Abstract
Background
Traditionally, patients with large liver tumors (≥ 50 mm) have been considered for anatomic major hepatectomy. Laparoscopic resection of large liver lesions is technically challenging and often performed by surgeons with extensive experience. The current study aimed to evaluate the surgical and oncologic safety of laparoscopic parenchyma-sparing liver resection in patients with large colorectal metastases.
Methods
Patients who primarily underwent laparoscopic parenchyma-sparing liver resection (less than 3 consecutive liver segments) for colorectal liver metastases between 1999 and 2019 at Oslo University Hospital were analyzed. In some recent cases, a computer-assisted surgical planning system was used to better visualize and understand the patients’ liver anatomy, as well as a tool to further improve the resection strategy. The surgical and oncologic outcomes of patients with large (≥ 50 mm) and small (< 50 mm) tumors were compared. Multivariable Cox-regression analysis was performed to identify risk factors for survival.
Results
In total 587 patients met the inclusion criteria (large tumor group, n = 59; and small tumor group, n = 528). Median tumor size was 60 mm (range, 50–110) in the large tumor group and 21 mm (3–48) in the small tumor group (p < 0.001). Patient age and CEA level were higher in the large tumor group (8.4 μg/L vs. 4.6 μg/L, p < 0.001). Operation time and conversion rate were similar, while median blood loss was higher in the large tumor group (500 ml vs. 200 ml, p < 0.001). Patients in the large tumor group had shorter 5 year overall survival (34% vs 49%, p = 0.027). However, in the multivariable Cox-regression analysis tumor size did not impact survival, unlike parameters such as age, ASA score, CEA level, extrahepatic disease at liver surgery, and positive lymph nodes in the primary tumor.
Conclusion
Laparoscopic parenchyma-sparing resections for large colorectal liver metastases provide satisfactory short and long-term outcomes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Minimally invasive procedures have revolutionized the surgical practice in many surgical sub-specialties as well as in hepatobiliary surgery. Laparoscopic liver surgery has shown its numerous advantages over conventional open surgery and has been established as a first-line surgical approach in specialized centers, despite its relatively slow implementation [1,2,3,4].
Over the last two decades, the evidence level of laparoscopic liver surgery has increased significantly, from small case series of selected patients to large multi-center series and randomized control trials [5,6,7]. This minimally invasive liver surgery has been well reported for benign and malignant liver tumors, including primary and secondary liver malignancies [8,9,10,11]. In 2017 the European consensus guidelines meeting for laparoscopic liver surgery held in Southampton, United Kingdom, it was advocated that the laparoscopic approach should be considered standard practice for lesions in the left lateral and the anterior segments, while technically challenging resections, such as repeated resections or 2-staged hepatectomies, resections for large lesions, and lesions close to the liver hilum were considered possible by surgeons with extensive experience in laparoscopic liver surgery[12]. Earlier, in the first international consensus meeting (the Louisville Statement, 2008), it was stated that the patients with solitary lesions, 50 mm or less, located in the antero-lateral segments are acceptable indications for laparoscopic liver resection [13].
In our center, the main indication for laparoscopic liver resections is colorectal liver metastases (CRLM), where the parenchyma-sparing strategy is the method of choice [1, 14, 15]. However, the laparoscopic parenchyma-sparing approach to resect large lesions is challenging, and careful pre-operative surgical planning is essential for evaluating the chosen resection strategy. In this context, the use of computer-assisted resection planning systems can provide surgeons with an accurate characterization of the resection in terms of trajectory, safety margins, and resection volumetry [16]. To the best of our knowledge, most of the studies on laparoscopic parenchyma-sparing liver resections (LPSLR) reported the results of single small metastases. Earlier, we reported our experience in LPSLR for patients with multiple CRLM and metastases located in the postero-superior liver segments [17, 18]. The current analysis aimed to evaluate the surgical and oncologic outcomes after LPSLR in patients with large (≥ 50 mm) CRLM.
