Abstract
Objective of the study
In esophageal surgery, anastomotic leak (AL) remains one of the most severe and critical adverse events after oncological esophagectomy. Endoscopic vacuum therapy (EVT) can be used to treat AL; however, in the current literature, treatment outcomes and reports on how to use this novel technique are scarce. The aim of this study was to evaluate the outcomes of patients with an AL after IL RAMIE and to determine whether using EVT as an treatment option is safe and feasible.
Material and methods
This study includes all patients who developed an Esophagectomy Complications Consensus Group (ECCG) type II AL after IL RAMIE at our center between April 2017 and December 2021. The analysis focuses on time to EVT, duration of EVT, and follow up treatments for these patients.
Results
A total of 157 patients underwent an IL RAMIE at our hospital. 21 patients of these (13.4%) developed an ECCG type II AL. One patient died of unrelated Covid-19 pneumonia and was excluded from the study cohort. The mean duration of EVT was 12 days (range 4–28 days), with a mean of two sponge changes (range 0–5 changes). AL was diagnosed at a mean of 8 days post-surgery (range 2–16 days). Closure of the AL with EVT was successful in 15 out of 20 patients (75%). Placement of a SEMS (Self-expandlable metallic stent) after EVT was performed in four patients due to persisting AL. Overall success rate of anastomotic sealing independently of the treatment modality was achieved in 19 out of 20 Patients (95%). No severe EVT-related adverse events occurred.
Conclusion
This study shows that EVT can be a safe and effective endoscopic treatment option for ECCG type II AL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal malignancy is the sixth most common cause of cancer-associated deaths worldwide [1]. The current curative standard treatment is multimodality therapy combined with transthoracic esophagectomy and 2-field lymph node dissection. However, esophagectomy is a complex surgical procedure associated with substantial morbidity and mortality [2,3,4]. The evolution towards minimally invasive techniques has led to reduced cardiopulmonary complication rates and reduced pain after esophagectomy.
The acceptance of robot-assisted minimally invasive esophagectomy (RAMIE) is mostly attributed to improved clinical outcomes through better vision and dissection capabilities [5]. Although RAMIE has shown to further reduce anastomotic leak rates compared to the hybrid minimal invasive approach, anastomotic leaks (AL) are still among the most critical adverse events [6, 7]. The impact of AL is severe and can increase hospital stay, morbidity, and have a negative impact on the long-term outcome [8, 9].
Over the last few decades, the management of AL has shifted from surgery to a variety of endoscopic interventional techniques [10,11,12,13,14,15]. Using stents is an established therapeutic option, and endoscopic vacuum therapy (EVT) has become a promising alternative [16, 17]. Several studies have demonstrated high closure rates of approximately 90% [18]. EVT can be used in two different ways: intraluminal positioning of the sponge in the esophageal lumen and intracavitary placement to treat a para-esophageal wound cavity [19]. Due to the lack of comparative studies, no firm conclusions concerning the superiority of one treatment method can be drawn at this point. Overall treatment time can be short, but the sponge-system needs to be changed frequently, so repeated endoscopies are necessary.
However, reports of the use of EVT for AL especially in large cohorts and after a minimally invasive i.e., robotic techniques do not exist. Therefore, our goal was to evaluate the safety and feasibility of EVT in patients with an Esophagectomy Complications Consensus Group (ECCG) type II AL after an Ivory-Lewis (IL) RAMIE procedure at a European High-Volume Center.
Materials and methods
Patient cohort
A retrospective chart review was performed at the Department of General, Visceral, Cancer, and Transplant Surgery in cooperation with the Interdisciplinary Endoscopy Unit at the University Hospital Cologne. Patients that underwent an oncological IL RAMIE with transthoracic gastric pull-up reconstruction and presented with an ECCG AL type II were included in the analysis.
Esophageal AL is defined according to the ECCG classification as a full thickness GI defect involving esophagus, anastomosis or staple line irrespective of presentation or method of identification. The leak were further classified as, Type I: local defect requiring no change in therapy or treated medically or with dietary modification; Type II: localized defect requiring interventional but not surgical therapy; Type III: localized defect requiring surgical therapy [20].
