Abstract
Introduction
Revisional bariatric surgery is being increasingly performed and is associated with higher operative risks. Optimal techniques to minimize complications remain controversial. Here, we report a retrospective review of the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Participant User Files (PUF) database, comparing outcomes between revision RBS and LBS.
Methods
The 2015 and 2016 MBSAQIP PUF database was retrospectively reviewed. Revision cases were identified using the Revision/Conversion Flag. Selected cases were further stratified by surgical approach. Subgroup analysis of sleeve gastrectomy and gastric bypass cases was performed. Case–controlled matching (1:1) was performed of the RBS and LBS cohorts, including gastric bypass and sleeve gastrectomy cohorts separately. Cases and controls were match by demographics, ASA classification, and preoperative comorbidities.
Results
26,404 revision cases were identified (93.3% LBS, 6.7% RBS). 85.6% were female and 67% white. Mean age and BMI were 48 years and 40.9 kg/m2. 1144 matched RBS and LBS cases were identified. RBS was associated with longer operative duration (p < 0.0001), LOS (p = 0.0002) and a higher rate of ICU admissions (1.3% vs 0.5%, p = 0.05). Aggregate bleeding and leak rates were higher in the RBS cohort. In both gastric bypass and sleeve gastrectomy cohorts, the robotic-assisted surgery remain associated with longer operative duration (p < 0.0001). In gastric bypass, rates of aggregate leak and bleeding were higher with robotic surgery, while transfusion was higher with laparoscopy. For sleeve gastrectomy cases, reoperation, readmission, intervention, sepsis, organ space SSI, and transfusion were higher with robotic surgery.
Conclusion
In this matched cohort analysis of revision bariatric surgery, both approaches were overall safe. RBS was associated with longer operative duration and higher rates of some complications. Complications were higher in the robotic sleeve cohort. Robotic is likely less cost-effective with no clear patient safety benefit, particularly for sleeve gastrectomy cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Metabolic and bariatric surgery remains the most effective treatment for severe obesity [1,2,3,4,5,6,7]. As the prevalence of obesity has grown increasingly pervasive in our society, more patients are turning to surgical treatment modalities for weight loss, and for improvement in comorbid conditions and quality of life [2, 3, 6,7,8]. Despite the reported effectiveness of bariatric surgery [2, 6,7,8], weight recidivism remains a significant challenge for bariatric patients and surgeons. An estimated 10–20% of patients will regain significant weight long-term post-bariatric surgery or fail to achieve a significant amount of weight loss [2, 7, 9, 10]. This has significant health and economic implications, as weight regain results in relapse of obesity-related comorbid conditions, such as diabetes [2, 8, 10], disability, a reduction in quality of life, and loss of productivity due to lost work days [7, 11], all of which contribute to increased healthcare-related costs. This high rate of weight recidivism following bariatric surgery is also consistent with the reported twofold increase in revisional bariatric procedures in recent literature [12, 13].
The optimal treatment modality for weight recidivism post-bariatric surgery remains controversial. Most practitioners agree that early recognition and intervention for weight recidivism post-bariatric surgery is important in containing obesity-related healthcare costs in this cohort of patients [3, 4, 14]; however, standardized practice guidelines for managing these patients are lacking. The spectrum of treatment recommendation includes behavior modification [7, 10], medication [10], endoscopic bariatric therapy [15], and revisional bariatric surgery [1,2,3,4,5,6,7, 9, 12, 16,17,18,19,20], with varying results. Revisional bariatric surgery is often recommended for those with inadequate weight loss or significant weight regain, as well as persistence comorbid conditions following primary bariatric surgery [1, 4, 9]. Other reasons for revisional or conversional bariatric surgery vary and are related to physiologic and anatomic complications associated with the index surgical procedure [1, 2, 6, 7].
Outcomes following revision or conversion bariatric surgery are not similar to outcomes following primary bariatric surgery [3, 4, 18, 19]. While some small cohorts and meta-analyses have reported no difference in complication rates between primary and revisional bariatric cases [6, 7, 18, 19], others have reported that weight loss is less and complication rates are higher in revisional bariatric surgery [14, 17, 20]. The optimal surgical approach also remains a point of controversy. As technical approaches to surgical weight loss continue to evolve, the robotic platform continues to be increasing used; however, the role, safety, and cost-effectiveness of this platform remain unclear for both primary and revisional bariatric surgery. There are limited published studies on revisional or conversional robotic bariatric surgery [5, 16, 17]. Most are small retrospective cohorts, limiting our understanding of outcomes following robotic revisional bariatric surgery. We present the largest retrospective cohort analysis of revisional bariatric surgery comparing conventional laparoscopic and the robotic-assisted techniques.