Methods
Study design and definitions
The study was conducted at Oslo University Hospital, a tertiary referral center for hepato-pancreato-biliary surgery for South-Eastern Norway Health Authority, serving about three million population. Patients who primarily underwent laparoscopic parenchyma-sparing (defined as a resection of less than three consecutive liver segments) liver resection for colorectal liver metastases between 1999 and 2019 at Oslo University Hospital were identified from the prospectively registered database and included in this study. Patients that had previously undergone liver resection were excluded. The surgical and oncologic outcomes of patients with large (≥ 50 mm) and small (< 50 mm) tumors were retrospectively analyzed and compared. The Institutional Review Board approved the study and due to the retrospective nature of the study written consents from the patients was not required.
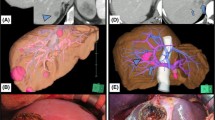
Perioperative management and surgical techniques have been described previously [19]. Standard preoperative investigations included clinical biochemistry, liver ultrasound, contrast-enhanced computed tomography (CT) scans and/or magnetic resonance imaging (MRI) of the thorax and abdomen, and positron emission tomography (PET) scan - if required (cases with suspicion of extrahepatic disease that cannot be confirmed by CT or MRI). In some cases, three-dimensional (3D) patient-specific liver models were created based on pre-operative CT and MRI images and used for virtual resection planning (Fig. 1).
Preoperative virtual resection planning
Virtual resection planning systems are computer-assisted systems that help surgeons define anatomy, resections and measure properties (e.g., volumetry, distances, safety margins, geometry, etc.) before the actual operation. While LPSLR can be performed using state-of-the-art medical imaging and surgical technology, the use of virtual resection planning systems can provide surgeons with information about the spatial distribution of relevant anatomical structures and the path of planned resection. This information can aid in the decision-making process during the planning and ultimately validate the resection plan.
In our workflow, preoperative CT or MRI are first segmented (images are annotated in 3D) and then reconstructed into a 3D patient-specific liver anatomy and pathology model. These 3D models contain liver parenchyma, portal, hepatic veins, and the relevant liver lesions [20,21,22]. Using a virtual resection planning system, a virtual deformable surface can be placed inside the patient-specific models, enabling the physicians to place and manipulate virtual resections to create a satisfactory resection plan (Fig. 2). Our implementation of a virtual resection planning system uses the software 3D Slicer and a custom-developed software module providing the resection and analysis tools [16, 23]. The necessary preparations (segmentation, 3D model reconstruction, and clinical validation of this information) are performed by a team of computer scientists, biomedical engineers, and clinicians. Surgeons generate and tailor virtual resection plans for individual clinical cases (Fig. 2).
Virtual resection planning steps (1. positioning a resection line and a virtual deformable surface, 2. manipulating the resection plane with control points (green balls) and 3. creating final virtual resection plan). Two proposed resection plans by a computer-assisted system (a). an atypical/non anatomic segmentectomy. (b). an atypical/non-anatomic bi-segmentectomy, and the final decision is made by the surgeon (Color figure online)
Definitions and statistics
The 90 days after surgery definition was used to report postoperative mortality, and the Accordion classification was applied to grade postoperative complications [24]. Tumor size was measured following specimen fixation in formaldehyde during the histopathologic analyses of resected specimens. Resection margins were assessed microscopically, and a resection margin of less than 1 mm was defined as positive (R1).
Data are presented as median (range) or mean (SD) and number (percentage). Categorical variables were compared using the Fisher’s exact test or the Chi-square test as appropriate and presented as number (percentage). Non-normally distributed continuous variables were compared using the Mann–Whitney U test and are presented as median (range), while normally distributed data are presented as mean (standard deviation [SD]), and Student’s T-test was applied to compare these variables.
Overall survival was estimated from the date of liver resection until death or censoring. Survival probabilities were calculated using the Kaplan–Meier method, and the Log-rank test was applied to compare survival times between the groups. Time-defined survivals are presented in percentage (± standard error). Uni- and multivariable Cox-regression analysis was performed to identify risk factors associated with poor survival. P-values less than or equal to 0.05 were considered statistically significant.
SPSS software (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, version 27.0, Armonk, NY, USA: IBM corp.) was used for statistical analysis.
