Abstract
Background
Many centers worldwide are shifting from laparoscopic to robotic minimally invasive hepato-pancreato-biliary resections (MIS-HPB) but large single center series assessing this process are lacking. We hypothesized that the introduction of robot-assisted surgery was safe and feasible in a high-volume center.
Methods
Single center, post-hoc assessment of prospectively collected data including all consecutive MIS-HPB resections (January 2010–February 2022). As of December 2018, all MIS pancreatoduodenectomy and liver resections were robot-assisted. All surgeons had participated in dedicated training programs for laparoscopic and robotic MIS-HPB. Primary outcomes were in-hospital/30-day mortality and Clavien-Dindo ≥ 3 complications.
Results
Among 1875 pancreatic and liver resections, 600 (32%) were MIS-HPB resections. The overall rate of conversion was 4.3%, Clavien-Dindo ≥ 3 complications 25.7%, and in-hospital/30-day mortality 1.8% (n = 11). When comparing the period before and after the introduction of robotic MIS-HPB (Dec 2018), the overall use of MIS-HPB increased from 25.3 to 43.8% (P < 0.001) and blood loss decreased from 250 ml [IQR 100–500] to 150 ml [IQR 50–300] (P < 0.001). The 291 MIS pancreatic resections included 163 MIS pancreatoduodenectomies (52 laparoscopic, 111 robotic) with 4.3% conversion rate. The implementation of robotic pancreatoduodenectomy was associated with reduced operation time (450 vs 361 min; P < 0.001), reduced blood loss (350 vs 200 ml; P < 0.001), and a decreased rate of delayed gastric emptying (28.8% vs 9.9%; P = 0.009). The 309 MIS liver resections included 198 laparoscopic and 111 robotic procedures with a 3.6% conversion rate. The implementation of robotic liver resection was associated with less overall complications (24.7% vs 10.8%; P = 0.003) and shorter hospital stay (4 vs 3 days; P < 0.001).
Conclusion
The introduction of robotic surgery was associated with greater implementation of MIS-HPB in up to nearly half of all pancreatic and liver resections. Although mortality and major morbidity were not affected, robotic surgery was associated with improvements in some selected outcomes. Ultimately, randomized studies and high-quality registries should determine its added value.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Minimally invasive surgery (MIS) has become routine practice in most types of gastrointestinal surgery. After a slow adoption in hepato-pancreato-biliary (HPB) surgery in the 1990s and 2000s [1], MIS-HPB has seen a rapid development in high-volume centers. Over the last decade the use of robotic surgery is increasing rapidly.
For MIS distal pancreatectomy, two randomized trials reported faster functional recovery, shorter hospital stay, and less blood loss after the laparoscopic as compared to the open approach [2, 3]. Therefore, the recent Miami guidelines now advise MIS distal pancreatectomy for benign and low-grade malignant tumors. For laparoscopic pancreatoduodenectomy, four randomized trials showed shorter hospital stay although safety concerns were reported in one multicenter randomized trial [3,4,5,6]. The Miami guidelines advice a minimum annual center volume of 20 MIS pancreatoduodenectomies [7]. Good outcomes were reported from (mostly single) high volume centers for robotic pancreatoduodenectomy but randomized trials are lacking [8,9,10,11].
For MIS liver surgery, two randomized trials reported less complications and shorter hospital stay after laparoscopic resection of colorectal liver metastases [12, 13]. The Southampton consensus guidelines recommend laparoscopy as standard of care for minor liver resections [14]. Again, good outcomes were reported from (mostly single) high volume centers for robotic MIS liver surgery but randomized studies are lacking [15,16,17,18,19,20].
However, MIS-HPB resections are technically demanding and associated with long learning curves [21, 22] requiring structured training programs [7, 14] which were implemented in the Netherlands for MIS pancreas resections [8, 23, 24] and liver resections [25]. Robotic surgery may facilitate surgeons when performing the relatively challenging MIS-HPB resections but studies focusing on a HPB unit perspective (i.e., including both pancreatic and liver resections) are lacking. We hypothesize that the introduction of robot-assisted surgery might be safe and feasible and might enhance the overall implementation of MIS-HPB resections.
Materials and methods
This is a single center study with post-hoc assessment of prospectively maintained database of all MIS-HPB resections, both laparoscopic and robot-assisted (hereafter: robotic) surgery between January 2010 and February 2022 at Amsterdam UMC. All consecutive procedures were included, including the first procedures for each type of resection. Most procedures were performed at the AMC hospital of Amsterdam UMC (January 2010 – May 2021). In June 2021, the AMC team moved to and merged with the HPB team in the VUMC hospital of Amsterdam UMC and the first 8 months hereafter were also included (June 2021 – February 2022). Inclusion ended when 600 MIS-HPB resections (i.e., including conversions) had been performed. All data were collected from the mandatory and anonymized, prospective Dutch Pancreatic Cancer Audit (DPCA) and Dutch Hepatobiliary Audit (DHBA) registries. The study was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [26]. The ethical committee of the Amsterdam UMC decided that ethical approval was not needed for this study since participants were not subject to additional interventions. Hence, no written informed consent was collected.
Eligibility criteria
Included were all MIS-HPB resections for which indications did not change during the study period. For MIS pancreatic resections this included laparoscopic and robotic pancreatoduodenectomy and distal pancreatectomy. Indications for MIS pancreatoduodenectomy included tumors < 8 cm, absence of portomesenteric vein or arterial involvement (3-5 mm free margin) on a computed tomography of maximum 4 weeks old, absence of chronic pancreatitis, and a BMI < 35 kg/m2. Indications for MIS distal pancreatectomy included tumors < 8 cm, absence of portomesenteric vein and celiac trunc involvement (3–5 mm free margin) on a computed tomography of maximum 4 weeks old.