Materials and methods
Data source
We performed a retrospective analysis of the 2015 and 2016 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program Participant Use File (MBSAQIP PUF) database for this study. We compared outcomes in revision or conversion metabolic and bariatric surgery performed with conventional laparoscopic or robotic-assisted techniques. The MBSAQIP accredits bariatric surgical facilities in the United States, who are then required to report bariatric surgical outcomes to the MBSAQIP PUF. The MBSAQIP PUF serves as a file registry that contains prospective, risk-adjusted data based on preoperative, intraoperative, and post-operative variables specific to bariatric surgery. Data is collected by trained Metabolic and Bariatric Surgery (MBS) Clinical Reviewers at each bariatric center and audited similar by the National Surgical Quality Improvement Program (NSQIP). De-identified data is reported on patient characteristics, operative details, and intraoperative and perioperative outcomes. As our study utilized deidentified data from a national clinical database, neither institutional review board (IRB) approval nor consent was required.
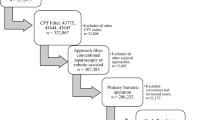
There are 355,675 bariatric cases in the combined 2015 and 2016 MBSAQIP PUF. We first excluded cases without the revision/conversion flag in the database. This excluded all primary MBS procedures. We then excluded cases by surgical approach, including only revision cases performed by either conventional laparoscopic or robotic-assisted techniques. From this cohort, we identified patients who had a revision/conversion bariatric operation using Current Procedure Terminology (CPT) codes for laparoscopic gastric proximal gastric bypass (43,644), laparoscopic distal gastric bypass (43,645), laparoscopic sleeve gastrectomy (43,775), laparoscopic gastric band (43,770), and laparoscopic duodenal switch (43,659). Our case selection algorithm resulted in exclusion of primary bariatric cases, all cases not performed by conventional laparoscopic or robotic-assisted techniques, revision cases that were not a revision/conversion to another bariatric procedure, as well as cases in our final study cohort with missing data points.
Case–control matching
In order to control for possible confounding variables, we performed 1:1 case–control matching of the entire cohort. Cases and controls were matched by patient demographics (age, gender, race/ethnicity, and body mass index (BMI) closest to surgery), ASA classification and preoperative comorbid conditions (history of myocardial infarction (MI), hypertension requiring medication, hyperlipidemia, renal insufficiency, need for dialysis, venous thrombosis requiring therapy, history of pulmonary embolism (PE), ambulation status, functional dependence, diabetes mellitus, steroid and immunosuppressant use, smoking status within 1 year of surgery, obstructive sleep apnea (OSA), chronic obstructive pulmonary disease (COPD), and oxygen dependence) (Table 1). Procedure-specific subgroup analyses were also performed, comparing case–control matched robotic-assisted versus conventional laparoscopic sleeve gastrectomy (SG) cases and robotic-assisted versus conventional laparoscopic roux-en-y gastric bypass (RnYGB) cases.
Outcome measures
Thirty primary outcomes variables were assessed, including operative time, hospital length of stay, conversion rate, discharge status, 30-day ICU admission, reoperation, readmission, intervention, or mortality, death likely related to bariatric surgery, drain present at 30-days, renal failure, progressive renal insufficiency, cardiopulmonary resuscitation (CPR), coma > 24 h, stroke, myocardial infarction, venous thrombosis requiring therapy, pulmonary emboli, transfusion, pneumonia, on ventilator > 48 h, unplanned intubation, peripheral nerve injury, urinary tract infection (UTI), sepsis, septic shock, superficial soft tissue infection (SSI), deep SSI, and organ space SSI. Seven aggregate complications were also assessed, including aggregate leak—as previously described by Berger et al. [21], bleeding, renal failure, cardiovascular and pulmonary complications, venous thromboembolic events and surgical site infection. Aggregate methodology is reported in Table 2. Primary and aggregate outcomes were analyzed for the entire unmatched cohort and case–control matched cohorts.
