Abstract
Background
Safety data on perioperative outcomes of laparoscopic antireflux surgery (LARS) after lung transplantation (LT) are lacking. We compared the 30-day readmission rate and short-term morbidity after LARS between LT recipients and matched nontransplant (NT) controls.
Methods
Adult patients who underwent LARS between January 1, 2015, and October 31, 2021, were included. The participants were divided into two groups: LT recipients and NT controls. First, we compared 30-day readmission rates after LARS between the LT and NT cohorts. Next, we compared 30-day morbidity after LARS between the LT cohort and a 1-to-2 propensity score-matched NT cohort.
Results
A total of 1328 patients (55 LT recipients and 1273 NT controls) were included. The post-LARS 30-day readmission rate was higher in LT recipients than in the overall NT controls (14.5% vs. 2.8%, p < 0.001). Compared to matched NT controls, LT recipients had a lower prevalence of paraesophageal hernia, a smaller median hernia size, and higher peristaltic vigor. Also compared to the matched NT controls, the LT recipients had a lower median operative time but a longer median length of hospital stay. The proportion of patients with a post-LARS event within 30 postoperative days was comparable between the LT and matched NT cohorts (21.8% vs 14.5%, p = 0.24).
Conclusions
Despite a higher perceived risk of comorbidity burden, LT recipients and matched NT controls had similar rates of post-LARS 30-day morbidity at our large-volume center with expertise in transplant and foregut surgery. LARS after LT is safe.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lung transplantation (LT) improves quality of life and longevity in patients with end-stage lung disease; however, post-LT long-term survival and mortality are largely driven by chronic lung allograft dysfunction (CLAD) [1]. CLAD is associated with gastroesophageal reflux disease (GERD), a modifiable risk factor that is prevalent after LT [2]. GERD causes CLAD through aspiration of gastric contents and lung parenchymal injury. Immunologic biomarkers in bronchoalveolar lavage fluid and serum are associated with GERD and CLAD [3]. Medical management of GERD with antisecretory medications alkalinizes the refluxate, but does not minimize volume reflux of nonacid contents; in contrast, antireflux surgery creates a physical barrier at the esophagogastric junction, preventing all reflux. This potentially modulates the pulmonary inflammatory milieu in LT recipients [4] and stabilizes long-term allograft function [5, 6].
Based on several prospective, randomized multicenter trials with short- and long-term outcomes in the nontransplant population, laparoscopic antireflux surgery (LARS) is considered superior to medical management for symptomatic relief, quality of life improvement, and objective control of reflux. LARS is associated with minimal perioperative morbidity and short hospital stays [7,8,9,10,11,12,13,14,15,16,17]. Our group has demonstrated that CLAD-free survival in LT recipients who underwent LARS was superior to that of medically managed LT recipients with a DeMeester score ≥ 30 (results presented at the Western Thoracic Surgical Association annual meeting, 2021). However, despite documented benefits of LARS, there is reluctance within the transplant community to offer LARS to this medically complex group of patients, likely stemming from the perception of increased post-LARS morbidity in LT recipients due to their comorbidities and immunosuppressed state. Although the benefits of LARS on allograft function outcomes in LT recipients have been well described, safety and operative morbidity data are sparse and come from small series without comparative data from nontransplant controls. Assurance of the safety and feasibility of LARS in LT recipients may remove referral and consultation barriers and potentially improve long-term survival of LT recipients with GERD.
We studied the 30-day readmission rate after LARS in LT recipients and nontransplant (NT) controls, compared the operative outcomes and short-term morbidity after LARS in LT recipients and matched NT controls, and assessed objective control of GERD in LT recipients after LARS.
Patients and methods
Institutional Review Board approval with waiver of patient consent for this retrospective study was obtained at Norton Thoracic Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona (PHXU-21-500-137-73-18; March 31, 2021). All adult patients (age ≥ 18 years) who underwent laparoscopic or robotic antireflux surgery at our center between January 1, 2015, and October 31, 2021, were included. Demographic characteristics, pre-LARS medical comorbidities, operative details, and morbidity within 30 days after surgery were obtained from data gathered for the Society of Thoracic Surgeons database.