Results
In total, 587 patients met the inclusion criteria (large tumor group, n = 59; and small tumor group, n = 528). Patient age and CEA level were higher in those with large tumors. Other baseline characteristics were similar between the groups (Table 1).
Median tumor size was 60 mm (range, 50–110) in the large tumor group and 21 mm (range, 3 to 48) in the small tumor group (p < 0.001) (Table 2).
In the large tumor group, 56% of the patients had resection in the postero-superior segments (technically major resections), versus 49% of patients in the small tumor group. Fourteen (24%) patients in the large tumor group and 78 (15%) in the small tumor group had other simultaneous abdominal procedures (p = 0.073). Operation time and conversion rate were similar, while median blood loss was higher in the large tumor group (500 ml vs. 200 ml, p < 0.001). Other perioperative outcomes, including postoperative morbidity and mortality, were similar. No difference in positive resection margins was found between the groups (Table 2).
Patients in the large tumor group had significantly shorter median overall survival, 47 (95%CI 35 to 59) months versus 57 (95%CI 46 to 68) months) (p = 0.027). 5 year overall survival was 34% (± 8.6) in the large tumor group and 49% (± 3.1) in the small tumor group (Table 3; Fig. 3). However, in the multivariable Cox-regression analysis, tumor size did not impact survival, unlike parameters such as patients’ age, ASA score, CEA level, presence of extrahepatic disease at liver surgery, and positive lymph nodes in the primary tumor that were independent predictors for poor overall survival (Table 4).
The preoperative virtual resection planning method was used in some of the more advanced cases included in this study during the last years and thus not systematically implemented in routine practice throughout the study period. Therefore, it is not presented as a variable to evaluate its impact on the surgical outcomes but as a tool for verification of the resection strategy and decision support.
Discussion
Laparoscopic approach to resect large liver lesions remains debatable and may still be a relative contraindication in many centers. Parenchyma-sparing liver resection performed by laparoscopic access in patients with large tumors can be technically challenging and is preserved for surgeons with extensive experience. The findings of the current analysis show that LPSLR for patients with large CRLM can achieve satisfactory results, similar to those with small lesions. It was associated with higher blood loss, while other perioperative outcomes were similar.
The parenchyma-sparing strategy for CRLM has shown its advantages and has been widely used [25, 26]. These resections are associated with decreased morbidity and increased salvageability and may improve the patients’ survival by facilitating future liver resections in case of liver recurrences [27,28,29]. In the report from Torzilli and colleagues, the authors distinct the parenchyma-sparing liver surgery as a minimally invasive surgery in a hepatic-centered perspective even if the surgery is performed by open approach [30].
In expert centers as well as in our center, laparoscopic liver surgery has been safely adopted and is used to perform numerous types of liver resections1. In a systematic review and meta-analysis by Kalil et al., the laparoscopic approach to perform parenchyma-sparing liver resections was defined as “maximally minimally invasive” surgery of the liver [31]. However, large liver malignancies to be removed by laparoscopic approach remains questionable, and the current data is limited by the case series with a relatively small number of patients [32, 33]. To the best of our knowledge, our report consists of the largest series focusing on laparoscopic liver resection for large CRLM and is the first study reporting parenchyma-sparing strategy for these patients. Comparison with the group of patients with smaller metastases shows that laparoscopic resection of large liver tumors in expert hands can achieve similar surgical outcomes. The higher blood loss seen in this series is in line with the previous studies on laparoscopic liver resection for large liver tumors [32, 34]. The worse overall survival in the large tumor group was somehow expected since the size of the tumor is a prognostic factor and has been included in clinical scoring systems [35, 36]. However, in this series, the tumor size did not significantly impact patients' survival in multivariable analysis (Table 4). It might be explained by the higher median value of the CEA level in patients with large tumors, which might have adjusted the impact of the tumor size.
LPSLR has become a standard surgical method in our center and is our preferred approach, especially in patients with CRLM, and it is preferred whenever possible. However, computer-assisted systems for patient-specific anatomy visualization and surgery planning could further improve the procedure. Through medical image segmentation and reconstruction techniques, 3D patient-specific liver models can help in surgery planning, especially in challenging cases, such as large tumors, tumors located in the “difficult” segments, deep located tumors, and tumors with close proximity to the major vessels. Moreover, resection planning using these imaging advancements and taking into account both inflow and outflow to the resection area can provide surgeons a better understanding of individual patient liver anatomy, tumor location, and its relation to the vessels, as well as a precise trajectory of the resection plane.