Statistical analysis
Pearson’s Chi squared test for categorical variables (i.e., gender, race, ASA class, and preoperative comorbidities) and an independent two sample t test and Mann–Whitney test for normally and non-normally distributed continuous variables (age, weight, and BMI), respectively, was performed to provide a univariate analysis of patient demographics, preoperative co-morbid conditions, 30-day outcomes, perioperative complications, and aggregate complications. Categorical variables were reported as frequency and percentage, and continuous variables as mean ± standard deviation (SD). All statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC). A p value < 0.05 was considered statistically significant.
Results
Of the 355,675 cases in the 2015 and 2016 MBSAQIP PUF database, 26,404 were identified as being laparoscopic or robotic-assisted revision/conversion cases and were included in this analysis. Descriptive statistics of the entire unmatched cohort is reported in Table 3. Mean age was 48 years. The majority of patients were white (67.0%) and female (85.6%). On univariate analysis of the entire cohort, the robotic-assisted cohort had a higher preoperative weight (lbs) (248.9 lbs vs. 253.2 lbs, p = 0.004) and BMI (40.9 kg/m2 vs. 41.5 kg/m2, p = 0.006) closest to surgery. This unmatched robotic-assisted revision/conversion cohort also had an increased incidence of hyperlipidemia (22.6% vs 19.4%, p = 0.0009), diabetes mellitus (18.6% vs. 16.2%, p = 0.007), and oxygen dependence (0.9% vs 0.5%, p = 0.0401) when compared to the unmatched laparoscopic revisional/conversional cohort.
Perioperative and aggregate outcomes for the entire unmatched conventional laparoscopic and robotic-assisted cohorts are described in Table 4. Operative duration (min) (121.7 ± 67.5 vs. 177.4 ± 79.4, p < 0.0001) and hospital length of stay (days) (2.2 ± 3.1 vs. 2.4 ± 3.1, p = 0.01) were significantly longer in the robotic-assisted cohort. 30-day reoperation (4.3% vs. 3.2%, p = 0.01), readmission (8.5% vs. 6.6%, p = 0.0024), and intervention (4.4% vs. 3.1%, p = 0.003) were also significantly higher in the robotic-assisted cohort. Perioperative complications were similar between the two cohorts, except for a higher rate of intraoperative or post-operative transfusion (1.5% vs. 0.9%, p = 0.04) in the conventional laparoscopic cohort. Aggregate complications were also similar between the cohorts, except for a significantly higher rate of leak (1.7% vs. 0.9%, p = 0.003) in the robotic-assisted cohort. Aggregate bleeding trended toward being significantly higher in the conventional laparoscopic cohort (p = 0.05). There was no mortality difference between the two cohorts (0.2% vs. 0.23%, p = 0.8).
Perioperative and aggregate outcomes following matched cohort analysis of all included bariatric procedures are described in Table 5. After 1:1 case–control matching for patient demographics and preoperative comorbidities (Table 1), 2288 cases and controls were identified. Operative duration (min) (119.5 ± 64.1 vs. 173.7 ± 78.9, p < 0.0001) and hospital length of stay (days) (1.9 ± 1.8 vs. 2.3 ± 2.2, p = 0.0002) remained significantly longer in the robotic-assisted cohort. 30-day outcomes were similar between the two cohorts, except for a higher rate of unplanned ICU admission in the robotic-assisted cohort (1.3% vs. 0.5%, p = 0.05). All perioperative complications were also similar between the two cohorts, including intraoperative or post-operative transfusion with 72-h, which was significantly higher for the conventional laparoscopic cohort in the unmatched cohort analysis (p = 0.04 vs. 1.0). Aggregate bleeding (1.0% vs. 0.4%, p = 0.07) and leak (1.3% vs. 0.6%, p = 0.09) remained higher in the robotic-assisted cohort, trending toward statistical significance. All other aggregate complications were similar between the two cohorts (Table 5).