MIS liver resections included both minor and major laparoscopic and robotic liver resections. Indications for MIS liver resections include tumors < 10 cm, absence of portal vein involvement (5-10 mm free margin) in the first 50 procedures, absence of caval vein involvement, and absence of need for a hilar resection. Previous abdominal surgery was not a contra-indication for MIS-HPB surgery. All MIS-HPB drainage surgery (such as for acute pancreatitis, chronic pancreatitis, liver cysts) and less common resections such as MIS central pancreatic and MIS total pancreatectomy were excluded from the current analysis. Patient undergoing conversion were included according to the intention-to-treat principle.
Surgical techniques
The techniques of laparoscopic and robotic MIS pancreatoduodenectomy and MIS distal pancreatectomy have previously been described in the LAELAPS-1, -2, and -3 training programs [8, 23, 24]. The da Vinci® Xi Robotic Surgical System (Intuitive Surgical®, Inc., Sunnyvale, CA, USA) was used since December 2018 for all MIS pancreatoduodenectomy procedures and essentially all MIS liver resections. In all robotic procedures, the table-side surgeon has a large standalone 3D-display on the patients’ right side. For laparoscopic liver resection, as previously described, a standardized approach was used [25]. For robotic liver resection, superficial parenchymal transection was performed by monopolar cautery and a vessel sealer. For deep parenchymal transection, a vessel sealer was used in combination with a robotic Maryland bipolar device. Indocyanine green (ICG) fluorescence imaging was administered perioperatively for different applications: 24 h prior to surgery for tumor imaging, within 1 h prior to surgery for biliary tract mapping, and intra-operatively for liver perfusion assessment. Vascular and biliary structures were divided between Hem-o-Lok clips (Weck Closure Systems, Research Triangle Park, USA) or a vessel sealer, or endoscopic stapling as required. Anesthetic management generally involved a restrictive intravenous fluid approach during liver transection combined with a low central venous pressure. Pringle was used on demand. Specimens were extracted in a plastic endoscopic bag via a widened trocar incision for small lesions or a Pfannenstiel incision for larger lesions or anatomically major resections.
Training surgical team
For laparoscopic pancreatoduodenectomy, which started in 2014, the surgical team consisted of 2 (out of 3) staff surgeons who all participated also in the LAELAPS-2 and -3 training programs [8, 24]. Per December 2018, 4 staff surgeons participated in the LAELAPS-3 training program for robotic pancreatoduodenectomy. Again, the surgical team per procedure involved 2 (out of 4) staff surgeons with one surgeon (MGB) present during all procedures to minimize the learning curve effect. Surgeons switched roles between the resection- and reconstruction phase. For MIS distal pancreatectomy, the surgical team involved 1 (out of 2) staff surgeons who participated also in the LAELAPS-1 training program surgeons, along with a fellow or senior resident [23]. Laparoscopic liver resection, which started in 2010, was performed by 1 or 2 (out of 4) staff surgeons, who participated also in the LAELIVE training program, along with a fellow or senior resident [27]. Per December 2018, 3 staff surgeons started with robotic liver resection. The surgical team involved 1 or 2 (out of 3) staff surgeons, along with a fellow or senior resident [23]. One surgeon (RJS) was present during all robotic resections to minimize the learning curve.
Data collection and definitions
Baseline patient characteristics included age, sex, body mass index (BMI, kg/m2), American Society of Anaesthesiologists (ASA) grade, cirrhosis, neoadjuvant chemotherapy, previous extrahepatic abdominal surgery, previous liver surgery and origin of the tumor. Tumor and procedure characteristics included histological diagnosis, number of lesions, size of the largest lesion, type of resection (minor, technically major, anatomically major resection [28]), and extent of resection.
Operative outcome included 30-day overall postoperative complications (defined according to the Clavien-Dindo classification; severe/major complications were defined as Clavien-Dindo grade III or higher) [29], 30-day readmission, 30-day reoperation, postoperative length of hospital stay and 30-day/in hospital mortality and, according to the International Study Group of Pancreatic Surgery (ISGPS) grade B/C definitions: pancreatic fistula (POPF) [30], delayed gastric emptying (DGE) [31], and post-pancreatectomy hemorrhage (PPH) [32]. Postoperative bile leakage grade B/C was defined according to the International Study Group of Liver Surgery [33].
Minor liver resection was classified as any resection from the anterolateral segments, i.e., 2, 3, 4b, 5, and 6 [28]. Anatomically major liver resection included resection of three or more Couinaud’s segments [28]. Technically major liver resection was considered any resection from the posterosuperior segments, i.e., 7, 8, 4a and 1 [28].
Outcome measures
The primary outcomes were in-hospital/30-day mortality and severe/major morbidity (≥ Grade 3a) according to the Clavien-Dindo classification [29]. Secondary outcomes included operative time, length of stay, blood loss, conversion, and R0/R1 resection margin.
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows version 27.0 (IBM Corp., Orchard Road Armonk, New York, US). Student’s t, Mann Whitney U, Chi-square, or Fisher’s exact tests will be used as appropriate. Categorical data are presented as proportions, continuous data are presented as either mean and standard deviation or median and inter-quartile-range as appropriate.