Data were retrieved from our prospectively maintained hospital database “Orbis” (version 08,043,703; Agfa HealthCare N.V., Belgium) and from our prospectively maintained endoscopic database “Clinic WinData” (version 8.06; E&L medical system GmbH, Erlangen, Germany). The following information was collected: demographic and clinical patient characteristics, details of the disease, leak characteristics, time to EVT, duration of EVT, and follow-up treatments.
Statement of ethics
The manuscript was submitted to the local ethics committee, which stated that we are exempt from applying for ethical approval—under German law, no separate ethics application and statement of ethical approval by the local ethics committee is required for performing purely retrospective clinical studies.
Surgical approach
Ivor-Lewis robotic-assisted minimally invasive transthoracic esophagectomy (IL RAMIE) using a gastric conduit for reconstruction of the gastrointestinal passage was performed either completely robotic (robotic gastrolysis, robotic transthoracic esophagectomy) or in hybrid from, totally minimally invasive (laparoscopic gastrolysis, robotic-assisted transthoracic esophagectomy) using the Da Vinci X or the Da Vinci Xi System (Da Vinci X/Xi system, Intuitive Surgical Inc. Sunnyvale, CA, USA). An updated robotic technique using a 28 mm circular stapler (Medtronic, Covidien, EEA 28 mm DST Circular Stapler, Medtronic GmbH Meerbusch/Germany) and indocyanine green for angiography of the gastric conduit and anastomotic region was implemented in 2019. In cases with a narrow esophagus, a 25 mm circular stapling device was used. We have published our technique previously [21].
Leak detection and treatment pathway
Several diagnostic criteria are available for AL detection. First of all, clinical signs were evaluated. The symptoms for AL range from no signs to fulminant sepsis, however, patients often present with arterial fibrillation, fever, chest pain or dyspnea. Additional blood tests were performed daily. A high level of blood inflammatory biomarkers (C-reactive protein (CRP), procalcitonin, and white blood cell counts) are good indicators for an AL. Based on clinical symptoms, overall state of the patient and the biochemical analysis the choice for additional diagnostics was made. In line with the current literature, a CT scan and EGD are the standard in our clinic [22]. Patients with a suspected AL received a flexible video esophagogastroduodenoscopy (EGD) (e.g., GIF-H190; GIF-XP180N; Olympus Medical Systems, Tokyo, Japan) with photo and video documentation. All procedures were performed under sedation with propofol (e.g., Fresenius Kabi Germany GmbH) or using general anaesthesia, if performed at the intensive care unit. EGD was performed by a board certified surgical attending, with expertise in surgical endoscopy at our interdisciplinary surgical endoscopy unit, which is a certified national reference center for upper gastrointestinal cancers. The choice between EVT and surgical revision as treatment options was based on an evaluation of defect size, perfusion of the conduit, postoperative day and clinical state of the patient. Cases of early AL combined with conduit necrosis usually underwent surgical revision, while cases of late AL were treated endoscopically. However, the postoperative day was not used as a strict cut off criteria. Previous studies have shown that patients with an early anastomotic leak benefit from surgical revision [23].The decision whether the AL is managed surgically or endoscopically is made by the operating surgeon in consensus with the endoscopist who has completed surgical training and is familiar with esophageal cancer surgery. The EGD is either directly shown to the respected surgeon or video recorded. Revisional cases are further discussed in an interdisciplinary clinical conference of the surgical department, where the EGD and CT scans are shown, and the clinical state of the patient is presented.
The CT scan was performed to detect mediastinal fluid collections and an additional drain was placed if needed. An empiric antibiotic and antifungal therapy was administered immediately. After successful EVT, defined as complete closure of the defect detected by EGD, sufficient keeping of air and fluids of the gastric conduit, the EVT was removed. Additionally, a swallowing study was performed to confirm the successful treatment. The feeding was slowly started after completion with liquids and gradually increased to a normal diet within a few days.
