting peritoneal violation. The peritoneal violations that occurred, happened all ≤ 12 cm from the anal verge. When comparing peritoneal violation, the present review identified seven cases (2.1%) while Kim et al. [38] found peritoneal entry in 6.0% of the L-TAMIS procedures. The studies with peritoneal entry complications were all in the setting of full thickness excisions [34].
Regarding oncologic pathology, a positive resection margin of 3.7% was found for R-TAMIS, which is slightly lower than for L-TAMIS with a positive resection margin of 7% to 8.6% [37, 38]. Lesion fragmentation occurred in three cases (0.9%) of the R-TAMIS, which is lower than for L-TAMIS (5%) as reported by Lee et al. [37]. However, only a few studies stated follow-up time, and no study had follow-up longer than a median of two years making it difficult to properly assess oncologic quality. The studies with the longest median follow-up time were Yao et al. [30] with 23.6 months, Ngu et al. [23] with 18.2 months, Ruiz et al. [20] with 18 months, and Tomassi et al. [25] with 16.9 months. These studies had a total of four recurrences out of 97 excisions (4.1%)—similar to L-TAMIS (6%) [4, 37]. Further studies are required, to establish whether the theoretical advantages of R-TAMIS indeed result in better oncologic excisions/outcomes.
Today, no published study comparing R-TAMIS and endoscopic polypectomy (endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD)) exists. A recently published study by Witjes et al. [39] have evaluated the oncological outcomes of endoscopic excisions compared to TEM and L-TAMIS finding a R1 rate of 56% (nl = 30 patients) for EMR/ESD and 15% (n = 67) for L-TAMIS/TEM. Witjes et al. found no differences in 5 year overall survival of patients who had polypectomy, TEM/TAMIS or major resection (96% versus 90% versus 88%, respectively, p = 0.89).
R-TAMIS comes with an additional cost compared to L-TAMIS, but only a few studies include anycosts parameters. Lee et al. [9] reports a price increase of 878.9 USD compared to an average L-TAMIS procedure. Hompes et al. [15] reported a similar level of additional cost of 926.8 USD, whereas Huang et al. [27] described an even larger increase of 2000 USD.
In general, R-TAMIS is intuitively perceived as an advancement in the treatment of rectal neoplasms. Lo et al. [33] and Warren et al. [24] among others found the articulated instruments and stabilized camera particularly useful in the upper parts of the rectum. Ngu et al. [23] described the advantage of maintaining pneumorectum due to lower torque force at the ports in contrast to L-TAMIS. Ruiz et al. [20] mention having another instrument available from the transanal assistant as an advantage. Finally, Lee et al. [9] pointed toward better ergonomic, ease of suturing, and being more aggressive with the excision due to increased vision and maneuverability compared to L-TAMIS. However, R-TAMIS was also found to have limitations. Marks et al. [34] (and others) experienced the da Vinci Si & Xi robotic arms colliding due to the narrow working area and challenges dealing with long docking times. This particular problem was addressed with the da Vinci SP robotic system, hence this system is designed for single port use.
A considerable weakness of our study is the heterogeneous or insufficient data available. We strived to retrieve the missing data by contacting authors of included studies with limited success making comparison of specific parameters difficult. Furthermore, R-TAMIS is a procedure in its infancy with only limited data regarding the technique available while the sparse outcome data derive from few institutions. The presented data came primarily from case- reports or small series that by nature are highly susceptible to selection bias. The few cohort studies on this topic had a “certainty of evidence” ranging from “very low” to “low” primarily due to small sample size [40]. In accordance with the IDEAL framework [41], we find the R-TAMIS procedure placed at the end of the stage 2b, i.e., at the exploration level. The current literature on the field comes with a high risk of bias, however, all found R-TAMIS to be safe and feasible. To judge the procedures justification, the next stage according to the IDEAL framework will be to conduct large prospective cohort studies, RCTs with an L-TAMIS comparator arm as well as studies with longer follow-up to also assess oncologic quality.