First, the included subjects were divided into 2 groups: LT recipients and NT controls; the primary outcome was the 30-day readmission rate after LARS. Then, a 1-to-2 propensity score-matched control group was established and balanced with patients in the LT cohort based on 8 baseline characteristics: (1) age, (2) pre-LARS body mass index, and pre-LARS medical comorbidities including (3) hypertension, (4) coronary artery disease, (5) pulmonary hypertension, (6) major vascular disease, (7) history of smoking, and (8) diabetes mellitus. Subjects in the LT and matched NT cohorts were then compared for differences in symptoms, objective reflux characteristics, and LARS perioperative outcomes; the secondary outcome was 30-day morbidity after LARS. Post-LARS morbidity was defined as an event that deviated from an uneventful postoperative course until discharge or 30 days after surgery, whichever was longer. Finally, for patients in the LT cohort, medical records were accessed to obtain and compare the results of pre- and post-LARS high-resolution manometry and 24- or 48-h reflux testing (when available).
Institutional practice for the management of GERD
At our institution, surgical management of GERD is considered after a review of symptoms and esophageal function testing with esophagogastroduodenoscopy, high-resolution manometry, and 24- or 48-h reflux testing (as indicated), as well as barium esophagography and gastric scintigraphy (selectively). Esophageal function testing is routinely performed before and within 6 months of LT.
Patients are referred by pulmonary transplant providers to Thoracic Surgery for LARS evaluation. Our group has previously reported that additional case-by-case considerations in LT recipients include medical fitness, prior abdominal surgery, body mass index, and change in foregut function after LT [2]. Medically stable and surgically suitable LT recipients with significant volume reflux, paraesophageal hernia, and sufficient esophageal motility are offered LARS.
The surgical technique involves full dissection and mobilization of the esophageal hiatus, reduction of any hernia, crural repair with interrupted nonabsorbable sutures, and a posterior 270° Toupet fundoplication. When appropriate, the preference of the surgical team is use of bioabsorbable Bio-A synthetic mesh (W. L. Gore & Associates, Flagstaff, Arizona) for cruroplasty reinforcement. Patients are given a liquid diet for a week and slowly transitioned to a solid diet. Acid suppression medications are discontinued after LARS unless indicated for ulcer prophylaxis.
Esophageal function testing
High-resolution manometry is performed with a 36-channel catheter (Given Imaging Ltd., Los Angeles, California) and interpreted with the ManoView ESO software version 3.3 (Given Imaging Ltd.) using the Chicago classification of esophageal motility disorders version 3.0 diagnostic criteria [18]. Ambulatory esophageal pH monitoring is performed using a dual (proximal and distal esophagus) catheter-based system for 24 h (Sandhill Scientific Inc, Highlands Ranch, Colorado) or a wireless probe for 48 h (Bravo capsule; Medtronic, Minneapolis, Minnesota), and interpreted using the Reflux Reader software version 6.1 (Medtronic). All pH testing is performed off acid suppression therapy (10 days for PPI and 3 days for H2 receptor blockers). Patient-reported heartburn, regurgitation, difficulty swallowing, chest pain, and abdominal bloating are recorded on a questionnaire as 0 (none), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe) at the time of esophageal function testing; for this study, symptoms were defined as absent (0) or present (1–4).
Statistical analysis
Analyses were performed using IBM SPSS Statistics for Windows, version 23.0. Armonk, NY: IBM Corp. released 2015, with “psmatching3.03” extension bundle and R package 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Propensity-score matching was performed using a logistic regression model with the nearest-neighbor method without replacement. The selection of 1-to-2 matched control patients was processed through maximized execution performance without caliper, based on age, pre-LARS body mass index, and pre-LARS medical comorbidities with a match tolerance of 2, 2, and 0, respectively. In select instances where no successful matches were obtained, medical comorbidities were sequentially removed from the match criteria. Covariate balance among the matched groups was assessed based on the standardized mean difference in each baseline characteristic. Small, medium, and large effect sizes were defined based on an absolute standardized difference (Cohen’s d) values of < 0.3, 0.4–0.6, and 0.7–1.2, respectively [19].
Data were expressed as count (percentage) or median (interquartile range). χ2 or Fischer’s exact tests were used to compare categorical variables, and nonparametric Kruskal–Wallis and Wilcoxon signed-rank sum tests were used to compare continuous variables in independent and paired samples, respectively. The Nelson–Aalen cumulative hazard function and Cox proportional hazard analysis were used to compare 30-day readmission rates between the groups. Statistical significance was set at p < 0.05.
