Abstract
Background
Several studies report on a learning curve for robotic pancreatoduodenectomy (R-PD) ranging between 20 and 80 operations, with conversion rates varying between 1.1 and 35%. However, as these publications mostly refer to initial robotic experiences and do not take into account the previous surgical background in pancreatic surgery (PS) and in robotic-assisted surgery (RAS), the center’s volume, as well as the platform used, we aimed to perform a surgical outcomes analysis with a particular view to these aspects.
Methods
Intraoperative and perioperative outcomes of the first 50 consecutive R-PD performed with the da Vinci Xi by the same surgeon, within a tertiary referral high-volume center, between January 2018 and March 2022, were analyzed. The surgeon was previously experienced in both PS and RAS. Shewhart control chart and cumulative sum (CUSUM) analysis were used to evaluate the learning curve of R-PD.
Results
All the operations were performed with a full-robotic technique, without any conversion to open surgery. Twenty of 50 patients (40%) had a BMI ≥ 25 kg/m2, while 24/50 (48%) had undergone previous abdominal surgery. Mean console time was 276.30 ± 31.16 min. The median post-operative length of hospital stay was 10 days, while 20/50 (40%) patients were discharged within post-operative day 8. Six patients (12%) had major complications (Clavien-Dindo grade 3 or above). There was no 30-day mortality. Shewhart control chart and CUSUM analysis did not show a significant learning curve during the study period.
Conclusions
An extensive prior experience in both PS and RAS, within a tertiary referral high-volume center with availability of the da Vinci Xi platform, can significantly flatten the learning curve and, therefore, enable safe performance of challenging operations, i.e., pancreatoduodenectomies with a minimally invasive approach, with very low risk of conversion to open surgery, even in the first 50 operations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreatoduodenectomy (PD) is one of the most challenging abdominal operations, still performed mainly by an open approach. During the last twenty years, several authors have demonstrated the feasibility and safety of laparoscopic pancreatoduodenectomy (L-PD); however, in spite of the well-known benefits of the minimally invasive approach, its widespread has been limited by the steep learning curve, high conversion rates, and issues concerning surgical and oncological safety [1,2,3].
The use of the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) has over the years progressively gained popularity among pancreatic surgeons. Indeed, this technology, by providing enhanced surgical dexterity, overcame some of the limitations of laparoscopic surgery. Hence, after the initial successful application to less complex operations, the robotic approach by the da Vinci Surgical System was applied and its safe use and benefit confirmed in minimally invasive PD [4, 5].
Robotic pancreatoduodenectomy (R-PD), first described in 2003 by Giulianotti et al. [6], has been reported to provide better post-operative outcomes and a shorter hospital stay when compared to open PD (O-PD) [7], while resulting in comparable results in terms of safety and short-term oncologic outcome [8, 9].
Since then, the growing interest in robotic technology led several authors to analyze the possible advantages of the robotic assistance in surgical practice for several operations. The benefits of robotic-assisted surgery (RAS) include the short learning curve and reduced conversion rates to open surgery. Indeed, studies using CUSUM analysis of operating time (OT) to investigate the R-PD learning curve have shown that the number of R-PD needed to train a single surgeon range from 20 to 80. This number corresponds to half of the operations required for L-PD training [2]. Conversion rates for R-PD, although lower than those reported for L-PD [10, 11], remain quite high and vary widely in the reported literature, ranging from 1.1 to 35% [12, 13]. Risk factors currently associated with higher conversion rates are vascular involvement, older age (≥ 75 years), high BMI, tumor size > 40 mm, chronic pancreatitis, poor nutrition (low albumin levels), and smoking [11, 14, 15].
However, most of the published studies have overlooked the possible role of previous experience in pancreatic surgery (PS) and in RAS, of the centers’ volume, as well as of the robotic platform used.
On the hypothesis, these parameters are likely to impact on the learning curve of R-PD, and we analyzed the outcome of the first 50 R-PD performed with the da Vinci Xi, by a single surgeon working in a Tertiary Referral high-volume center for PS and for RAS.