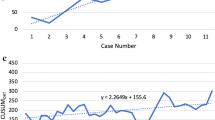
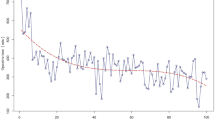
The learning effects were assessed by determining the correlation between consecutive procedures number (binning of 10 procedures) and the learning curve outcomes as determined by Müller et al.: (1) Competency: decrease in operative time; (2) Proficiency: decrease in major morbidity and mortality; and (3) Mastery: increase in textbook outcome. When a significant correlation was found, a CUSUM analysis was used to assess the learning curve. The top of the CUSUM graph thus represented the total operative time expressed in standard deviations above average up to that case. When interpreting the CUSUM graph, ‘slope’ is the informative part, wherein an uphill slope indicates an outcome above average and a downhill slope indicates an outcome below average for that consecutive case number. The turning point of curvature indicate the transition from one phase to another and overcomes the specific learning curve. For liver procedures, the CUSUM analysis was adjusted for either minor-, technically major-, or anatomically major resection.
Results
During the study period, 1875 HPB resections were performed including 600 MIS-HPB procedures (32%) consisting of 291 MIS pancreatic, of which 163 pancreatoduodenectomy, and 309 MIS liver resections (Fig. 1).
MIS-HPB patient characteristics and outcome
Table 1 shows the patient characteristics and outcome of all 600 patients undergoing a MIS-HPB resection. Median age was 64 years [IQR 53–72] with ASA 1–2 (73.6%). The median operation time was 281 min [IQR 171–374] with a median blood loss of 200 ml [IQR 100–400] and a conversion rate of 4.3%. Major morbidity occurred in 25.7% (n = 154) of patients. The median length of hospital stay was 5 days [IQR 3–8 days]. The overall in-hospital/30-day mortality was 1.8% (n = 11).
Trends in time
Figure 2 shows the implementation rates of MIS-HPB per year. The overall use of MIS-HPB increased from 30% in 2014 to 45% in 2021 (P < 0.001). When comparing the period before and after the introduction of robotic MIS-HPB (Dec 2018), the overall use of MIS-HPB increased from 25.3% to 43.8% (P < 0.001). The median overall blood loss decreased from 250 ml [IQR 100–500] with laparoscopic MIS-HPB to 150 ml [IQR 50–300] with robotic MIS-HPB (P < 0.001).
MIS pancreatectomy (all procedures)
Overall, 291 patients underwent MIS pancreatectomy during the study period including 175 laparoscopic and 116 robotic procedures. The implementation rate per year increased from 21.5% in 2014 to 44.2% in 2021 (P = < 0.001), Supplementary Fig. 1. The vast majority of MIS distal pancreatectomy procedures (96.1%) was performed laparoscopically. The median annual volume of MIS pancreatectomy was 28 [IQR 23–49] which increased from 23 in 2014 to 68 in 2021 (P < 0.001).
MIS pancreatoduodenectomy
Patient characteristics are shown in Table 2; 52 patients underwent laparoscopic and hereafter 111 patients robotic pancreatoduodenectomy. The median annual volume of MIS pancreatoduodenectomy was 25 [IQR 15–37], including 45 procedures in the last study year, 2021. The use of MIS pancreatoduodenectomy increased from 28.4% in 2015 to 36.3% of all pancreatoduodenectomies in 2021 (P = 0.253). Gland texture was most often soft (67.3% in laparoscopic and 63.1% in robotic).
Outcomes are shown in Table 3. The conversion rate was 7.7% for laparoscopic and 2.7% for robotic pancreatoduodenectomy (p = 0.143). The median operation time was 450 [IQR 411–518] for laparoscopic and 361 min [IQR 330 – 406] for robotic pancreatoduodenectomy (P < 0.001), see Fig. 3. Median blood loss was 350 ml [IQR 250–500] for laparoscopic and 200 ml [IQR 100–350] for robotic pancreatoduodenectomy (P < 0.001). Overall, after MIS pancreatoduodenectomy, major morbidity occurred in 51.5% (n = 84), the rate of POPF grade B/C was 35% (n = 57), and in-hospital/30-day mortality 3.1%. There was no significant difference in (major) complications between both approaches except for a decreased rate of delayed gastric emptying after robotic pancreatoduodenectomy (28.8% vs 9.9%; P = 0.009). The lymph node retrieval in case of malignancy was higher with the robotic approach (14 [IQR 11–17] vs 11 [IQR 8–15], P = 0.016).
Competency learning curve of operative time in minimally invasive pancreatoduodenectomy and liver resections. A & C The X-axis indicated groups of 10 consecutive cases, color indicated per approach (laproscopic = blue squares, robotics = red dots), and the Y-axis indicates the combined operative time expressed in minutes (for liver resection, this way adjusted for extend of resection). The fit line indicated the mean operative time during the maturation of experience with grey lines indication in 95% confidence interval. B & D The X-axis indicates consecutive cases, color indicated per approach (laproscopic = blue squares, robotics = red dots) and the Y-axis indicates the CUSUM operative time expressed in standard deviations. In pancreatoduodenectomy, the label (n = 54) indicated the top turning point of the learning curve, hereafter, the learning curve follows a downward slope. In liver resections, the label [10] indicates the turning point where after both technically major- and anatomically major liver resections were performed. Hereafter, the label [174] indicates the top turning point of the learning curve for liver resections overall
A total of 18 reoperations was performed in all pancreatoduodenectomies (laparoscopic n = 4 and robotic n = 14). Reasons for re-operation consisted of anastomotic leakage (n = 7), bleeding without endovascular options (n = 6), evacuation of a hematoma after bleeding (n = 3), bowel ischemia (n = 1) and trocar site herniation (n = 1).