The current analysis has several limitations. Firstly, information bias is possibly present due to the retrospective nature of this analysis. The presented computer-assisted resection planning method was not presented as a variable, and we could not evaluate its impact. Further investigations are in process, and more results will be available in the future. False-negative findings are possible when comparing the groups, caused by the significant difference in the number of patients in the groups, which is another weakness of this study.
Conclusion
Based on our experience and the current analysis results, we may conclude that laparoscopic liver surgery is safe and provides good surgical and oncologic outcomes even for challenging tumors. Laparoscopic parenchyma-sparing liver resections should be preferred whenever technically possible. The continuous advancements in medical technologies can potentially improve these procedures.
References
Aghayan DL, Kazaryan AM, Fretland ÅA, Røsok B, Barkhatov L, Lassen K, Edwin B (2021) Evolution of laparoscopic liver surgery: 20-year experience of a Norwegian high-volume referral center. Surgical Endoscopy. https://doi.org/10.1007/s00464-021-08570-3
Görgec B, Fichtinger RS, Ratti F, Aghayan D, Van der Poel MJ, Al-Jarrah R, Armstrong T, Cipriani F, Fretland ÅA, Suhool A, Bemelmans M, Bosscha K, Braat AE, De Boer MT, Dejong CHC, Doornebosch PG, Draaisma WA, Gerhards MF, Gobardhan PD, Hagendoorn J, Kazemier G, Klaase J, Leclercq WKG, Liem MS, Lips DJ, Marsman HA, Mieog JSD, Molenaar QI, Nieuwenhuijs VB, Nota CL, Patijn GA, Rijken AM, Slooter GD, Stommel MWJ, Swijnenburg RJ, Tanis PJ, Te Riele WW, Terkivatan T, Van den Tol PMP, Van den Boezem PB, Van der Hoeven JA, Vermaas M, Edwin B, Aldrighetti LA, Van Dam RM, Abu Hilal M, Besselink MG, Group ftDLC (2021) Comparing practice and outcome of laparoscopic liver resection between high-volume expert centres and nationwide low-to-medium volume centres. Br J Surg 108:983–990
Fretland AA, Dagenborg VJ, Bjornelv GMW, Kazaryan AM, Kristiansen R, Fagerland MW, Hausken J, Tonnessen TI, Abildgaard A, Barkhatov L, Yaqub S, Rosok BI, Bjornbeth BA, Andersen MH, Flatmark K, Aas E, Edwin B (2018) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 267:199–207
Fretland AA, Dagenborg VJ, Waaler Bjornelv GM, Aghayan DL, Kazaryan AM, Barkhatov L, Kristiansen R, Fagerland MW, Edwin B, Andersen MH (2019) Quality of life from a randomized trial of laparoscopic or open liver resection for colorectal liver metastases. Br J Surg 106:1372–1380
Aghayan DL, Kazaryan AM, Dagenborg VJ, Rosok BI, Fagerland MW, Waaler Bjornelv GM, Kristiansen R, Flatmark K, Fretland AA, Edwin B (2021) Long-term oncologic outcomes after laparoscopic versus open resection for colorectal liver metastases: a randomized trial. Ann Intern Med 174:175–182
Robles-Campos R, Lopez-Lopez V, Brusadin R, Lopez-Conesa A, Gil-Vazquez PJ, Navarro-Barrios A, Parrilla P (2019) Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 33:3926–3936
Syn NL, Kabir T, Koh YX, Tan HL, Wang LZ, Chin BZ, Wee I, Teo JY, Tai BC, Goh BKP (2019) Survival advantage of laparoscopic versus open resection for colorectal liver metastases: a meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann Surg. https://doi.org/10.1097/SLA.0000000000003672
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263:761–777
Ban D, Tanabe M, Kumamaru H, Nitta H, Otsuka Y, Miyata H, Kakeji Y, Kitagawa Y, Kaneko H, Wakabayashi G, Yamaue H, Yamamoto M (2020) Safe dissemination of laparoscopic liver resection in 27,146 cases between 2011 and 2017 from the national clinical database of Japan. Ann Surg. https://doi.org/10.1097/SLA.0000000000003799