Materials and methods
Data of the first consecutive patients who underwent R-PD by the single surgeon (LM) from January 2018 to March 2022 at our Institution were retrieved from a prospectively collected computer database and a retrospective analysis was performed. All R-PD were performed at a Multidisciplinary Tertiary Referral Center for Robotic Surgery (> 1000 robotic operations/year), and within a high-volume PS unit (> 60 operations/year). All the operations were performed by the same academic surgeon with extensive previous experience in PS (> 400 operations) and minimally invasive surgery (both laparoscopic and robotic-assisted surgery, > 800 operations). All the R-PD operations were performed with the da Vinci Xi platform. At the initial phase of the reported series, the surgeon had not performed R-PD with da Vinci Si or other robotic platforms, nor laparoscopic or minimally access PD of any kind. The only surgical exclusion criteria for minimally invasive approach were the presence of vascular involvement (borderline resectable or locally advanced tumors) and previous major open surgery in the supra-mesocolic compartment. Also, availability of the robot was another reason for exclusion as some patients with malignant tumors diagnosed during a period of low robot availability (mainly in the initial period of the series and during the COVID-19 pandemic outbreak) were operated with the conventional open surgical approach to avoid undue delays in the time interval between diagnosis and surgical resection. High BMI and other previous abdominal surgery were not considered exclusion criteria.
Pre-operative data included patient’s demographic characteristics, age, sex, BMI, American Society of Anesthesiologists (ASA) Score, comorbidities, and previous abdominal surgery. Intraoperative data included overall operative time (OT) and console time (CT), reconstructive technique, androbotic instrument used. The risk of fistula was calculated using the fistula risk score (FRS) [16]. Post-operatively, all events occurring within 90 days of surgery were considered. Post-operative pancreatic fistula (POPF) and delayed gastric emptying (DGE) were classified according to the 2016-ISGPS criteria [17], post-operative morbidity according to Clavien Dindo [18], defining as severe complications those requiring treatment under general anesthesia or intensive care (grade III and higher). Length of hospital Stay (LOS), pathological findings, and mortality were also evaluated.
The pre‐operative workup included standard plasma biochemical analysis, abdominal ultrasonography and/or endoscopy and endoscopic ultrasonography, chest radiography and/or abdomen computed tomography (CT) scan, and magnetic resonance imaging (MRI). All patients underwent pre-operative multidisciplinary discussions.
The study was approved by the Ethics Committee for Clinical Trial Area Vasta Nord-Ovest CEAVNO.
Operative technique
The open technique previously described for open PD [19] was adopted for the R-PD, with some modifications and technical refinements needed for RAS. The patient is placed supine with the legs parted and the assistant surgeon stands between the patient’s legs. Four robotic trocars are placed about 1–2 cm above the transverse umbilical line at least 8 cm from each other. The 12 mm assistant port is placed immediately below or above the umbilicus. The right mid-clavicular line trocar is used for the camera. The robot is docked from the right side of the patient. Instruments routinely used are the monopolar scissors and the endowrist vessel sealer extend for the right hand, the bipolar maryland forceps for the left hand, and a grasper for the fourth arm. Only one needle driver is used for the anastomoses. Dissection and reconstruction proceed as previously described [19, 20]. The pancreatojejunostomy is performed in all cases using a barbed suture without fashioning the “classical” Wirsung‐jejunostomy, as previously reported in detail [21]. The hepatico–jejunostomy is performed using two half running sutures of 4/0 or 5/0, barbed or non-barbed, polydioxanone sutures, depending on the surgeon’s choice. The pylorus is preferably preserved, but a distal resection of the stomach can be performed in selected cases, if necessary. Two or three gravity drains are placed at the end of the procedure. One or two drains are placed in front and behind the pancreatojejunostomy, and one is placed behind the hepatico–jejunostomy.