The mean operative time was 402 min. Naturally, there was a gradual decrease of operative during the maturation of experience (Rho = -0.542, P < 0.001). For laparoscopic pancreatoduodenectomy, the mean operative time was 492 min in the first 10 procedures and 472 min in the last 10 procedures. Hereafter, in the first 10 robotic pancreatoduodenectomies, the mean operative time was 442 min and 355 min in the last 10 procedures. Overall, operative time did reach a stable plateau of 360 min. See Fig. 3A for more details. CUSUM analysis on all pancreatoduodenectomy, identified a turning point of 54 procedures, whereafter the operative time was markedly shorter than during the first 54 procedures (450 [412–513] min vs 360 [330–403] min, P < 0.001). The laparoscopic procedures alone did not reach a CUSUM analysis turning point. In the robotic procedures alone, there was a turning point after 7 procedures. See Fig. 3B for more details. Mortality and major morbidity did not demonstrate a significant decrease (Rho = -0.070, P = 0.374; Rho = 0.116, P = 0.142, respectively). We found no significant changes in vascular resection rates, multi-visceral resection rates, lesion size, or length of stay.
MIS distal pancreatectomy
In total, 123 procedures were performed laparoscopically with a median patient age of 66 years.
[IQR 54–73] and 73.2% ASA 1–2 (n = 90). The median annual volume of laparoscopic distal pancreatectomy was 17 [IQR 9 – 20]. The annual rate of MIS distal pancreatectomy remained stable over the years from 76% to 76.6% (P = 0.953). The median operation time was 251 min [IQR 206 – 322] and the median blood loss 200 ml [IQR 50–350]. Major morbidity was 29.3% with an in-hospital/30-day mortality 1.6% (n = 2). The rate of POPF grade B/C was 35.8% (n = 44). Robotic distal pancreatectomy was implemented only recently and therefore not included in this analysis.
MIS liver resection (all procedures)
A total of 309 patients underwent MIS liver resection including 198 laparoscopic and 111 robotic procedures. As of December 2018, the MIS liver resection program became nearly completely robotic. The median annual volume of MIS liver resection during the study period was 24 resections [IQR 18–33 resections], with 50 robotic MIS liver resections in 2021. The annual implementation rate of MIS liver resection increased from 10% in 2010 to 47% in 2021 (P < 0.001), Supplementary Fig. 2. Since the application of the robotic approach, the use of robotics within the MIS liver resection group increased from 75% in 2019 to 96% in 2021 (P = 0.005).
Baseline characteristics are shown in Table 4. In the robotic group, there were more patients with ASA 3–4 (19.7% vs. 34.5%; P = 0.004), more patients received neo-adjuvant chemotherapy (13.1% vs 31.5%; P < 0.001), and more patients had previous extrahepatic abdominal surgery (30.8% vs 58.6%; P < 0.001). In addition, 156 minor (78.8%), 26 technically major (13.1%) and 16 anatomically major (8.1%) liver resections were performed in the laparoscopic group, compared to 61 minor (55.0%), 42 technically major (37.8%) and 8 anatomically major (7.2%) resections in the robotic group. The 2 other anatomically major liver resections were anatomical resections of 3 contiguous segments.
Table 5 shows the operative outcomes of all MIS liver resections stratified for approach. Overall, the median blood loss was 200 mL [IQR 50–400] with a conversion rate of 3.6%. The rate of severe complications was 10.4% and median hospital stay 4 days [IQR 2–5]. The rate of 30-day/in-hospital mortality was 1.3% (n = 4). Reoperations occurred in 9 patients (3.7%) and indications for reoperation included anastomotic leakage after simultaneous colorectal resection (n = 4), intra-abdominal bleeding (n = 4) and a strangulated inguinal hernia (n = 1). Comparing outcomes before and after introduction of the robotic approach, blood loss decreased (288 ml vs. 100 ml; P < 0.001) and hospital stay shortened (4 vs 3 days; P < 0.001) as compared to the laparoscopic approach, whereas the 30-day/in-hospital mortality did not differ significantly between both approaches (1.0% vs. 1.8%; P = 0.555).
Additionally, Supplementary Table 1 shows the operative outcomes stratified for minor, technically major and anatomically major laparoscopic and robotic MIS liver resection. Median blood loss was less with the robotic approach in both minor (250 mL vs 50 mL; P < 0.001) and technically major (300 mL vs 150 mL; P = 0.001) resections, whilst there was no difference in the anatomically major group. Complications occurred less often with the robotic approach after both minor (17.9% vs 4.9%; P = 0.014) and technically major (42.3% vs. 16.7%; P = 0.020) MIS liver resections, as compared to the laparoscopic approach. Hospital stay was shorter with the robotic approach for both minor (4 vs. 3 days; P < 0.001) and technically major (6 vs. 3 days; P < 0.001) MIS liver resections. Operation time, conversion rates and 30-day/in-hospital mortality did not differ between both approaches in all subgroups.