Endoscopic vacuum therapy
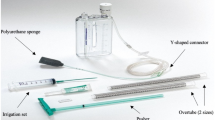
An open-pore polyurethane sponge (EsoSponge®; Braun Medical, Melsungen, Germany) was adapted in size to correspond to the extent of the leakage. The sponge was then inserted into the para-esophageal wound cavity (intracavitary) or into the esophageal lumen (intraluminal) using an overtube (Fig. 1). The placement of the sponge was performed under continuous endoscopic vision, and the area of the AL was completely covered by the Sponge. The connected tube was then repositioned to the nasal cavity. Afterwards, a defined negative pressure of 125 mm of mercury (mmHg) was applied using an electronic vacuum pump (e.g., VivanoTec®, Hartmann AG, Germany). The sponge was removed endoscopically every 3–5 days and replaced if needed. However, if the patients’ clinical status or the blood inflammatory biomarkers worsened the endoscopy and EVT change was performed immediately. Successful completion of EVT was defined as complete closure of the defect detected by EGD and sufficient keeping of air and fluids of the gastric interpona. Hence the treatment was stopped. In our manuscript we described four patients with a persisting AL. Due to a persisting leakage and insufficient closure of the anastomosis, the placement of a SEMS was indicated. SEMS placement was performed if no healing progress was seen over a course of three EVT changes.
Additional treatment
A triple-lumen diverted nasogastric feeding tube (Freka® Trelumina, Fresenius Kabi Germany GmbH) was endoscopically placed additionally right before the insertion of the EVT-device (EsoSponge®). The endoscopist made sure that the triple-lumen nasogastric feeding tube was positioned on the opposite side of the anastomotic leak so that the parallel introduced EVT had full contact to the area. The gastric tube of the triple-lumen nasogastric feeding tube was used to decompress the gastric conduit and to evacuate gastric and duodenal reflux. The jejunal tube of the triple-lumen nasogastric feeding tube secured the enteral nutrition of the patient and remained at least until the leak was fully resolved or longer if needed. We routinely place a triple-lumen nasogastric feeding tubes in addition in every patient receiving an EVT for securing an enteral feeding. We started feeding patients through the tripple-lumen diverted nasogastric feeding tube immediately after the endoscopy and started with 20 ml/h of Fresubin.
Statistics
Statistical comparison and analysis were performed using Fisher’s exact test for nominal data, and student’s t-test for continuous data. A p-value of less than 0.05 was considered statistically significant. Continuous variables are presented as means and range. Categorical data are presented as numbers and percentages. Data were analyzed by GraphPad Software (San Diego, California, USA) and Microsoft Excel Version 2013 for Windows (Microsoft Corp, Redmond, WA).
Results
From April 2017 until December 2021 a total of 157 patients underwent an IL RAMIE for cancer at our clinic. No patients showed a type I leak, 21 patients developed an ECCG type II AL (13.4%) and 8 patients developed an ECCG type III AL (5.1%). Oncological and demographic data of patients with and without an AL are depicted in Table 1. The mean BMI was 25.35 kg/m2 (range 18.52–33.90 kg/m2) in the group of patients with type II compared to a mean BMI of 25.57 kg/m2 (range 15.62–35.35 kg/m2) in the cohort without any leak, which constitutes no statistically significant difference.
Endoscopic vacuum therapy and postoperative course
All cases (21 patients) with an ECCG type II AL received EVT as a first line therapy. Two patients died prior to the completion of endoscopic therapy: one of sepsis with an unknown focus. before EVT therapy could be successfully terminated. One patient was infected with COVID 19 on POD 3 and transferred to ICU for better surveillance. During the clinical course, the patient developed an anastomotic leak which was treated with EVT. A sepsis related to the AL was not found. Additionally, as the COVID 19 pneumonia progressed multi organ failure and especially a distinctive respiratory insufficiency was seen. The patient died on POD 26 respiratory insufficiency due to the COVID 19 pneumonia. Therefore, the latter patient was excluded from the study cohort. Two patients in our cohort received a combined therapy with a hybrid stent, which combines endoluminal endoscopic vacuum therapy with SEMS treatment (VACStent®).