References
Buess G, Kipfmüller K, Hack D, Grüßner R, Heintz A, Junginger T (1988) Technique of transanal endoscopic microsurgery. Surg Endosc 2(2):71–75. https://doi.org/10.1007/BF00704356
Clancy C, Burke JP, Albert MR, O’Connell PR, Winter DC (2015) Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum 58:254–261. https://doi.org/10.1097/DCR.0000000000000309
Moore JS, Cataldo PA, Osler T, Hyman NH (2008) Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 51:1026–1031. https://doi.org/10.1007/s10350-008-9337-x
Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S (2014) A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol 18:775–788. https://doi.org/10.1007/s10151-014-1148-6
Atallah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24:2200–2205. https://doi.org/10.1007/s00464-010-0927-z
Van den Eynde F, Jaekers J, Fieuws S, D’Hoore AM, Wolthuis AM (2019) TAMIS is a valuable alternative to TEM for resection of intraluminal rectal tumors. Tech Coloproctol 23:161–166. https://doi.org/10.1007/s10151-019-01954-7
Vallribera Valls F, Espín Bassany E, Jiménez-Gómez LM, Ribera Chavarría J, Armengol Carrasco M (2014) Robotic transanal endoscopic microsurgery in benign rectal tumour. J Robot Surg 8:277–280. https://doi.org/10.1007/s11701-013-0429-9
Liu S, Suzuki T, Murray BW, Parry L, Johnson CS, Horgan S, Ramamoorthy S, Eisenstein S (2019) Robotic transanal minimally invasive surgery (TAMIS) with the newest robotic surgical platform: a multi-institutional North American experience. Surg Endosc 33:543–548. https://doi.org/10.1007/s00464-018-6329-3
Lee SG, Russ AJ, Casillas MA (2019) Laparoscopic transanal minimally invasive surgery (L-TAMIS) versus robotic TAMIS (R-TAMIS): short-term outcomes and costs of a comparative study. Surg Endosc 33:1981–1987. https://doi.org/10.1007/s00464-018-6502-8
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021:372
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy KMPF (2020) Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z (eds) JBI Manual for Evidence Synthesis. JBI
Schünemann H, Brożek J, Guyatt G, Oxman (2013) A GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group
Bardakcioglu O (2013) Robotic transanal access surgery. Surg Endosc 27(4):1407–1409. https://doi.org/10.1007/s00464-012-2581-0
Buchs NC, Pugin F, Volonte F, Hagen ME, Morel P, Ris F (2013) Robotic transanal endoscopic microsurgery: technical details for the lateral approach. Dis Colon Rectum 56:1194–1198
Hompes R, Rauh SM, Ris F, Tuynman JB, Mortensen NJ (2014) Robotic transanal minimally invasive surgery for local excision of rectal neoplasms. Br J Surg 101:578–581. https://doi.org/10.1002/bjs.9454
Atallah S, Martin-Perez B, Parra-Davila E, deBeche-Adams T, Nassif G, Albert M, Larach S (2015) Robotic transanal surgery for local excision of rectal neoplasia, transanal total mesorectal excision, and repair of complex fistulae: clinical experience with the first 18 cases at a single institution. Tech Coloproctol 19:401–410. https://doi.org/10.1007/s10151-015-1283-8
Bocanegra MG, Ruiz MG, Fernandez CC, Martin JA, Parra JIM, Fleitas MG (2015) Robot-assisted transanal polypectomy, a new approach. Preliminary results of our series. Colorectal Dis 2015:18
Murray S, Fisher K, Johnson C (2016) Robotic transanal minimally invasive surgery, a case series. Dis Colon Rectum 59:e252–e253
Erenler I, Aytac E, Bilgin IA, Baca B, Hamzaoglu I, Karahasanoglu T (2017) Robotic transanal minimally invasive surgery (R-TAMIS) with the da Vinci Xi system—a video vignette. Colorectal Dis 19:401
Gómez Ruiz M, Cagigas Fernández C, Alonso Martín J, Cristobal Poch L, Manuel Palazuelos C, Javier Barredo Cañibano F, Gómez Fleitas M, Castillo Diego J (2017) Robotic assisted transanal polypectomies: is there any indication? Cir Esp 95:601
Arnott S, Skancke M, Obias V (2018) Robotic transanal microsurgery for high early rectal neoplasia (T0–T1, N0 lesions), case series of 10 patients. Int J Med Robot Comput Assist Surg. https://doi.org/10.1002/rcs.1956
Chang SW, Kuo LJ (2018) Robotic transanal minimally invasive surgery for a neuroendocrine rectal tumour—a video vignette. Colorectal Dis 20:936–937
Ngu JCY, Kuo LJ, Kung CH, Chen CL, Kuo CC, Chang SW, Chen CC (2018) Robotic transanal minimally invasive surgery for rectal cancer after clinical complete response to neoadjuvant chemoradiation. Int J Med Robotics Comput Assist Surg. https://doi.org/10.1002/rcs.1948
Warren CD, Hamilton AER, Stevenson ARL (2018) Robotic transanal minimally invasive surgery (TAMIS) for local excision of rectal lesions with the da Vinci Xi (dVXi): technical considerations and video vignette. Tech Coloproctol 22:529–533. https://doi.org/10.1007/s10151-018-1816-z