For MIS liver resection, mean operative time was 209 min. Naturally, there was a gradual decrease of operative during the maturation of experience (Rho = -0.162, P < 0.001). For laparoscopic liver resections, the mean operative time was 96 min in the first 10 procedures and 186 min in the last 10 laparoscopic liver resection. In the first 10 robotic liver resections, the mean operative time was 194 min and 182 min in the last 10 RPD procedures. Both the mean operative time and the decrease in operative time differed between minor liver resection, technically major liver resection and anatomically major resection: 181(± 95) min, Rho = 0.121; 235(± 93), Rho -0.116; and 384(± 117), Rho -0.271, P < 0.001, respectively. See Fig. 3C for more details. CUSUM analysis identified a turning point of 52 liver resections, whereafter both technically major- and anatomically major liver resections were performed. The turning point of 174 liver resections indicated that, hereafter, the operative time was markedly shorter compared to the operative times of liver resections before the turning point (consecutive 52 to 174 procedures): 229 [146–333] min vs 179 [130–240] min, P = 0.003). The laparoscopic liver resections alone did not reach a CUSUM analysis turning point. In the robotic cases alone, there was a turning point after 86 procedures. See Fig. 3B for more details. Mortality did not demonstrate a significant decrease (Rho = -0.100, P = 0.079. However, major morbidity did demonstrate a significant decrease (Rho = -0.144, P = 0.011) CUSUM analysis revealed a turning point after 128 cases, the difference in mortality rate before and this tuning point was an odds ratio of 0.669, P = 0.280 (12.6% vs 8.8%).
Discussion
This first single-center study to describe the transition from laparoscopic to robotic surgery in 600 MIS-HPB resections found the latter approach to be associated with increased implementation up to nearly half of all pancreatic and liver resections. Furthermore, the introduction of the robotic approach was associated with improved outcomes in these selected patients and low mortality. Ultimately, randomized studies will have to confirm the benefits of the robotic approach.
Large single-center studies assessing the implementation of MIS-HPB resections including both laparoscopic and robotic pancreatic and liver resections are lacking. For MIS-HPB surgery in general, one single center study reported on the stepwise implementation of 77 robotic liver resections and 68 robotic pancreatoduodenectomies, but did not include laparoscopic resections [34]. Most other single center implementation studies focused on either pancreas or liver resections and, similarly, either laparoscopic or robot surgery. The largest series on the introduction of robotic pancreatoduodenectomy (n = 500) comes from Pittsburgh and reported a plateau for operating time after 240 procedures of 390 min [9]. This series compares favorably with a median operating time of 360 min but this is most likely explained by the LAELAPS-3 training program wherein the Dutch surgeons were trained by the Pittsburgh team [8]. Recently, for MIS liver resection, a single center and single-surgeon retrospective Belgium study assessed the transition from 120 laparoscopic to 71 robotic liver resections [35]. The authors concluded that experience with laparoscopic MIS seems to overcome the learning curve of robotic MIS as early outcomes of robotic MIS were similar to the mastery phase of laparoscopic MIS. According to a systematic review by Müller et al., the proficiency learning curve (major complication) has not been reported for robotic pancreatoduodenectomy and may extend 25 to 80 laparoscopic pancreatoduodenectomies [36]. In this study major morbidity and mortality rates did not decrease significantly with the maturation of experience, however, it could be a result of the limited power and expanding the indication to patients with higher risks for complications. For example, mortality of pancreatoduodenectomy decreased from 8.6% to 0% after 58 cases in this study. According to a systematic review by Chua et al., the learning curve for minimally invasive hepatectomy is 50 procedures for laparoscopic- and 25 for robotic procedures [37]. Conversely, Krenzien et al., demonstrated that the learning phase for MILS adjusting for complexity is about 4 times longer, i.e., 117 LLR and 93 RLR [38]. This resonates with the findings in the current study where we could adjust the CUSUM analysis of liver resections for the extent of the resection (minor, technically major, anatomically major). As complexity has not been adequately represented in learning curve models, the current study provides a nuance to studies looking at either minor or major resection only. The learning curve for major complications was 128 cases, however without a significant impact before and after the turning point.
The introduction of robotic pancreatoduodenectomy in this study was associated with a reduction in operation time and blood loss. This reduction could also be explained by increasing experience, yet Fig. 3 suggests that the reduction extends beyond the previously observed trend and is quite ‘steep’. Previous studies reporting on a shift from laparoscopic to robotic pancreatoduodenectomy are lacking. A systematic review found robotic pancreatoduodenectomy associated with a shorter hospital stay, less blood loss, and less conversion, as compared to laparoscopic pancreatoduodenectomy [39]. Notably, randomized trials focusing on robotic pancreatoduodenectomy are lacking. The recent Miami guidelines advice a minimum center volume of 20 MIS pancreatoduodenectomies per year [7]. These criteria were met in each year in our center after implementation of robotic pancreatoduodenectomy with a median of 34 procedures per year which should be taken into account when interpreting these findings.
The use of MIS liver surgery increased during the study period from 10 to 47% per year. The earlier mentioned Belgium single-center study reported an increase in the use of MIS liver resection from 45% in 2012 to 79% in 2020 [35]. Although substantially higher than the annual rates in the present series, this might be related to the referral practice for hilar cholangiocarcinoma in our center [40]. Furthermore, a single high-volume experience from Singapore reported increased use of MIS liver resections from 3.3% to 44.1% which is highly similar to the 47% in the present series [41].
The overall rate of POPF after MIS pancreatoduodenectomy in the current study (41.1%) is higher than previously reported, whereas the mortality rate (3.1%) is comparable to other retrospective studies describing first experiences with robotic and laparoscopic pancreatoduodenectomy (1.7% vs 1.4% and 4.0%) [9, 42, 43]. The high rate of POPF may be partially explained by the proactive drainage approach as was implemented in the nationwide randomized PORSCH trial which led to a 50% reduction of mortality after pancreatoduodenectomy [44]. Clearly, also a learning curve effect cannot be excluded. Only six patients used neoadjuvant therapy in this study. This is explained by the use of MIS pancreatoduodenectomy only in patients without vascular contact in the study period wherein neoadjuvant therapy was only used in patient with borderline and resectable disease.