With EVT as mono-therapy, a closure rate was achieved in 15 out of 20 patients (75%). Placement of a SEMS after EVT was performed in 4 patients due to persisting AL. Overall success of anastomotic sealing independently of the treatment modality was achieved in 19 out of 20 Patients (95%).
ECCG Type II AL was diagnosed at a median of 8 days post-surgery (range 2–16 days). The mean duration of EVT was 12 days (range 4–28 days) with a mean of two endoscopic sponge changes (range 0–5 changes). No patient with an initial ECCG type II needed additional surgical therapy. The median postoperative length of stay was 24 days (range 16–59 days) for patients with a type II leak compared to a median of 15 days (range 10–123 days) for patients with no AL. More details about the postoperative course are depicted in Table 2.
Discussion
In this study, we present the clinical outcomes of EVT in the management of ECCG type II AL after IL RAMIE at our institution between 2017 and 2021. Our study aimed to report our method of application of EVT for anastomotic leak in patients that underwent a minimally invasive esophagectomy. The analysis showed that EVT is both safe and technically feasible. Additionally, we demonstrated excellent clinical outcomes and low morbidity of this endoscopic treatment modality for this patient cohort that includes all patients who underwent IL RAMIE at our institution.
While an open or hybrid minimally invasive transthoracic esophagectomy is still considered the gold standard for the treatment of esophageal cancer in many centers [24], minimally invasive robotic operating techniques are becoming more popular and depict the standard of care at our high-volume center. While an open technique may be associated with higher rates of postoperative complications according to recent literature, the use of RAMIE may reduce the negative impact without compromising oncological outcomes [7, 25]. Despite the lower morbidity rate of RAMIE, the occurrence of an AL is still one of the most feared life-threating complications. Timely and appropriate treatment is crucial in the management of esophageal AL [8, 9].
Our study cohort showed an ECCG AL type II in 21 of 157 patients (13.4%) after IL RAMIE. This corresponds to the data by van der Sluis et al., Pointer et al., and Egberts et al., who report a leak rate of 11, 13.5, and 13.2%, respectively [26,27,28]. Compared to the studies of the DCDP Database and the ESO Benchmark database which report an AL rate of 15 and 15.9%, IL RAMIE showed a lower AL rate. This suggests that IL RAMIE may reduce the surgical trauma and can lead to better postoperative outcomes. A large nationwide data analysis supports these findings [29].
The management of AL represents a clinical challenge with an associated risk of mortality and morbidity [30]. One minimally invasive endoscopic treatment approach for this diagnosis is the use of EVT [14]. Several studies have demonstrated high closure rates of approximately 90% with a mortality rate of 10% [18]. In our cohort, all 21 patients with an ECCG type II leak received EVT. One patient died of unrelated Covid-19 pneumonia and was excluded from the study cohort. Successful treatment was observed in 15 of 20 patients (75%) which is in line with our earlier study in which we reported a rate of 71.5% [14, 15]. Moreover, our data showed a similar mean treatment duration of 12 days (4–28 days) as our earlier study and, compared to a recently published register study by Richter et al., a shorter treatment time [15, 31]. The rate of clinical success with this short treatment duration suggests promising results compared to previous studies that focused on alternative endoscopic treatment options for AL such as SEMS treatment [13, 16]. However, the results of the ongoing phase 2 randomized trial (ESOLEAK) that compares EVT with SEMS for the treatment of AL after IL esophagectomy is expected to give us an indication of whether this trend is significant [32].
EVT can be applied in two different ways—intraluminal positioning of the sponge or intracavitary placement to treat a para-intestinal wound cavity [19]. In our series, all patients (n = 20) received an intraluminal EVT as primary treatment. In 6 out of 20 patients, the treatment method was switched from intraluminal to intracavitary because a large wound cavity developed during EVT treatment. Three of these received a SEMS as a final treatment due to the unsuccessful closure of intracavitary EVT treatment. An explanation for this may a need for additional treatment options; EVT alone may not be sufficient to treat complex and large wound cavities. Surgical or radiological drainage of any deep thoracic collections, antibiotics, and antifungal therapy should be introduced additionally in the management of these patients. So far we cannot formulate a recommendation for the ideal position of the sponge, and more studies are required to clarify the influence of the sponge position on the healing process.