Perioperative care
The orotracheal and the nasogastric tubes are usually removed at the end of the procedure. The need for intensive care unit admission is selective, being evaluated at the end of the operation, but it is not routine. Prophylactic somatostatin or somatostatin analogs are not administrated routinely in the post-operative period. Evaluation of POPF and management of peritoneal drains are standardized and in accordance with latest guidelines [22]. The drain output volume is measured daily, and its amylase content is assayed on post-operative day (POD) 3 and 5, and when positive, every 3 days until drain removal. The drainage tubes are removed on POD 5 in patients judged as ISGPS grade none, negative amylase content of the drainage and without any signs of intra-abdominal infection. An abdominal ultrasound scan is performed as first-level exam in case of clinical suspicion of intra-abdominal complications and followed by CT-scan when indicated. Intra-abdominal collections caused by POPF are usually drained with an interventional ultrasound-guided procedure, or with a CT-scan-guided pigtail placement. Amylase activity is also measured in fluid samples obtained by aspiration of intra-abdominal collections or ascites. Urinary catheter is removed as soon as the patient is independently ambulant. Early and active mobilization is encouraged from POD 1 [22].
Statistical analysis
Categorical variables are enumerated as number of cases and percentages, while continuous variables were expressed as mean ± standard deviation and range or median and [25–75 percentile] or [minimum; maximum value] as appropriate, depending on their distribution.
Control chart and CUSUM analysis
The Shewhart control chart and cumulative sum (CUSUM) analysis were used to evaluate the learning curve of the procedures based on OT and on CT, being the latter considered more representative of the operative surgeon act. The same methods were also used to analyze the quality of procedure considering LOS as a proxy. Control chart for proportion was used to evaluate the occurrence of POPF and DGE in consecutive cases considering groups of five interventions.
The Shewhart control chart is a graphical display of values (or summary of values i.e., mean), of a continuous variable together with control limits, on a time-series line that allows to visually asses the pattern of a process indicator and helps determine large shift and process being out-of-control. Assuming a Gaussian distribution, control limits are usually computed at ± 3σ from the center, in the case represented by the overall mean.
On the other hand, given observations of a continuous variable x chronologically ordered, CUSUM is an iterative calculus that consisting in the running total differences between single values (xi) and their mean (μ)
differently from Shewhart chart, CUSUM chart works well even with limited number of observations and also helps easily detect even small changes. Control limits are displayed also in CUSUM chart helping identifying process out of control, and they are generally defined in terms of number of standard errors of the summary statistics, and a value of 5 is generally used.
Results
None of the 50 R-PDs performed were converted to open surgery; although 24 patients (48%) had previously undergone abdominal surgery, 17 (34%) had a BMI ≥ 25 kg/m2 and < 30 kg/m2 and 3 (6%) of them had a BMI ≥ 30 kg/m2. Pre-operative data are reported in Table 1.
Mean OT was 426.24 ± 59.11 min, and mean CT was 276.30 ± 31.16 min. Forty-two out of 50 (84%) were pylorus-preserving PD while the remaining 8 (16%) were Whipple procedures. All the operations were performed with a fully robotic technique, both for the resective and the reconstructive phases, including all anastomoses (pancreatojejunostomy, hepaticojejunostomy, and duodenojejunostomy or gastrojejunostomy). Intraoperative blood loss was negligible and no major bleeding occurred in the whole series. Patients were stratified according to the FRS in four groups: negligible risk (2 patients, 0 FRS points), low risk (10 patients, 1–2 FRS points), intermediate risk (27 patients, 3–6 FRS points), and high risk (11 patients, 7–10 FRS points). Only one patient underwent to an unplanned concomitant vascular resection, and in one case, a combined right partial nephrectomy was performed.