Regarding MIS liver resection, the recent Southampton guidelines advise a stepwise implementation [14]. Several studies showed that conversion may worsen blood loss, prolong operation time, increase hospital stay, morbidity rates, and even mortality [45,46,47]. The conversion rate in the current study for all MIS liver resections (3.6%) was favorable and in line with previous large retrospective cohort studies [19, 46, 48, 49]. In addition, acceptable rates of major morbidity and mortality after laparoscopic and robotic MIS liver resection were observed which are in line with previous reports [19, 46, 49,50,51]. Randomized studies comparing laparoscopic and robotic MIS liver resection are lacking and are warranted to explore the exact value of robotic MIS liver resection in the current MIS liver resection practice.
The current findings may reflect or forecast the use of MIS-HPB resections in high-volume HPB centers worldwide. This has important implications for training of surgeons and operating staff in HPB units worldwide, including the availability of robotic systems [10, 52]. For instance, in our center we have only recently obtained a third day per week on one of the Xi surgical robots. This will allow us to now further expand the MIS-HPB program to include robotic distal pancreatectomy. It is expected that many, if not most, HPB units currently struggle to obtain sufficient robotic operating time or have no access to robotic systems at all. Future studies are needed to assess whether the higher operative costs with robotic surgery are compensated by improved outcome and shorter hospital stay. Although the costs of robotic surgery are expected to decrease with the arrival of new robotic systems, ultimately randomized studies will have to confirm the benefit of robotic as compared to laparoscopic and open MIS-HPB surgery.
The current study has several limitations which should be taken into account. First, procedures were performed in selected patients. However, the selection criteria are provided in the methods section and aim to safeguard good patient outcome. Second, this study was performed in a high volume setting by a small group of specifically trained, and procedure dedicated surgeons. One surgeon was present during all laparoscopic and robotic pancreatoduodenectomy procedures and another surgeon during all robotic liver resections. This was done by design in order to minimize negative learning curve effects. As a result, the impact of surgeon training and experience could not be assessed and the results may not be generalizable to centers who disperse MIS-HPB resections over a larger group of surgeons. However, all surgeons performing minimally invasive pancreatic surgery had participated in the LAELAPS-1, -2, and -3 training programs [8, 23, 24]. Third, the outcomes of robotic and laparoscopic MIS-HPB resections cannot be compared directly because of the ongoing learning curve. It may be that with ongoing experience outcomes in most recent years would also have improved. This cannot be excluded, but when assessing operating times for MIS pancreatoduodenectomy (Fig. 3) there seems to be a rather steep improvement with the introduction of robotic surgery. This also the reasons that no (propensity-score) matching was performed. Fourth, the overall generalizability of our findings might be hampered by the use of specific training programs in our center (i.e., LAELAPS 1–2-3). However, the LEARNBOT training program for robot pancreatoduodenectomy is currently ongoing in Europe for high volume centers (www.e-mips.com/learnbot) and soon the LIVEROBOT robot liver training program will start. Both are endorsed by E-AHPBA and provide European HPB units which aim to start a robot MIS-HPB program the option to follow designated training programs.
A major strength of this study is that it demonstrates the implementation and outcome of robotic surgery in a large cohort of 600 MIS-HPB pancreatic and liver resections using a structured approach to patient selection and surgical training.
In conclusion, the implementation of robotic surgery in MIS-HPB appeared to be safe and feasible in a high-volume center leading to an implementation approaching half of all pancreatic and liver resections. Adequate training, a high-volume practice, and a deliberate team-based approach to these procedures may be beneficial. Ultimately, randomized studies will have to confirm the benefits of MIS-HPB resections including robot-assisted surgery.