Preclinical basic research focusing on the underlying mechanisms of EVT is still very limited. Morykwas et al. showed in a porcine model that an effective increase of wound blood flow was reached at a negative pressure of − 125 mmHg, which lead to optimal oxygenation, neoangiogenisis and elaboration of growth factors and consequently to a faster tissue granulation [33]. Contrary to this, Jung et al. presented a clinical trial in which they achieved a sealing rate of 78.3% in an AL subgroup analysis for the mono-therapy of EVT with a pressure between − 20 and − 50 mmHg [34]. We have used − 125 mmHg since we introduced this treatment option in our clinic [14]. Since then, we have observed that the treatment with − 125 mmHg is sufficient for sealing the leak and achieved sufficient granulation of the wound bed. Using this negative pressure, we had less technical complication in terms of dislocation of the sponge or a blocked tube. While these are promising results, a larger patient cohort is needed to verify our results concerning the ideal negative pressure.
Another factor for which we still lack enough evidence to draw firm conclusions is the question of when to exchange the sponge-system. The accepted time frame varies between 2 and 3 days. At our institution, sponge-systems were renewed every 3 to 4 days. This interval was chosen based on the availability of the endoscopy service for routine procedures on weekends. Consequently, in our study the sponge-system may have been exchanged too late. On the other hand, our clinical results confirm our treatment pathway. Overall, the efficacy of leak closure might have been even higher if we had exchanged it more often, and the optimal exchange interval has yet to be determined.
Laukoetter et al. reported that EVT-associated complications occurred in 4.1% of all interventions and with minor bleeding in 1.3% of the patients [35]. They reported two cases of fatal bleeding related to the rupture of adjacent cardiovascular structures. Minor bleeding can often be observed when the sponge is removed. To reduce the risk of this complication, we switched off the vacuum pump 2 hours before the extraction and moistened the sponge. These precautions may have contributed to the fact that no serious adverse events occurred during the EVT treatment. Additionally, the mortality rate of our cohort of patients with an AL was lower than in previously published studies (9.5% versus 26%) [36]. The reason for these positive effects could be the minimally invasive robotic surgical approach combined with a standardized minimally invasive endoscopic treatment with EVT. While these are promising results, a larger patient cohort is needed to verify our results.
Based on the results of our study, EVT seems to be a safe and feasible procedure for treating AL as there were no serious adverse events associated with the application of the device itself in our cohort.
Regarding the retrospective design, our study has certain limitations, including a lack of a comparable cohort with alternative endoscopic treatment options and a limited number of included patients. We included all patients from the very first IL RAMIE case at our institution, a fact that constitutes the inclusion of a learning curve. According to published learning curves of RAMIE and our own published data, this affects at least the first 20 patients in this collective [21, 37]. Some may see this fact as a limitation, whereas we believe this is a strength of our study. Reporting the results of an entire, standardized collective from a single high-volume center is important and insightful. Overall success rate of anastomotic sealing with purely endoscopic technology in ECCG type II AL independently of the treatment modality was achieved in 95%, a fact that underlines the effectivity of our treatment pathway.