Ratti F, Cipriani F, Fiorentini G, Burgio V, Ronzoni M, Della Corte A, Cascinu S, De Cobelli F, Aldrighetti L (2021) Evolution of surgical treatment of colorectal liver metastases in the real world: single center experience in 1212 cases. Cancers 13:1178
Berardi G, Van Cleven S, Fretland AA, Barkhatov L, Halls M, Cipriani F, Aldrighetti L, Abu Hilal M, Edwin B, Troisi RI (2017) Evolution of laparoscopic liver surgery from innovation to implementation to mastery: perioperative and oncologic outcomes of 2,238 patients from 4 European specialized centers. J Am Coll Surg. https://doi.org/10.1016/j.jamcollsurg.2017.08.006
Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, Aroori S, Belli G, Besselink M, Briceno J, Gayet B, D’Hondt M, Lesurtel M, Menon K, Lodge P, Rotellar F, Santoyo J, Scatton O, Soubrane O, Sutcliffe R, Van Dam R, White S, Halls MC, Cipriani F, Van der Poel M, Ciria R, Barkhatov L, Gomez-Luque Y, Ocana-Garcia S, Cook A, Buell J, Clavien PA, Dervenis C, Fusai G, Geller D, Lang H, Primrose J, Taylor M, Van Gulik T, Wakabayashi G, Asbun H, Cherqui D (2017) The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. https://doi.org/10.1097/SLA.0000000000002524
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: the louisville statement, 2008. Ann Surg 250:825–830
Kazaryan AM, Marangos IP, Rosok BI, Rosseland AR, Villanger O, Fosse E, Mathisen O, Edwin B (2010) Laparoscopic resection of colorectal liver metastases: surgical and long-term oncologic outcome. Ann Surg 252:1005–1012
Aghayan DL, Pelanis E, Avdem Fretland A, Kazaryan AM, Sahakyan MA, Rosok BI, Barkhatov L, Bjornbeth BA, Jakob Elle O, Edwin B (2018) Laparoscopic parenchyma-sparing liver resection for colorectal metastases. Radiol Oncol 52:36–41
Palomar R, Cheikh FA, Edwin B, Fretland A, Beghdadi A, Elle OJ (2017) A novel method for planning liver resections using deformable Bezier surfaces and distance maps. Comput Methods Programs Biomed 144:135–145
Kazaryan AM, Aghayan DL, Barkhatov LI, Fretland AA, Edwin B (2019) Laparoscopic multiple parenchyma-sparing concomitant liver resections for colorectal liver metastases. Surg Laparosc, Endosc Percutaneous Tech 29:187–193
Aghayan DL, Fretland AA, Kazaryan AM, Sahakyan MA, Dagenborg VJ, Bjornbeth BA, Flatmark K, Kristiansen R, Edwin B (2019) Laparoscopic versus open liver resection in the posterosuperior segments: a sub-group analysis from the OSLO-COMET randomized controlled trial. HPB (Oxford) 21:1485–1490
Kazaryan AM, Rosok BI, Marangos IP, Rosseland AR, Edwin B (2011) Comparative evaluation of laparoscopic liver resection for posterosuperior and anterolateral segments. Surg Endosc 25:3881–3889
Kumar RP, Barkhatov L, Edwin B, Albregtsen F, Elle OJ (2018) Portal and Hepatic Vein Segmentation with Leak Restriction: A Pilot Study. In: Eskola H, Väisänen O, Viik J, Hyttinen J (eds) EMBEC & NBC 2017. Springer, Singapore, pp 823–826
Pelanis E, Kumar RP, Aghayan DL, Palomar R, Fretland AA, Brun H, Elle OJ, Edwin B (2020) Use of mixed reality for improved spatial understanding of liver anatomy. Minim Invasive Ther Allied Technol 29:154–160
Kumar RP, Pelanis E, Bugge R, Brun H, Palomar R, Aghayan DL, Fretland ÅA, Edwin B, Elle OJ (2020) Use of mixed reality for surgery planning: assessment and development workflow. J Biomed Inform 8:100077
Pieper S, Halle M, Kikinis R (2004) 3D Slicer. 2004 2nd IEEE international symposium on biomedical imaging: nano to macro (IEEE Cat No. 04EX821), IEEE, pp 632–635