Change history
27 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00464-022-09848-w
References
Cuschieri A (1994) Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 39:178–184
De Rooij T, Van Hilst J, Van Santvoort H et al (2019) Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg 269:2–9. https://doi.org/10.1097/SLA.0000000000002979
Björnsson B, Sandström P, Larsson AL et al (2019) Laparoscopic versus open distal pancreatectomy (LAPOP): study protocol for a single center, nonblinded, randomized controlled trial. Trials 20:1–5. https://doi.org/10.1186/S13063-019-3460-Y/TABLES/1
Poves I, Burdío F, Morató O et al (2018) Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the Padulap randomized controlled trial. Ann Surg 268:731–739. https://doi.org/10.1097/SLA.0000000000002893
van Hilst J, De Rooij T, Bosscha K et al (2019) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol. Hepatol. 4:199–207. https://doi.org/10.1016/S2468-1253(19)30004-4
Wang M, Li D, Chen R et al (2021) Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 6:438–447. https://doi.org/10.1016/S2468-1253(21)00054-6
Asbun HJ, Moekotte AL, Vissers FL et al (2020) The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 271:1–14. https://doi.org/10.1097/SLA.0000000000003590
Zwart MJW, Nota CLM, de Rooij T et al (2021) Outcomes of a multicenter training program in robotic pancreatoduodenectomy (LAELAPS-3). Ann Surg. https://doi.org/10.1097/sla.0000000000004783
Zureikat AH, Beane JD, Zenati MS et al (2021) 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg 273:966–972. https://doi.org/10.1097/SLA.0000000000003550
Napoli N, Kauffmann EF, Vistoli F et al (2021) State of the art of robotic pancreatoduodenectomy. Updates Surg 73:873–880. https://doi.org/10.1007/S13304-021-01058-8
Zhou ZP, Tan XL, Zhao ZM et al (2021) Robotic resection of duodenal gastrointestinal stromal tumour: Preliminary experience from a single centre. World J. Gastrointest Oncol 13:706–715. https://doi.org/10.4251/wjgo.v13.i7.706
Fretland AA, Dagenborg VJ, Bjørnelv GMW et al (2018) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 267:199–207. https://doi.org/10.1097/SLA.0000000000002353
Robles-Campos R, Lopez-Lopez V, Brusadin R et al (2019) Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc. https://doi.org/10.1007/s00464-019-06679-0
Abu Hilal M, Aldrighetti L, Dagher I et al (2018) The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 268:11–18. https://doi.org/10.1097/SLA.0000000000002524
Nota CL, Woo Y, Raoof M et al (2019) Robotic versus open minor liver resections of the posterosuperior segments: a multinational, propensity score-matched study. Ann Surg Oncol 26:583–590. https://doi.org/10.1245/s10434-018-6928-1
Croner RS, Perrakis A, Hohenberger W, Brunner M (2016) Robotic liver surgery for minor hepatic resections: a comparison with laparoscopic and open standard procedures. Langenbeck’s Archives of Surgery 401:707–714. https://doi.org/10.1007/s00423-016-1440-1
Da CP, Wu CY, Hu RH et al (2017) Robotic versus open hepatectomy for hepatocellular carcinoma: a matched comparison. Ann Surg Oncol 24:1021–1028. https://doi.org/10.1245/s10434-016-5638-9
Daskalaki D, Gonzalez-Heredia R, Brown M et al (2017) Financial impact of the robotic approach in liver surgery: a comparative study of clinical outcomes and costs between the robotic and open technique in a single institution. J Laparoendosc Adv Surg Tech 27:375–382. https://doi.org/10.1089/lap.2016.0576
Cipriani F, Fiorentini G, Magistri P et al (2021) Pure laparoscopic versus robotic liver resections: Multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepatobiliary Pancreat Sci 00:1–16. https://doi.org/10.1002/JHBP.1022
Chong CC, Fuks D, Lee K-F et al (2022) Propensity score-matched analysis comparing robotic and laparoscopic right and extended right hepatectomy. JAMA Surg. https://doi.org/10.1001/JAMASURG.2022.0161
Vigano L, Laurent A, Tayar C et al (2009) The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. https://doi.org/10.1097/SLA.0b013e3181bd93b2
van der Poel MJ, Besselink MG, Cipriani F et al (2016) Outcome and learning curve in 159 consecutive patients undergoing total laparoscopic hemihepatectomy. JAMA Surg 151:923. https://doi.org/10.1001/jamasurg.2016.1655
De Rooij T, Van Hilst J, Boerma D et al (2016) Impact of a nationwide training program in minimally invasive distal pancreatectomy (LAELAPS). Ann Surg 264:754–762. https://doi.org/10.1097/SLA.0000000000001888
De Rooij T, van Hilst J, Topal B et al (2019) Outcomes of a multicenter training program in laparoscopic pancreatoduodenectomy (LAELAPS-2). Ann Surg 269:344–350. https://doi.org/10.1097/SLA.0000000000002563
van der Poel MJ, Fichtinger RS, Bemelmans M et al (2019) Implementation and outcome of minor and major minimally invasive liver surgery in the Netherlands. HPB 21:1734–1743. https://doi.org/10.1016/j.hpb.2019.05.002
Vandenbroucke JP, von Elm E, Altman DG et al (2014) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg 12:1500–1524. https://doi.org/10.1016/j.ijsu.2014.07.014
van der Poel MJ, Fichtinger RS, van Dam RM, Besselink MG (2018) Outcomes of laparoscopic minor and major liver surgery in the Netherlands (LAELIVE): nationwide retrospective cohort. HPB. https://doi.org/10.1016/j.hpb.2018.06.210
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12:351–355. https://doi.org/10.1007/s00534-005-0999-7
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications. Ann Surg 250:187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591. https://doi.org/10.1016/J.SURG.2016.11.014
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768. https://doi.org/10.1016/J.SURG.2007.05.005
Wente MN, Veit JA, Bassi C et al (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25. https://doi.org/10.1016/J.SURG.2007.02.001
Koch M, Garden OJ, Padbury R et al (2011) Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 149:680–688. https://doi.org/10.1016/j.surg.2010.12.002