Subgroup analyses of SG and RnYGB cohorts were then performed. Perioperative and aggregate outcomes for the unmatched revision SG and RnYGB cohorts are detailed in Table 6. In the unmatched RnYGB cohort (n = 7901), 9.2% were performed robotically. In comparison with conventional laparoscopic cases, robotic-assisted cases were associated with significantly longer operative duration (195.4 ± 73.1 min vs. 154.9 ± 73.2 min, p < 0.0001) and higher rates of conversion (1.8% vs. 0.8%, p = 0.008) and aggregate bleeding (1.8% vs. 0.9%, p = 0.02). In contrast, the conventional laparoscopic cohort had significantly higher rates of transfusion requirement (2.1% vs. 0.8%, p = 0.02), aggregate leak (1.1% vs. 0.9%, p = 0.04), and pulmonary complications (1.4% vs. 0.6%, p = 0.05). Mortality, morbidity, 30-day adverse outcomes, and other complications were not significantly different in the unmatched revision robotic and laparoscopic bypass cohorts. In the unmatched sleeve gastrectomy cohort (n = 11,525), 5.9% were performed robotically. The robotic-assisted approach was associated with significantly longer operative duration (145.3 ± 54.4 min vs. 103.9 ± 48.5 min, p < 0.0001), length of stay (2.01 ± 3.2 days vs. 1.8 ± 1.9 days, p < 0.0001), and higher rates of conversion (0.9% vs. 0.3%, p = 0.008), 30-day adverse outcomes including reoperation (3.1% vs. 1.6%, p = 0.003), readmission (5.6% vs. 3.9%, p = 0.03) and intervention (3.4% vs. 1.7%, p = 0.001), use of ventilator > 48 h (0.3% vs. 0.06%, p = 0.04), unplanned intubation (0.4%, vs. 0.1%, p = 0.02), post-operative UTI (0.9% vs. 0.3%, p = 0.005), post-operative sepsis (0.7% vs. 0.1%, p = 0.0002), organ space SSI (1.3% vs. 0.4%, p = 0.0007), and aggregate leak (1.3% vs. 0.6%, p = 0.03).
Peri-operative and aggregate outcomes for procedure-specific matched cohorts are detailed in Table 7. Following 1:1 case–control matching, 668 revisional gastric bypass (338 robotic-assisted and 338 conventional laparoscopic) and 778 revisional sleeve gastrectomy (389 robotic-assisted and 389 conventional laparoscopic) cases were compared. In the matched revisional gastric bypass cohort, outcomes were preserved and similar to the unmatched analysis. Robotic-assisted RnYGB was associated with longer operative duration (186.6 ± 68.0 vs. 151.4 ± 67.6, p < 0.0001) and conventional laparoscopy was associated with fivefold higher rate of transfusion requirement (2.9% vs. 0.6%, p = 0.02). All other outcome measures were similar between the two surgical approaches for gastric bypass cases. In matched sleeve gastrectomy cohort analysis, robotic-assisted surgery remains associated with significantly longer operative duration (143.8 ± 56.6 min vs. 106.9 ± 47.4 min, p < 0.0001) and a higher rate of post-operative sepsis (1.0% vs. 0%, p = 0.04). However, post-operative length of stay and outcome measures that were significantly different in unmatched analysis, were similar among the two surgical approaches in matched sleeve gastrectomy cases.
Discussion
As the number of total bariatric procedure performed annually continues to increase, it is expected that a concomitant increase will be seen in the total number of complications, cases with weight recidivism, and other post-operative morbidities that may require the need for revisional/conversional bariatric procedures [6, 7, 10, 22]. This is a challenging cohort. In a recent systematic review of re-operative bariatric surgery, mortality was estimated to be 2%, which is significantly higher than the 0.1–1.1% reported for primary bariatric procedures [2]. In a case-matched analysis comparing primary and revisional laparoscopic Roux-en-Y gastric bypass (LRYGB), the revisional cohort was found to have significantly longer length of stay (3.8 vs. 2.4, p = 0.02), conversion to laparotomy (10.8% vs. 0%, p = 0.01), and 30-day morbidity (27% vs. 8.1%, p = 0.02) [3]. A meta-analysis comparing bariatric reoperations after adjustable gastric banding (ABG) found that conversion to sleeve gastrectomy had the lowest long-term complication rates (2.6%), while conversion to RYGB had the highest short-term and long-term complication rate at 10.7% and 22.0%, respectively [4]. The current literature suggests that revisional bariatric surgery is associated with higher rates of mortality and morbidity and outcomes may be related to the primary and re-operative operation performed. However, there have been limited studies evaluating outcomes in revisional bariatric surgery comparing conventional laparoscopic- and robotic-assisted surgical approaches [5, 16, 17]. This study represents the largest case–controlled retrospective review of the MBSAQIP PUF database comparing perioperative outcomes in laparoscopic- and robotic-assisted revisional/conversional bariatric surgery.