The median LOS was 10 days [8–18,75]. Twenty out of 50 (40%) patients were discharged within POD 8. Mean intensive care unit stay was 1.2 ± 0.76 days. Pathological diagnoses were pancreatic adenocarcinoma (n = 24), ampullary adenocarcinoma (n = 7), cholangiocarcinoma (n = 6), neuroendocrine tumor (n = 3), intraductal papillary mucinous neoplasm (n = 3), and other (n = 7). Six patients (12%) had major complications (Clavien-Dindo grade 3 or above), while only 3 (6%) developed a clinically relevant (grade B) POPF. Clinically relevant DGE was reported in 7 patients (14%). Re-operation was required in 2/50 patients (4%), due to bile leakage in one case and bleeding in the other one. There was no 30-day mortality, while the 90-day mortality rate was 2% (1/50 patients). Intra- and post-operative data are reported in Table 2 and 3.
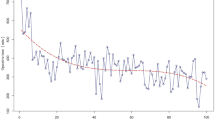
The Shewhart chart showed that for all the 50 procedures considered in the present analysis, OT and CT lied within lower (LCL) and upper control limits (UCL), suggesting no major shift from the overall mean (Fig. 1a and Fig. 2a). Similarly, the CUSUM chart highlights that for all the observations, the cumulative sum does not exceed limits (Fig. 1b and Fig. 2b), though for all the last 7 interventions, the cumulative sum were negative and, thus, suggestive of a slight but not significant reduction of OT only (Fig. 1b).
With respect to LOS, the Shewhart chart and CUSUM highlighted that just for two of the procedures performed (case number 21 and 26), the LOS was higher than the overall mean determining a temporary increase of the parameter (Fig. 3a, b).
For both POPF and DGE, no significant shift was observed over the five consecutive groups (Fig. 4). Groups of different size were also considered obtaining the same results (data not shown).
Discussion
R-PD is a challenging procedure, with varying reported rates of learning curves and exhibiting significant biases, rendering robust evaluation difficult if not impossible. Indeed, although several reported studies have analyzed learning curve for the R-PD [23, 24], scrutiny of their reported data fails to categorize their results as meaningful or robust to the robotic platforms used by centers, as well as attending surgeons at the start of their robotic experience. The Pittsburgh group in 2015 [24] reported in retrospective study on 200 consecutive patients who underwent R-PD at a large academic center, dividing them into sequential groups of 20. They reported statistical improvements in estimated blood loss and conversion to open surgery after 20 cases, incidence of pancreatic fistula after 40 cases and reduction of operative time after 80 cases (581 min vs. 417 min). Notably, their reported learning curve was representative of a pancreatic surgery team rather than a single surgeon. In addition, trainees played an increasing role throughout the experience and assumed increasing responsibilities during the study period. Furthemore, although the reported cases comprised the first 200 consecutive R-PD, these were performed between October 2008 and March 2014, hence, including results from operations performed using the precursor da Vinci master slave manipulator, the Si platform at an early stage of their robotic experience, and not exclusively with the Xi. Hence, their conclusions may noy be strictly applicable at today’s major Robotic Surgery Centers that use the Xi da Vinci platform.
Zureikat et al. [8], in a multi-institutional non-randomized study, reported 80 cases as the cutoff to obtain comparable results to the open approach in terms of surgical morbidity, risks and oncological outcomes. Chen et al. [1] also concluded that R-PD is associated with a significant learning curve, with respect to operative time, blood loss and morbidity, achieving better patient outcomes in their last 20 cases out of the entire reported series of 60 patients. Also these reports, however, refer to experiences of over an entire decade ago, therefore performed with precursor da Vinci. Moreover, although the reported series was performed by surgical teams with extensive experience in pancreatic surgery, they were also in the initial phase of their robotic-assisted experience. More recent reports [25, 26], from centers that have used the latest Xi version of the da Vinci, have certainly already identified shorter learning curves. Ryoo et al. [25] included in their study two surgeons who completed a robotic training protocol before undertaking R-PDs. The operative time of these two surgeons plateaued after 10 cases reaching the mean operating time of the series. Another study, from Schmidt et al. [26] suggests that formal robotic training facilitates a safe and efficient adoption of R-PD for new programs, significantly reducing the learning curve. In fact, OT in their analysis does not have a plateau or significant decline suggesting the absence of a true learning curve.