Results
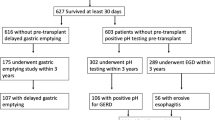
A total of 1328 patients who underwent LARS at our center during the study period were included: 55 LT recipients and 1273 NT controls.
Baseline characteristics of the LT recipients
The median (IQR) age, body mass index, and lung allocation score at the time of LT were 62.4 (55.7, 69.4) years, 24.2 (20.9, 28.5) kg/m2, and 38.15 (34.97, 43.41), respectively. The underlying end-stage lung conditions included obstructive lung disease (38%, n = 21), restrictive lung disease (49%, n = 27), cystic fibrosis (5%, n = 3), pulmonary vascular disease (2%, n = 1), COVID-19 induced adult respiratory distress syndrome (2%, n = 1), and redo-transplant (4%, n = 2). The median interval between LT and LARS was 8.2 (5.4, 14.5) months.
Post-LARS 30-day readmissions of LT and overall NT cohorts
The post-LARS 30-day readmission rate was higher in the LT cohort than in the overall NT cohort (14.5% [8/55] vs 2.8% [36/1273], p < 0.001; hazard ratio [95% CI] 5.466 [2.540–11.760], p < 0.001; Fig. 1). The indications for readmission in the two groups are shown in Table 1. Notably, 3 of the 8 readmissions in the LT cohort were for planned interventions (plasmapheresis for antibody-mediated rejection) rather than surgery-related complications. Even after excluding planned readmissions of the LT recipients, the post-LARS 30-day readmission rate of the LT cohort was higher than that of the overall NT cohort (9.1% [5/55] vs 2.8% [36/1273], p = 0.009).
Post-LARS 30-day readmissions of LT and matched NT cohorts
A propensity-matched NT control cohort was balanced with patients in the LT cohort on matched characteristics, i.e., age, pre-LARS body mass index, hypertension, coronary artery disease, pulmonary hypertension, major vascular disease, history of smoking, and diabetes mellitus with a small effect size. However, unmatched characteristics, i.e., male sex and pre-LARS serum creatinine level, were significantly higher in the LT cohort than in the matched NT cohort (Table 2). The post-LARS 30-day readmission rate of the LT cohort was significantly higher than that of the matched NT cohort (14.5% [8/55] vs 3.6% [4/110], p = 0.01). However, after excluding planned readmissions of the LT recipients, the post-LARS 30-day readmission rate of the LT cohort and the matched NT cohort was comparable (9.1% [5/55] vs 3.6% [4/110], p = 0.16).
Pre-LARS esophageal function of LT and matched NT cohorts
Patients in the LT cohort had a lower prevalence of manometric paraesophageal hernia (45.5% vs 70%, p = 0.002), a smaller median hernia size (3.6 cm vs 4.5 cm, p = 0.04), and higher esophageal body peristaltic vigor (1695 mmHg-cm-s vs 1124 mmHg-cm-s, p = 0.05) than those in the matched NT cohort; however, the esophageal body motility diagnoses and severity of reflux were comparable between the two cohorts. Patients in the matched NT cohort were more symptomatic of heartburn (p < 0.001), dysphagia (p < 0.001), regurgitation (p < 0.001), chest pain (p = 0.02), and abdominal bloating (p = 0.03) than those in the LT cohort.
LARS operative outcomes in LT and matched NT cohorts
The use of mesh was comparable between the two cohorts. Compared to the matched NT controls, LT recipients had a significantly lower median operative time for LARS (86 min vs 103 min, p = 0.002) but a significantly longer median length of hospital stay for LARS (2 days vs 1 day, p = 0.003; Table 3).