In conclusion, our study demonstrates that using EVT for ECCG type II AL is safe und feasible. No patient required surgical reoperation and no patient died due to EVT-related complications.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G, Davies A, D’Journo XB, Gisbertz SS, Griffin SM, Hardwick R, Hoelscher A, Hofstetter W, Jobe B, Kitagawa Y, Law S, Mariette C, Maynard N, Morse CR, Nafteux P, Pera M, Pramesh CS, Puig S, Reynolds JV, Schroeder W, Smithers M, Wijnhoven BPL (2019) Benchmarking complications associated with esophagectomy. Ann Surg 269:291–298. https://doi.org/10.1097/SLA.0000000000002611
Schmidt HM, Gisbertz SS, Moons J, Rouvelas I, Kauppi J, Brown A, Asti E, Luyer M, Lagarde SM, Berlth F, Philippron A (2017) Defining benchmarks for transthoracic esophagectomy: a multicenter analysis of total minimally invasive esophagectomy in low risk patients. Ann Surg 266:814–821. https://doi.org/10.1097/SLA.0000000000002445
Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD (2008) Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 85:424–429. https://doi.org/10.1016/j.athoracsur.2007.10.007
van der Horst S, de Maat MFG, van der Sluis PC, Ruurda JP, van Hillegersberg R (2019) Extended thoracic lymph node dissection in robotic-assisted minimal invasive esophagectomy (RAMIE) for patients with superior mediastinal lymph node metastasis. Ann Cardiothorac Surg 8:218–225. https://doi.org/10.21037/acs.2019.01.04
Rutegård M, Lagergren P, Rouvelas I, Lagergren J (2012) Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 19:99–103. https://doi.org/10.1245/s10434-011-1926-6
Babic B, Müller DT, Jung J-O, Schiffmann LM, Grisar P, Schmidt T, Chon SH, Schröder W, Bruns CJ, Fuchs HF (2022) Robot-assisted minimally invasive esophagectomy (RAMIE) vs hybrid minimally invasive esophagectomy: propensity score matched short-term outcome analysis of a European high-volume center. Surg Endosc. https://doi.org/10.1007/s00464-022-09254-2
Messager M, Warlaumont M, Renaud F, Marin H, Branche J, Piessen G, Mariette C (2017) Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol EJSO 43:258–269. https://doi.org/10.1016/j.ejso.2016.06.394
Grimminger PP, Goense L, Gockel I, Bergeat D, Bertheuil N, Chandramohan SM, Chen K, Chon S, Denis C, Goh K, Gronnier C, Liu J, Meunier B, Nafteux P, Pirchi ED, Schiesser M, Thieme R, Wu A, Wu PC, Buttar N, Chang AC (2018) Diagnosis, assessment, and management of surgical complications following esophagectomy. Ann N Y Acad Sci 1434:254–273. https://doi.org/10.1111/nyas.13920
Kähler G (2017) Anastomotic leakage after upper gastrointestinal surgery: endoscopic treatment. Visc Med 33:202–206. https://doi.org/10.1159/000475783
Chon SH, Toex U, Plum PS, Kleinert R, Bruns CJ, Goeser T, Berlth F (2020) Efficacy and feasibility of overstitch suturing of leaks in the upper gastrointestinal tract. Surg Endosc 34:3861–3869. https://doi.org/10.1007/s00464-019-07152-8
Chon SH, Scherdel J, Rieck I, Lorenz F, Dratsch T, Kleinert R, Gebauer F, Fuchs HF, Goeser T, Bruns CJ (2022) A new hybrid stent using endoscopic vacuum therapy in treating esophageal leaks: a prospective single-center experience of its safety and feasibility with mid-term follow-up. Dis Esophagus. https://doi.org/10.1093/dote/doab067
Plum PS, Herbold T, Berlth F, Christ H, Alakus H, Bludau M, Chang D-H, Bruns CJ, Hölscher AH, Chon SH (2019) Outcome of self-expanding metal stents in the treatment of anastomotic leaks after ivor lewis esophagectomy. World J Surg 43:862–869. https://doi.org/10.1007/s00268-018-4832-2
Bludau M, Fuchs HF, Herbold T, Maus MKH, Alakus H, Popp F, Leers JM, Bruns CJ, Hölscher AH, Schröder W, Chon SH (2018) Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc 32:1906–1914. https://doi.org/10.1007/s00464-017-5883-4