Strasberg SM, Linehan DC, Hawkins WG (2009) The accordion severity grading system of surgical complications. Ann Surg 250:177–186
Evrard S, Torzilli G, Caballero C, Bonhomme B (2018) Parenchymal sparing surgery brings treatment of colorectal liver metastases into the precision medicine era. Eur J Cancer (Oxford, England: 1990) 104:195–200
Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, Beal EW, Felekouras E, Vernadakis S, Fung JJ, Pawlik TM (2017) Liver transplantation in patients with liver metastases from neuroendocrine tumors: a systematic review. Surgery 162:525–536
Kingham TP, Correa-Gallego C, D’Angelica MI, Gonen M, DeMatteo RP, Fong Y, Allen PJ, Blumgart LH, Jarnagin WR (2015) Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg 220:471–479
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C (2016) Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg 263:146–152
Barkhatov L, Aghayan DL, Scuderi V, Cipriani F, Fretland ÅA, Kazaryan AM, Ratti F, Armstrong T, Belli A, Dagher I, Belli G, Aldrighetti L, Hilal MA, Troisi RI, Edwin B (2021) Long-term oncological outcomes after laparoscopic parenchyma-sparing redo liver resections for patients with metastatic colorectal cancer: a European multi-center study. Surg Endosc. https://doi.org/10.1007/s00464-021-08655-z
Torzilli G, McCormack L, Pawlik T (2020) Parenchyma-sparing liver resections. Int J Surg. https://doi.org/10.1016/j.ijsu.2020.04.047
Kalil JA, Poirier J, Becker B, Van Dam R, Keutgen X, Schadde E (2019) Laparoscopic parenchymal-sparing hepatectomy: the new maximally minimal invasive surgery of the liver-a systematic review and meta-analysis. J Gastrointest Surg 23:860–869
Shelat VG, Cipriani F, Basseres T, Armstrong TH, Takhar AS, Pearce NW, AbuHilal M (2015) Pure laparoscopic liver resection for large malignant tumors: does size matter? Ann Surg Oncol 22:1288–1293
Levi Sandri GB, Spoletini G, Vennarecci G, Francone E, Abu Hilal M, Ettorre GM (2018) Laparoscopic liver resection for large HCC: short- and long-term outcomes in relation to tumor size. Surg Endosc 32:4772–4779
Cheung T-T, Wang X, Efanov M, Liu R, Fuks D, Choi G-H, Syn NL, Chong CC, Sucandy I, Chiow AKH, Marino MV, Gastaca M, Lee JH, Kingham TP, D’Hondt M, Choi SH, Sutcliffe RP, Han H-S, Tang CN, Pratschke J, Troisi RI, Goh BKP, International R, Laparoscopic Liver Resection Study Group C (2021) Minimally invasive liver resection for huge (≥10 cm) tumors: an international multicenter matched cohort study with regression discontinuity analyses. Hepatobiliary Surg Nutr 10:587–597
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318 (discussion 318-321)
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG (2008) Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 247:125–135
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Davit L. Aghayan, Gabriella d’Albenzio, Åsmund A. Fretland, Egidijus Pelanis, Bård I. Røsok, Sheraz Yaqub, Rafael Palomar and Bjørn Edwin have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The material presented in this manuscript was partly reported as an oral presentation at the 14th Biennial E-AHPBA Congress (virtual) in September 2021 and at the ILLS 3rd World Congress (virtual) in June 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aghayan, D.L., d’Albenzio, G., Fretland, Å.A. et al. Laparoscopic parenchyma-sparing liver resection for large (≥ 50 mm) colorectal metastases. Surg Endosc 37, 225–233 (2023). https://doi.org/10.1007/s00464-022-09493-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09493-3