Post-LARS 30-day morbidity in LT and matched NT cohorts
The proportion of patients with post-LARS events before discharge or within 30 postoperative days (whichever was longer) was comparable between the LT and matched NT cohorts (21.8% vs 14.5%, p = 0.24; Table 3). Most complications in both cohorts were Clavien–Dindo grade I or II (Table 3). Of note, 1 patient in the LT cohort and 2 patients in the matched NT cohort had a life-threatening complication with need for ICU management (Clavien–Dindo grade IVa). One LT recipient for cystic fibrosis, status post pleurodesis for chylothorax, had an intra-abdominal hematoma after LARS and received 3 units of packed red blood cells in the ICU followed by an uneventful recovery. The intraoperative course of one patient in the NT cohort was complicated by acute hypotension, quickly progressing to ST segment elevation and cardiac arrest (known pre-LARS hypertension, negative cardiac stress test, and American Society of Anesthesiologists grade III). She was appropriately resuscitated and postoperative cardiac catheterization showed Takotsubo cardiomyopathy with ejection fraction of 30%. The patient was discharged on postoperative day 8 after stabilization. A second patient in the NT cohort had postoperative acute pre-renal kidney injury and was monitored in the ICU. Renal insufficiency resolved prior to discharge. No 30-day mortality was observed in either group.
Efficacy of LARS in objective control of reflux in LT cohort
Pre- and post-LARS reflux testing was ordered based on surgeon preference and was available for 13 patients in the LT cohort at a median (IQR) of 133 (63.5, 195.5) days post-LARS. Abnormal distal esophageal acid exposure resolved in all patients. After LARS, the median DeMeester score (39.5 vs 2.1, p = 0.001) and median total distal acid exposure time to pH < 4 (9.7% vs 0.2%, p = 0.002) significantly decreased (Table 4).
Discussion
GERD after LT adversely affects allograft and patient survival. Compared to medical management of GERD, LARS offers greater improvement in quality of life with less perioperative morbidity in nontransplant patients [7, 9, 10]. However, LARS is reluctantly offered to LT recipients with GERD because of limited evidence of safety in this population. LT recipients have unique pathophysiological characteristics of GERD, often with minimal or atypical symptoms, and are prone to silent tracheal aspiration [20]. Potential mechanical or hypothermia-induced trauma to the vagus nerve during the transplant surgery may result in posttransplant gastroparesis, loss of airway protective reflexes, and impaired mucociliary clearance in the implanted lungs. Additionally, LARS in LT recipients may be technically complex because of friable diaphragmatic tissue quality secondary to steroid use and challenging dissection of the esophagus within the posterior mediastinum secondary to pleural adhesions from transplant surgery [21].
In the present study (the largest to date), we report that the 30-day readmission rate after LARS was higher in the LT cohort than in the NT cohort; however, 38% of readmissions in the LT cohort were planned for medical issues not related to LARS. The second most common indication of readmission of LT recipients was intractable nausea, which was also the most common cause of readmission of NT controls. Similarly, others have reported post-LARS intractable nausea or bloating requiring readmission (up to 22%), recurrence of GERD symptoms (up to 11%), and significant weight loss in LT recipients [5, 6, 22, 23]. However, nausea or rectal flatulence, inability to belch or vomit, and dysphagia are well-described sequelae of LARS in the nontransplant population [9, 17]. Therefore, the perceived surgical risk from the comorbidity burden may not have posed an additional threat in the LT cohort of the current study. Additionally, although post-LARS weight loss may be desired and advantageous in the general population with GERD, it can be mitigated with a high-calorie diet or elective gastrostomy in LT recipients [5].
The post-LARS median length of hospital stay was longer for LT recipients than for matched NT controls. This can be at least partly attributed to the need to optimize multi-drug immunosuppressive regimens. Despite longer post-LARS hospital stays, the operative complication rate of LARS in the LT recipients was comparable to that in the matched NT controls in our study. Several other studies have reported comparative LARS safety data in LT and NT populations [6, 20, 21, 24]. O’Halloran et al. [24] compared the outcomes of laparoscopic Nissen fundoplication in 28 LT recipients and 63 NT reflux patients and reported significantly longer postoperative hospital stays (2.89 vs 0.71 days) and a higher 30-day readmission rate (25% vs 3.2%) in the LT cohort. Similarly, Lau et al. [6] reported that the number of hospital days after LARS was significantly higher (3.8 ± 4.0 days versus 1 day, p = 0.009) in the LT recipients (n = 18) than in the NT GERD patients (n = not available). Fisichella et al. [20] compared post-LARS outcomes in 29 LT recipients and 23 consecutive nontransplant GERD patients and demonstrated comparable operative time (180 min vs 143 min, p = 0.09), blood loss (20 cc vs 15 cc, p = 0.18), and length of stay (1 [1, 2] day vs 1 [1, 2] day, p = 0.75) between the two groups. Although a shorter LARS operative time in LT recipients in our study could be attributed to smaller hiatal hernias, the subjective technical complexity or lack of additional technical difficulty of LARS in LT recipients could be due to surgeon experience and foregut expertise at our LT center. Similar to our experience, Davis et al. [21] also did not find any difference in estimated blood loss, duration of surgery, or length of stay between 25 LT recipients and 23 NT controls. An additional handful of small, non-comparative studies of safety outcomes of LARS in LT recipients have reported satisfactory resolution of reflux symptoms after LARS [5, 22, 23, 25]. We have also demonstrated excellent control of GERD after LARS in LT recipients with objective measures such as a pH study. Notably, early death after LARS (postoperative day 17) and a few late deaths (3 months after LARS) have also been reported, with causes of death reported as unlikely related to LARS [24, 25].