Berlth F, Bludau M, Plum PS, Herbold T, Christ H, Alakus H, Kleinert R, Bruns CJ, Hölscher AH, Chon SH (2019) Self-expanding metal stents versus endoscopic vacuum therapy in anastomotic leak treatment after oncologic gastroesophageal surgery. J Gastrointest Surg 23:67–75. https://doi.org/10.1007/s11605-018-4000-x
Dasari BVM, Neely D, Kennedy A, Spence G, Rice P, Mackle E, Epanomeritakis E (2014) The role of esophageal stents in the management of esophageal anastomotic leaks and benign esophageal perforations. Ann Surg 259:852–860. https://doi.org/10.1097/SLA.0000000000000564
Scognamiglio P, Reeh M, Karstens K, Bellon E, Kantowski M, Schön G, Zapf A, Chon SH, Izbicki JR, Tachezy M (2020) Endoscopic vacuum therapy versus stenting for postoperative esophago-enteric anastomotic leakage: systematic review and meta-analysis. Endoscopy 52:632–642. https://doi.org/10.1055/a-1149-1741
Wichmann D, Fusco S, Werner CR, Voesch S, Duckworth-Mothes B, Schweizer U, Stüker D, Königsrainer A, Thiel K, Quante M (2022) Endoscopic management for post-surgical complications after resection of esophageal cancer. Cancers 14:980. https://doi.org/10.3390/cancers14040980
Loske G, Schorsch T, Müller C (2011) Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy 43:540–544. https://doi.org/10.1055/s-0030-1256345
Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SYK, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJB (2015) International consensus on standardization of data collection for complications associated with esophagectomy: esophagectomy complications consensus group (ECCG). Ann Surg 262:286–294. https://doi.org/10.1097/SLA.0000000000001098
Fuchs HF, Müller DT, Leers JM, Schröder W, Bruns CJ (2019) Modular step-up approach to robot-assisted transthoracic esophagectomy—experience of a German high volume center. Transl Gastroenterol Hepatol 4:62–62. https://doi.org/10.21037/tgh.2019.07.04
Fabbi M, Hagens ERC, van Berge Henegouwen MI, Gisbertz SS (2021) Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus. https://doi.org/10.1093/dote/doaa039.]
Schaheen L, Blackmon SH, Nason KS (2014) Optimal approach to the management of intrathoracic esophageal leak following esophagectomy: a systematic review. Am J Surg 208(4):536–543. https://doi.org/10.1016/j.amjsurg.2014.05.011.]
Mariette C, Markar S, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D’Journo XB, Brigand C, Perniceni T, Carrere N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G (2020) Health-related quality of life following hybrid minimally invasive versus open esophagectomy for patients with esophageal cancer, analysis of a multicenter, open-label, randomized phase iii controlled trial: the MIRO trial. Ann Surg 271:1023–1029. https://doi.org/10.1097/SLA.0000000000003559
van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, Kroese CC, Haj Mohammad N, Mook S, Vleggaar FP, Borel Rinkes IHM, Ruurda JP, van Hillegersberg R (2019) Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg 269:621–630. https://doi.org/10.1097/SLA.0000000000003031
van der Sluis PC, Babic B, Uzun E, Tagkalos E, Berlth F, Hadzijusufovic E, Lang H, Gockel I, van Hillegersberg R, Grimminger PP (2022) Robot-assisted and conventional minimally invasive esophagectomy are associated with better postoperative results compared to hybrid and open transthoracic esophagectomy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 48:776–782. https://doi.org/10.1016/j.ejso.2021.11.121
Pointer DT, Saeed S, Naffouje SA, Mehta R, Hoffe SE, Dineen SP, Fleming JB, Fontaine JP, Pimiento JM (2020) Outcomes of 350 robotic-assisted esophagectomies at a high-volume cancer center: a contemporary propensity-score matched analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000004317
Egberts J-H, Welsch T, Merboth F, Korn S, Praetorius C, Stange DE, Distler M, Biebl M, Pratschke J, Nickel F, Müller-Stich B, Perez D, Izbicki JR, Becker T, Weitz J (2022) Robotic-assisted minimally invasive Ivor Lewis esophagectomy within the prospective multicenter German da Vinci Xi registry trial. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-022-02520-w
Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H (2021) Comparing perioperative mortality and morbidity of minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: a nationwide retrospective analysis. Ann Surg 274:324–330. https://doi.org/10.1097/SLA.0000000000003500
Chevallay M, Jung M, Chon SH, Takeda FR, Akiyama J, Mönig S (2020) Esophageal cancer surgery: review of complications and their management. Ann N Y Acad Sci 1482:146–162. https://doi.org/10.1111/nyas.14492
Richter F, Hendricks A, Schniewind B, Hampe J, Heits N, von Schönfels W, Reichert B, Eberle K, Ellrichmann M, Baumann P, Egberts J-H, Becker T, Schafmayer C (2022) Eso-Sponge® for anastomotic leakage after oesophageal resection or perforation: outcomes from a national, prospective multicentre registry. BJS Open. https://doi.org/10.1093/bjsopen/zrac030
Tachezy M, Chon SH, Rieck I, Kantowski M, Christ H, Karstens K, Gebauer F, Goeser T, Rösch T, Izbicki JR, Bruns CJ (2021) Endoscopic vacuum therapy versus stent treatment of esophageal anastomotic leaks (ESOLEAK): study protocol for a prospective randomized phase 2 trial. Trials 22:377. https://doi.org/10.1186/s13063-021-05315-4
Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38:553–562. https://doi.org/10.1097/00000637-199706000-00001
Jung CFM, Hallit R, Müller-Dornieden A, Calmels M, Goere D, Chaput U, Camus M, Gonzalez JM, Barthet M, Jacques J, Legros R, Barrioz T, Kück F, Seif Amir Hosseini A, Ghadimi M, Kunsch S, Ellenrieder V, Wedi E, Barret M (2022) Endoscopic internal drainage and low negative-pressure endoscopic vacuum therapy for anastomotic leaks after oncologic upper gastrointestinal surgery. Endoscopy 54:71–74. https://doi.org/10.1055/a-1375-8151
Laukoetter MG, Mennigen R, Neumann PA, Dhayat S, Horst G, Palmes D, Senninger N, Vowinkel T (2017) Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 31:2687–2696. https://doi.org/10.1007/s00464-016-5265-3
Schniewind B, Schafmayer C, Voehrs G, Egberts J, von Schoenfels W, Rose T, Kurdow R, Arlt A, Ellrichmann M, Jürgensen C, Schreiber S, Becker T, Hampe J (2013) Endoscopic endoluminal vacuum therapy is superior to other regimens in managing anastomotic leakage after esophagectomy: a comparative retrospective study. Surg Endosc 27:3883–3890. https://doi.org/10.1007/s00464-013-2998-0
Kingma BF, Hadzijusufovic E, Van der Sluis PC, Bano E, Lang H, Ruurda JP, van Hillegersberg R, Grimminger PP (2020) A structured training pathway to implement robot-assisted minimally invasive esophagectomy: the learning curve results from a high-volume center. Dis Esophagus. https://doi.org/10.1093/dote/doaa047
Acknowledgements
We would like to thank all the nurses in the endoscopy department of the University Hospital of Cologne for their expert technical assistance. We thank Dr. phil. Lisa-Marie Teubler for proofreading.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Univ.-Prof. Dr. HF Fuchs and Univ.-Prof. Dr. CJ Bruns are members of the advisory board for Medtronic and received an educational grant from Intuitive (ESOMAP trial). Univ.-Prof. Dr. HF Fuchs is an advisor for Activ Surgical, Stryker, and Dexter and holds stock options of Fortimedix Surgical.Seung-Hun Chon, Stefanie Brunner, Dolores T Müller, Florian Lorenz, Raphael Stier, Lea Streller, Jennifer Eckhoff, Jennifer Straatman, Benjamin Babic, Lars M. Schiffmann,Wolfgang Schröder, Thomas Schmidt, Christiane J Bruns, Hans F Fuchs have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chon, SH., Brunner, S., Müller, D.T. et al. Time to endoscopic vacuum therapy—lessons learned after > 150 robotic-assisted minimally invasive esophagectomies (RAMIE) at a German high-volume center. Surg Endosc 37, 741–748 (2023). https://doi.org/10.1007/s00464-022-09754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09754-1