Nota CL, Molenaar IQ, te Riele WW et al (2020) Stepwise implementation of robotic surgery in a high volume HPB practice in the Netherlands. HPB 22:1596–1603. https://doi.org/10.1016/J.HPB.2020.01.018
D’Hondt M, Devooght A, Willems E et al (2022) Transition from laparoscopic to robotic liver surgery: clinical outcomes, learning curve effect, and cost-effectiveness. J Robot Surg 2022:1–10. https://doi.org/10.1007/S11701-022-01405-W
Müller PC, Kuemmerli C, Cizmic A et al (2022) Learning curves in open, laparoscopic, and robotic pancreatic surgery. Ann Surg Open 3:e111. https://doi.org/10.1097/AS9.0000000000000111
Chua D, Syn N, Koh YX, Goh BKP (2021) Learning curves in minimally invasive hepatectomy: systematic review and meta-regression analysis. Br J Surg 108:351–358. https://doi.org/10.1093/BJS/ZNAA118
Krenzien F, Benzing C, Feldbrügge L et al (2022) Complexity-adjusted learning curves for robotic and laparoscopic liver resection. Ann Surg Open 3:e131. https://doi.org/10.1097/AS9.0000000000000131
Kamarajah SK, Bundred J, Saint MO et al (2020) Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol 46:6–14. https://doi.org/10.1016/J.EJSO.2019.08.007
Franken LC, Benzing C, Krenzien F et al (2022) Right-sided resection with standard or selective portal vein resection in patients with perihilar cholangiocarcinoma: a propensity score analysis. HPB (Oxford) 24:391–397. https://doi.org/10.1016/J.HPB.2021.06.429
Goh BKP, Lee SY, Teo JY et al (2018) Changing trends and outcomes associated with the adoption of minimally invasive hepatectomy: a contemporary single-institution experience with 400 consecutive resections. Surg Endosc 32:4658–4665. https://doi.org/10.1007/S00464-018-6310-1/TABLES/3
Klompmaker S, Van Hilst J, Wellner UF et al (2020) Outcomes after minimally-invasive versus open pancreatoduodenectomy: A Pan-European propensity score matched study. Ann Surg 271:356–363. https://doi.org/10.1097/SLA.0000000000002850
Guerra F, Checcacci P, Vegni A et al (2019) Surgical and oncological outcomes of our first 59 cases of robotic pancreaticoduodenectomy. J Visc Surg 156:185–190. https://doi.org/10.1016/J.JVISCSURG.2018.07.011
Smits FJ, Henry AC, Van Eijck CH et al (2020) Care after pancreatic resection according to an algorithm for early detection and minimally invasive management of pancreatic fistula versus current practice (PORSCH-trial): design and rationale of a nationwide stepped-wedge cluster-randomized trial. Trials 21:1–16. https://doi.org/10.1186/S13063-020-4167-9/TABLES/4
Troisi RI, Montalti R, Van Limmen JGM et al (2014) Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB 16:75–82. https://doi.org/10.1111/HPB.12077
Halls MC, Cipriani F, Berardi G et al (2018) Conversion for unfavorable intraoperative events results in significantly worse outcomes during laparoscopic liver resection: lessons learned from a multicenter review of 2861 cases. Ann Surg. https://doi.org/10.1097/SLA.0000000000002332
Cauchy F, Fuks D, Nomi T et al (2015) Risk factors and consequences of conversion in laparoscopic major liver resection. Br J Surg 102:785–795. https://doi.org/10.1002/bjs.9806
Aghayan DL, Kazaryan AM, Fretland ÅA et al (2021) Evolution of laparoscopic liver surgery: 20-year experience of a Norwegian high-volume referral center. Surg Endosc 1:1–9. https://doi.org/10.1007/S00464-021-08570-3/FIGURES/4
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection—2,804 patients. Ann Surg 250:831–841. https://doi.org/10.1097/SLA.0b013e3181b0c4df
Berardi G, Van Cleven S, Fretland ÅA et al (2017) Evolution of laparoscopic liver surgery from innovation to implementation to mastery: perioperative and oncologic outcomes of 2,238 patients from 4 European specialized centers. J Am Coll Surg 225:639–649. https://doi.org/10.1016/J.JAMCOLLSURG.2017.08.006
Aziz H, Wang JC, Genyk Y, Sheikh MR (2021) Comprehensive analysis of laparoscopic, robotic, and open hepatectomy outcomes using the nationwide readmissions database. J Robot Surg. https://doi.org/10.1007/S11701-021-01257-W
Di Martino M, Caruso R, D’Ovidio A et al (2021) Robotic versus laparoscopic distal pancreatectomies: a systematic review and meta-analysis on costs and perioperative outcome. Int. J. Med. Robot. Comput. Assist. Surg. 17:e2295. https://doi.org/10.1002/RCS.2295
Acknowledgements
The authors would like to thank the Dutch Pancreatic Cancer Audit (DPCA) and the Dutch Hepato-Biliary Audit (DHBA) for support in providing data and the specialized operating team headed by S.I. Sussenbach for their support in performing these procedures.
Funding
This study was supported by the Intuitive Surgical, Ethicon, Medtronic.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosures
M.G. Besselink has received an Intuitive grant for the LEARNBOT European Robotic Pancreatoduodenectomy training program, the DIPLOMA-2 trial and the E-MIPS quality registry. A Medtronic grant for the investigator-initiated DIPLOMA trial. An Ethicon grant for the PANDORINA trial and the E-MIPS quality registry. A.M.L.H. Emmen, B. Görgec, M.J.W. Zwart, F. Daams, J. Erdmann, S. Festen, D.J. Gouma, T.M. van Gulik, J. van Hilst, G. Kazemier, S. Lof, S.I. Sussenbach, P.J. Tanis, B.M. Zonderhuis, O.R. Busch, R.J. Swijnenburg have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
S2aspringer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A.M.L.H. Emmen and B. Görgec authors shares first authorship.
R.J. Swijnenburg and M.G. Besselink author shares senior authorship.
Meeting presentation: Poster Presentation IHPBA 2022, New York.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emmen, A.M.L.H., Görgec, B., Zwart, M.J.W. et al. Impact of shifting from laparoscopic to robotic surgery during 600 minimally invasive pancreatic and liver resections. Surg Endosc 37, 2659–2672 (2023). https://doi.org/10.1007/s00464-022-09735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09735-4