Tomassi MJ, Taller J, Yuhan R, Ruan JH, Klaristenfeld DD (2019) Robotic transanal minimally invasive surgery for the excision of rectal neoplasia: clinical experience with 58 consecutive patients. Dis Colon Rectum 62:279–285. https://doi.org/10.1097/DCR.0000000000001223
Baker EJ, Waters PS, Peacock O, Narasimhan V, Larach T, McCormick J, Heriot AG, Warrier S, Lynch C (2020) Robotic transanal minimally invasive surgery—technical, oncological and patient outcomes from a single institution. Colorectal Dis 22:1422–1428. https://doi.org/10.1111/codi.15045
Huang YJ, Huang YM, Wang WL, Tong YS, Hsu W, Wei PL (2020) Surgical outcomes of robotic transanal minimally invasive surgery for selected rectal neoplasms: a single-hospital experience. Asian J Surg 43:290–296. https://doi.org/10.1016/j.asjsur.2019.04.007
Paull JO, Graham A, Parascandola SA, Hota S, Pudalov N, Arnott S, Skancke M, Obias V (2020) The outcomes of two robotic platforms performing transanal minimally invasive surgery for rectal neoplasia: a case series of 21 patients. J Robot Surg 14:573–578. https://doi.org/10.1007/s11701-019-01021-1
Studniarek A, Pan J, Gantt G, Mellgren A, Giulianotti PC, Nordenstam JF (2021) Single-port robot-assisted transanal excision of rectal lesion. Dis Colon Rectum 64(2):e25. https://doi.org/10.1097/DCR.0000000000001882
Yao HL, Ngu JCY, Lin YK, Chen CC, Chang SW, Kuo LJ (2020) Robotic transanal minimally invasive surgery for rectal lesions. Surg Innov 27:181–186. https://doi.org/10.1177/1553350619892490
Hannan E, Feeney G, Ullah MF, Amin K, Coffey JC, Peirce C (2021) The first robotic transanal minimally invasive surgery in Ireland: a case-based review. Ir J Med Sci 23:3050
Liu S, Kelley SR, Behm KT (2021) Single-port robotic transanal minimally invasive surgery (SPR-TAMIS) approach to local excision of rectal tumors. Tech Coloproctol 25:229–234. https://doi.org/10.1007/s10151-020-02286-7
Lo KW, Blitzer DN, Shoucair S, Lisle DM (2022) Robotic transanal minimally invasive surgery: a case series. Surg Endosc 36:793–799. https://doi.org/10.1007/s00464-020-08257-1
Marks JH, Kunkel E, Salem JF, Martin CT, Anderson B, Agarwal S (2021) First clinical experience with single-port robotic transanal minimally invasive surgery: phase II trial of the initial 26 cases. Dis Colon Rectum. https://doi.org/10.1097/DCR.0000000000001999
You YN, Baxter NN, Stewart A, Nelson H (2007) Is the increasing rate of local excision for stage I rectal cancer in the United States justified? A nationwide cohort study from the National Cancer Database. Ann Surg 245:726–733. https://doi.org/10.1097/01.sla.0000252590.95116.4f
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Freedman-Cass DA, Gregory KM, Gurski L (2018) Rectal cancer, version 2.2018 clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw 16:874–901
Lee L, Burke JP, Debeche-Adams T, Nassif G, Martin-Perez B, Monson JRT, Albert MR, Atallah SB (2018) Transanal minimally invasive surgery for local excision of benign and malignant rectal neoplasia. Ann Surg 267:910–916. https://doi.org/10.1097/SLA.0000000000002190
Kim MJ, Lee T-G (2021) Transanal minimally invasive surgery using laparoscopic instruments of the rectum: a review. World J Gastrointest Surg 13:1149–1165. https://doi.org/10.4240/wjgs.v13.i10.1149
Witjes CDM, Patel AS, Shenoy A, Boyce S, East JE, Cunningham C (2022) Oncological outcome after local treatment for early stage rectal cancer. Surg Endosc 36:489–497. https://doi.org/10.1007/s00464-021-08308-1
OCEBM Levels of Evidence Working Group*. The Oxford Levels of Evidence 2
Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J, Rovers M, Blencowe N, Pennell C, Quinn T, Rogers W, Cook J, Kolias AG, Agha R, Dahm P, Sedrakyan A, McCulloch P (2019) No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg 269:211–220. https://doi.org/10.1097/SLA.0000000000002794
Acknowledgements
None.
Funding
No funding has been received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
P. Cornelius Jakobsen, Peter-Martin Krarup and Andreas Nordholm-Carstensen have no conflicts of interest or financial ties to disclose. Kristian K. Jensen is a speaker for Intuitive Surgical Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jakobsen, P.C.H., Krarup, PM., Jensen, K.K. et al. Robot-assisted TAMIS: a systematic review of feasibility and outcomes. Surg Endosc 37, 3398–3409 (2023). https://doi.org/10.1007/s00464-022-09853-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09853-z