Our single surgeon’s experience in R-PD started with a background of extensive experience in RAS for other indications, in addition to experience in PS, within a high-volume center both for PS and for RAS. The results are comparable with those reported in the literature after the learning curve regarding all intra-operative data and post-operative clinical outcomes. Indeed, as showed by the Shewhart chart and CUSUM analysis, which are well-established methods for high-quality execution, widely used in healthcare, and considered as particularly useful for monitoring surgical quality, the OT and CT did not show a significant learning curve, as it was flattened up to be reset by the described background. The Shewhart and CUSUM charts for LOS just highlighted two values “out-of-control” and that could be explained by the inherent variability of cases treated.
This positive assessment is also enforced by the absence of conversions in the entire series. Indeed, conversion to open surgery is another key point, as well as another criterion on which the learning curve of minimally invasive procedures can be assessed, as its occurrence is associated with greater post-operative complications. Stiles et al. [10], analyzed the impact of unplanned conversion to an open procedure during minimally invasive pancreatectomy, reporting that in R-PD, conversion is associated with significantly longer operative times, greater post-operative morbidity, and 30-day mortality (> four times). In particular, patients undergoing unplanned conversion experienced significantly greater rates of pancreatic fistula, organ space infection, pneumonia, and perioperative bleeding, with results sometimes even worse than those of planned open resection. Furthermore, they identified vascular involvement/resection as a predictive factor of unplanned conversion, in accordance with previous published papers [24, 27]. Chen et al. [1] confirmed these data reporting their experience of 60 cases of R-PD with only one conversion (1.7%) due to the requirement of vein reconstruction. Boone et al.[24] reported a steep decline in conversion rate after 20 procedures from 35% to 3.3%. Reasons for conversion were failure to progress (n = 5), unexpected vascular involvement (n = 5), bleeding (n = 2), and inability to tolerate pneumoperitoneum (n = 1)[24]. In our experience, the only surgical exclusion criterion for minimally invasive approach was pre-operative evidence of vascular involvement (borderline resectable or locally advanced tumors). Therefore, only one patient, diagnosed with PDAC, needed superior mesenterico-portal vein tangential resection, performed with a full-robotic technique.
It is widely reported how the conversion to open surgery during any minimally invasive surgery is less common if procedures are performed with robotic assistance [10, 12], but, because of the complexity of pancreatoduodenectomy, to the best of our knowledge, there are no published series with zero conversion rate to date. Indeed, the conversion rate reported for R-PD remains quite high and varies widely in the literature, ranging from 1.1% [13] to 35%. However in our opinion, also in this regards, the reported data are affected by the same biases discussed earlier. Furthermore, they vary from several monocentric centers and few higher volume centers’ experiences to multi-institutional national sample with a mix of low and high-volume centers, but no one reported experiences putting together the same background reported in our experience. The international propensity score-matched comparison study published in 2020 by the European consortium on Minimally Invasive Pancreatic Surgery (E-MIPS) confirms that medium volume centers have a higher risk of conversion than high-volume centers (15.2% vs. 4.1%) [11]. The experience described in the present paper is reporting not only that of a high-volume PS center, where more than 60 pancreatectomies/years are performed, but also that of a multidisciplinary robotic surgery center, where more than 1000 procedure per year were performed. The high volume of activity, ranging from colorectal to pancreatic, from urological to gynecology and thoracic robotic procedures, have enabled the development of a major anesthesiologist service and high-quality nursing staff who are all extremely proficient with the requirements of and efficiently run da Vinci Surgical System. As previously reported, [28] this type of centralized hospital set and underpinning multidisciplinary organization should not be underestimated as it is certainly conducive to both clinical and oncological outcome, in addition to the essential, rather than desirable, the presence of experience multidisciplinary equipped not only to perform major RAS but equally proficient in dealing with complications.