Our study has limitations in addition to its single-center retrospective design. First, the LT patients selected for LARS were likely to be medically healthier than LT recipients who were not offered LARS, potentially limiting the generalization of our study results to all LT recipients with GERD. Second, we reported only the short-term, 30-day outcomes of LARS, but it is unlikely that perioperative surgical issues will affect long-term outcomes. Finally, definitive reflux control after LARS has been documented only in the short term. Nonetheless, our study shows that appropriately selected LT recipients are not more vulnerable to post-LARS adverse events than matched NT controls. We advocate that LARS should be offered to LT patients with pathological GERD, which will hopefully improve allograft and overall survival.
Despite a higher perceived risk of comorbidity burden, LT recipients and matched NT controls had similar rates of post-LARS 30-day morbidity at our large-volume center with expertise in transplant and foregut management. LARS after LT is safe and results in effective reflux control.
References
Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA, Ensor CR, Gottlieb J, Hachem RR, Lama V, Martinu T, Neil DAH, Singer LG, Snell G, Vos R (2019) Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 38:493–503
Masuda T, Mittal SK, Kovács B, Smith MA, Walia R, Huang JL, Bremner RM (2019) Foregut function before and after lung transplant. J Thorac Cardiovasc Surg 158:619–629
Sharma M, Ravichandran R, Perincheri S, Danziger-Isakov L, Heeger PS, Sweet SC, Mohanakumar T (2020) Distinct molecular and immunological properties of circulating exosomes isolated from pediatric lung transplant recipients with bronchiolitis obliterans syndrome—a retrospective study. Transpl Int 33:1491–1502
Fisichella PM, Davis CS, Lowery E, Pittman M, Gagermeier J, Love RB, Kovacs EJ (2012) Pulmonary immune changes early after laparoscopic antireflux surgery in lung transplant patients with gastroesophageal reflux disease. J Surg Res 177:e65-73
Burton PR, Button B, Brown W, Lee M, Roberts S, Hassen S, Bailey M, Smith A, Snell G (2009) Medium-term outcome of fundoplication after lung transplantation. Dis Esophagus 22:642–648
Lau CL, Palmer SM, Howell DN, McMahon R, Hadjiliadis D, Gaca J, Pappas TN, Davis RD, Eubanks S (2002) Laparoscopic antireflux surgery in the lung transplant population. Surg Endosc 16:1674–1678
Anvari M, Allen C, Marshall J, Armstrong D, Goeree R, Ungar W, Goldsmith C (2011) A randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for the treatment of patients with chronic gastroesophageal reflux disease (GERD): 3-year outcomes. Surg Endosc 25:2547–2554
Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Långström G, Lind T, Lundell L (2011) Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 305:1969–1977
Grant AM, Cotton SC, Boachie C, Ramsay CR, Krukowski ZH, Heading RC, Campbell MK (2013) Minimal access surgery compared with medical management for gastro-oesophageal reflux disease: five year follow-up of a randomised controlled trial (REFLUX). BMJ 346:f1908
Grant AM, Wileman SM, Ramsay CR, Mowat NA, Krukowski ZH, Heading RC, Thursz MR, Campbell MK (2008) Minimal access surgery compared with medical management for chronic gastro-oesophageal reflux disease: UK collaborative randomised trial. BMJ 337:a2664
Hatlebakk JG, Zerbib F, des Bruley Varannes S, Attwood SE, Ell C, Fiocca R, Galmiche JP, Eklund S, Långström G, Lind T, Lundell LR (2016) Gastroesophageal acid reflux control 5 years after antireflux surgery, compared with long-term esomeprazole therapy. Clin Gastroenterol Hepatol 14:678–685
Lundell L, Miettinen P, Myrvold HE, Hatlebakk JG, Wallin L, Engström C, Julkunen R, Montgomery M, Malm A, Lind T, Walan A (2009) Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clin Gastroenterol Hepatol 7:1292–1298 (quiz 1260)
Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Liedman B, Hatlebakk JG, Julkonen R, Levander K, Carlsson J, Lamm M, Wiklund I (2001) Continued (5-year) followup of a randomized clinical study comparing antireflux surgery and omeprazole in gastroesophageal reflux disease. J Am Coll Surg 192:172–179 (discussion 179-181)
Mahon D, Rhodes M, Decadt B, Hindmarsh A, Lowndes R, Beckingham I, Koo B, Newcombe RG (2005) Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton-pump inhibitors for treatment of chronic gastro-oesophageal reflux. Br J Surg 92:695–699
Mehta S, Bennett J, Mahon D, Rhodes M (2006) Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 10:1312–1316 (discussion 1316-1317)
Parrilla P, Martínez de Haro LF, Ortiz A, Munitiz V, Molina J, Bermejo J, Canteras M (2003) Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett’s esophagus. Ann Surg 237:291–298
Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, Raufman JP, Sampliner R, Schnell T, Sontag S, Vlahcevic ZR, Young R, Williford W (2001) Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 285:2331–2338
Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE (2015) The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 27:160–174
Schober P, Mascha EJ, Vetter TR (2021) Statistics From A (Agreement) to Z (z Score): a guide to interpreting common measures of association, agreement, diagnostic accuracy, effect size, heterogeneity, and reliability in medical research. Anesth Analg 133:1633–1641
Fisichella PM, Davis CS, Gagermeier J, Dilling D, Alex CG, Dorfmeister JA, Kovacs EJ, Love RB, Gamelli RL (2011) Laparoscopic antireflux surgery for gastroesophageal reflux disease after lung transplantation. J Surg Res 170:e279-286
Davis CS, Jellish WS, Fisichella PM (2010) Laparoscopic fundoplication with or without pyloroplasty in patients with gastroesophageal reflux disease after lung transplantation: how I do it. J Gastrointest Surg 14:1434–1441
Pegna V, Mickevičius A, Tsang C (2014) How useful is antireflux surgery in lung transplant patients with gastroesophageal reflux? Medicina 50:318–322
Robertson AG, Krishnan A, Ward C, Pearson JP, Small T, Corris PA, Dark JH, Karat D, Shenfine J, Griffin SM (2012) Anti-reflux surgery in lung transplant recipients: outcomes and effects on quality of life. Eur Respir J 39:691–697
O’Halloran EK, Reynolds JD, Lau CL, Manson RJ, Davis RD, Palmer SM, Pappas TN, Clary EM, Eubanks WS (2004) Laparoscopic Nissen fundoplication for treating reflux in lung transplant recipients. J Gastrointest Surg 8:132–137
Abbassi-Ghadi N, Kumar S, Cheung B, McDermott A, Knaggs A, Zacharakis E, Moorthy K, Carby M, Hanna GB (2013) Anti-reflux surgery for lung transplant recipients in the presence of impedance-detected duodenogastroesophageal reflux and bronchiolitis obliterans syndrome: a study of efficacy and safety. J Heart Lung Transplant 32:588–595
Acknowledgements
The authors thank Kristine Nally for editorial assistance and Marco Marchionni for graphic design.
Funding
No outside funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Deepika Razia, Sumeet K. Mittal, Rajat Walia, Sofya Tokman, Jasmine L. Huang, Michael A. Smith, and Ross M. Bremner have no conflicts of interest or financial ties to disclose.
Ethical approval
Institutional Review Board approval with waiver of patient consent for this retrospective study was obtained at Norton Thoracic Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona (PHXU-21-500-137-73-18; March 31, 2021).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These data were presented as a poster at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons, March 16–19, 2022; Denver, CO, USA.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Razia, D., Mittal, S.K., Walia, R. et al. Morbidity of antireflux surgery in lung transplant and matched nontransplant cohorts is comparable. Surg Endosc 37, 1114–1122 (2023). https://doi.org/10.1007/s00464-022-09598-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09598-9