Moreover, the reported case series was entirely performed with the latest released da Vinci multiport platform, the da Vinci Xi, by a surgeon who had already performed many robotically assisted operations with the Xi. To the best of our knowledge, no articles comparing different platforms for R-PD have been available so far. However, in our opinion, similarly to other indications, the da Vinci Xi with its increased flexibility can contribute to enhance the workflow of this long and complex operation [29,30,31]. In approaching the da Vinci Xi platform, the surgeon must deal with its novel aspects such as different trocar placements, robotic cart position, new functions (pointing, targeting, camera hopping, etc.), new docking system and robotic arms regulation, even if the surgeon is already expert in both laparoscopic and robotic laparoscopic pancreatic resections. Such innovative technologies help in the execution of the operation. Also its energy devices, especially the powerful and fast Vessel Sealer Extend, facilitate difficult tasks of the demolitive phase such as the retroportal lamina. Together with the improved workflow, they facilitate the execution of the entire procedure and contribute significantly to the low conversion rates. Accordingly, the results of this work are in line with the very low conversion rates previously reported for other indications [32]. Finally, it should be also noticed that the reported series was not highly selected, as the only surgical exclusion criteria for minimally invasive approach was the presence of vascular involvement or previous major open surgery in the supra-mesocolic compartment.
The retrospective nature is the main limitation of the study, together with the relatively small sample size.
In conclusion, previously published works have been very important in establishing the learning curve of something completely “new” and in a less favorable setting, but surgeons who are starting to perform this procedure, nowadays, may experience a different story. Indeed, in our opinion, with the spread of the new da Vinci Xi robotic platform, and increased experience in RAS within tertiary referral high-volume Centers, the learning curve of R-PD should be re-considered, particularly for surgeons starting their R-PDs experience from a solid background with PS.
References
Chen S, Chen JZ, Zhan Q, Deng XX, Shen BY, Peng CH (2015) Li HW (2015) robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc 2912(29):3698–3711. https://doi.org/10.1007/S00464-015-4140-Y
Napoli N, Kauffmann EF, Vistoli F, Amorese G, Boggi U (2021) State of the art of robotic pancreatoduodenectomy. Updates Surg 73:873–880. https://doi.org/10.1007/s13304-021-01058-8
Wang M, Li D, Chen R, Huang X, Li J, Liu Y, Liu J, Cheng W, Chen X, Zhao W, Li J, Tan Z, Huang H, Li D, Zhu F, Qin T, Ma J, Yu G, Zhou B, Zheng S, Tang Y, Han W, Meng L, Ke J, Feng F, Chen B, Yin X, Chen W, Ma H, Xu J, Liu Y, Lin R, Dong Y, Yu Y, Liu J, Zhang H, Qin R (2021) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 6(6):438–447. https://doi.org/10.1016/S2468-1253(21)00054-6
Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ (2013) 250 robotic pancreatic resections: safety and feasibility. Ann Surg 258:554–559. https://doi.org/10.1097/SLA.0B013E3182A4E87C
Cirocchi R, Partelli S, Trastulli S, Coratti A, Parisi A, Falconi M (2013) A systematic review on robotic pancreaticoduodenectomy. Surg Oncol 22:238–246. https://doi.org/10.1016/J.SURONC.2013.08.003
Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138:777–784. https://doi.org/10.1001/ARCHSURG.138.7.777
Zhang T, Zhao ZM, Gao YX, Lau WY, Liu R (2019) The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc 33:2927–2933. https://doi.org/10.1007/s00464-018-6595-0
Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, Weber SM, Abbott DE, Ahmad SA, Maithel SK, Hogg ME, Zenati M, Cho CS, Salem A, Xia B, Steve J, Nguyen TK, Keshava HB, Chalikonda S, Walsh RM, Talamonti MS, Stocker SJ, Bentrem DJ, Lumpkin S, Kim HJ, Zeh HJ, Kooby DA (2016) A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg 264:640–649. https://doi.org/10.1097/SLA.0000000000001869
Kornaropoulos M, Moris D, Beal EW, Makris MC, Mitrousias A, Petrou A, Felekouras E, Michalinos A, Vailas M, Schizas D, Papalampros A (2017) Total robotic pancreaticoduodenectomy: a systematic review of the literature. Surg Endosc 31:4382–4392. https://doi.org/10.1007/s00464-017-5523-z
Stiles ZE, Dickson PV, Deneve JL, Glazer ES, Dong L, Wan JY, Behrman SW (2018) The impact of unplanned conversion to an open procedure during minimally invasive pancreatectomy. J Surg Res 227:168–177. https://doi.org/10.1016/j.jss.2018.02.028
Lof S, Vissers FL, Klompmaker S, Berti S, Boggi U, Coratti A, Dokmak S, Fara R, Festen S, D’Hondt M, Khatkov I, Lips D, Luyer M, Manzoni A, Rosso E, Saint-Marc O, Besselink MG, Abu Hilal M (2021) Risk of conversion to open surgery during robotic and laparoscopic pancreatoduodenectomy and effect on outcomes: international propensity score-matched comparison study. Br J Surg 108:80–87. https://doi.org/10.1093/bjs/znaa026
Beane JD, Pitt HA, Dolejs SC, Hogg ME, Zeh HJ, Zureikat AH (2018) Assessing the impact of conversion on outcomes of minimally invasive distal pancreatectomy and pancreatoduodenectomy. HPB 20:356–363. https://doi.org/10.1016/j.hpb.2017.10.007
Shi Y, Wang W, Qiu W, Zhao S, Wang J, Weng Y, Huo Z, Jin J, Wang Y, Deng X, Shen B, Peng C (2021) Learning curve from 450 cases of robot-assisted pancreaticoduocectomy in a high-volume pancreatic center optimization of operative procedure and a retrospective study. Ann Surg 274:E1277–E1283. https://doi.org/10.1097/SLA.0000000000003664
Chao YJ, Liao TK, Su PJ, Wang CJ, Shan YS (2021) Impact of body mass index on the early experience of robotic pancreaticoduodenectomy. Updates Surg 73:929–937. https://doi.org/10.1007/s13304-021-01065-9
Nassour I, Wang SC, Porembka MR, Augustine MM, Yopp AC, Mansour JC, Minter RM, Choti MA, Polanco PM (2017) Conversion of minimally invasive distal pancreatectomy: predictors and outcomes. Ann Surg Oncol 24:3725–3731. https://doi.org/10.1245/s10434-017-6062-5
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM (2013) A Prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 216:1–14. https://doi.org/10.1016/j.jamcollsurg.2012.09.002
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M (2017) International study group on pancreatic surgery (isgps) the 2016 update of the international study group (isgps) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161(3):584–591. https://doi.org/10.1016/j.surg.2016.11.014
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Morelli L, Di Franco G, Guadagni S, Palmeri M, Furbetta N, Gianardi D, Del Chiaro M, Di Candio G, Mosca F (2017) Technical details and results of a modified end-to-side technique of pancreatojejunostomy: a personal series of 100 patients. J Gastrointest Surg. https://doi.org/10.1007/s11605-017-3587-7
Di Franco G, Lorenzoni V, Palmeri M, Furbetta N, Guadagni S, Gianardi D, Bianchini M, Pollina LE, Melfi F, Mamone D, Milli C, Di Candio G, Turchetti G, Morelli L (2021) Robot-assisted pancreatoduodenectomy with the da Vinci Xi: can the costs of advanced technology be offset by clinical advantages? A case-matched cost analysis versus open approach. Surg Endosc. https://doi.org/10.1007/s00464-021-08793-4
Morelli L, Furbetta N, Gianardi D, Guadagni S, Di Franco G, Bianchini M, Palmeri M, Masoni C, Di Candio G, Cuschieri A (2020) Use of barbed suture without fashioning the “classical” wirsung-jejunostomy in a modified end-to-side robotic pancreatojejunostomy. Surg Endosc. https://doi.org/10.1007/s00464-020-07991-w
Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, Wellge EB, Kunzler F, Besselink MG, Asbun H, Scott MJ, Dejong CHC, Vrochides D, Aloia T, Izbicki JR, Demartines N (2020) Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (eras) recommendations 2019. World J Surg 44:2056–2084. https://doi.org/10.1007/s00268-020-05462-w
Zureikat AH, Beane JD, Zenati MS, Al Abbas AI, Boone BA, Moser AJ, Bartlett DL, Hogg ME, Zeh HJ (2021) 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg 273:966–972. https://doi.org/10.1097/SLA.0000000000003550
Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, Zeh HJ, Zureikat AH (2015) Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 150:416–422. https://doi.org/10.1001/jamasurg.2015.17
Ryoo DY, Eskander MF, Hamad A, Li Y, Cloyd J, Manilchuk A, Tsung A, Pawlik TM, Dillhoff M, Schmidt C, Ejaz A (2021) Mitigation of the robotic pancreaticoduodenectomy learning curve through comprehensive training. HPB (Oxford) 23:1550–1556. https://doi.org/10.1016/J.HPB.2021.03.010
Schmidt CR, Harris BR, Musgrove KA, Rao P, Marsh JW, Thomay AA, Hogg ME, Zeh HJ, Zureikat AH, Boone BA (2021) Formal robotic training diminishes the learning curve for robotic pancreatoduodenectomy: implications for new programs in complex robotic surgery. J Surg Oncol 123:375–380. https://doi.org/10.1002/JSO.26284
Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC (2011) Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg 35:2739–2746. https://doi.org/10.1007/S00268-011-1276-3
Shyr BU, Chen SC, Shyr YM, Wang SE (2018) Learning curves for robotic pancreatic surgery-from distal pancreatectomy to pancreaticoduodenectomy. Med (United States). https://doi.org/10.1097/MD.0000000000013000
Morelli L, Di Franco G, Guadagni S, Rossi L, Palmeri M, Furbetta N, Gianardi D, Bianchini M, Caprili G, D’Isidoro C, Mosca F, Moglia A, Cuschieri A (2018) Robot-assisted total mesorectal excision for rectal cancer: case-matched comparison of short-term surgical and functional outcomes between the da Vinci Xi and Si. Surg Endosc 32:589–600. https://doi.org/10.1007/s00464-017-5708-5
Morelli L, Guadagni S, Di Franco G, Palmeri M, Caprili G, D’Isidoro C, Pisano R, Moglia A, Ferrari V, Di Candio G, Mosca F (2015) Use of the new Da Vinci Xi® during robotic rectal resection for cancer: technical considerations and early experience. Int J Colorectal Dis 30:1281–1283. https://doi.org/10.1007/s00384-015-2350-3
Morelli L, Di Franco G, Lorenzoni V, Guadagni S, Palmeri M, Furbetta N, Gianardi D, Bianchini M, Caprili G, Mosca F, Turchetti G, Cuschieri A (2019) Structured cost analysis of robotic TME resection for rectal cancer: a comparison between the da Vinci Si and Xi in a single surgeon’s experience. Surg Endosc 33:1858–1869. https://doi.org/10.1007/s00464-018-6465-9
Muaddi H, El HM, Choi WJ, Lillie E, de Mestral C, Nathens A, Stukel TA, Karanicolas PJ (2021) Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg 273:467–473. https://doi.org/10.1097/SLA.0000000000003915
Acknowledgements
The authors thank Arpa and Tizzi Foundations for the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Prof. Luca Morelli, Dr. Niccolò Furbetta, Dr. Matteo Palmeri, Dr. Simone Guadagni, Dr. Gregorio Di Franco, Dr. Desirée Gianardi, Dr. Rosa Cervelli, Dr. Valentina Lorenzoni, Dr. Annalisa Comandatore, Dr. Cristina Carpenito, Prof. Giulio Di Candio, and Prof. Alfred Cuschieri have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morelli, L., Furbetta, N., Palmeri, M. et al. Initial 50 consecutive full-robotic pancreatoduodenectomies without conversion by a single surgeon: a learning curve analysis from a tertiary referral high-volume center. Surg Endosc 37, 3531–3539 (2023). https://doi.org/10.1007/s00464-022-09784-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09784-9