Our case–control matched analysis of 2288 revisional bariatric cases revealed longer operative duration and hospital length of stay, and higher rates of ICU admission, aggregate leak and bleeding complications in the robotic-assisted bariatric surgery compared to conventional laparoscopy. This is in contrast with other studies. Buchs et al. performed a comparison of 60 consecutive revisional bariatric procedures performed laparoscopically, open, or robotic-assisted [16]. They found that while operative duration was significantly longer in the robotic-assisted cohort, there were less complications and a shorter hospital stay when the robotic platform was used. In another small series (n = 32) evaluating robotic-assisted revisional Roux-en-Y gastric bypass, the authors concluded that their complications and peri-operative outcomes were similar to the published results on conventional laparoscopic revisional bariatric surgery [5].
There remained some similarities and differences between the findings in our study and prior studies. In overall and procedure-specific match analysis, robotic-assisted surgery was associated with significantly longer operative duration, which is consistent with the published literature. While outcomes between robotic-assisted and conventional laparoscopic revisional gastric bypass were statistically similar, robotic-assisted surgery was associated with higher rates of aggregate bleeding (fivefold higher) and aggregate leak (2.5-fold higher). In our matched analysis of robotic and laparoscopic sleeve gastrectomy, most outcomes were statistically similar, as with the gastric bypass cohorts. However, robotic-assisted revisional sleeve gastrectomy was associated with higher rates of conversion (twofold higher), 30-day reoperation (3.3-fold higher), 30-day readmission (1.5-fold higher), 30-day intervention (2.5-fold higher), anticoagulation for presumed for confirmed VTE (twofold higher), transfusion requirement (fourfold higher), organ space SSI (sixfold higher), aggregate leak (1.25-fold higher), aggregate venous thromboembolism (1.9-fold higher), and aggregate SSI (1.8-fold higher).
Much of the higher complication rates observed in the robotic-assisted cohorts were not statistically different. This may be a reflection of the smaller sample size compared after our procedure-specific case–control matching. While unclear, this suggests that the robotic-assisted platform is associated with higher rates of adverse outcomes in sleeve gastrectomy revisional cases compared to gastric bypass revisional cases. The reasons for our findings remain unclear.
Our study represents the largest case–controlled matched study comparing these two surgical platforms for revision/conversion bariatric surgery. We show that while most peri-operative outcomes are similar after controlling for confounders, operative duration remains significantly higher in both robotic-assisted gastric bypass and sleeve gastrectomy. While the robotic platform was overall safe for both revisional gastric bypass and sleeve gastrectomy cases, we also showed that while most complications were statistically similar in matched gastric bypass (robotic vs. laparoscopic) and matched sleeve gastrectomy (robotic vs. laparoscopic) cohorts, robotic revisional metabolic and bariatric surgery was associated with non-significantly higher rates of some complications. These complications rates were overall higher in the sleeve gastrectomy cohort compared to the gastric bypass cohort. Giving these findings, the robotic platform seems overall safe, but is likely less cost-effective, and value added for patient safety remains unclear for revisional metabolic and bariatric surgical procedures, and particularly for revisional sleeve gastrectomy cases.
Our study has a number of limitations that should be highlighted. First, this study is limited to peri-operative outcomes only, so long-term outcomes cannot be assessed. Second, the database does not provide the details about the initial bariatric operation performed for cases designated as revision/conversion. As the primary bariatric operation may impact the level of difficulty of a revision/conversion bariatric procedure, the lack of detail about the initial bariatric operation performed is a possible confounding variable our study could not account for. Third, the dataset does not provide details about anastomotic techniques and surgeon experience, which are variables previously shown to impact outcomes following metabolic and bariatric surgery [23, 24]. The level of surgeon experience is not accounted for in this database, including where surgeons are on the laparoscopic or robotic learning curve. It is also unclear if anastomotic techniques varied by surgical approach. For instance, were more robotic anastomosis hand-sewn and laparoscopic stapled? It is also unclear what primary bariatric procedures were converted to what revisional procedures. Were more difficult conversion cases performed using the robotic platform versus conventional laparoscopy. These nuances could not be illicit from the MBSAQIP database, and may be confounding variables not accounted for in our study. Lastly, this is a retrospective analysis and is therefore susceptible to biases associated with retrospective analyses of clinical databases.
Taking into consideration the above outlined study limitations, the findings of this case-control matched analysis comparing these two surgical approaches for revision/conversion metabolic and bariatric surgery show that using the robotic platform is overall safe, but is associated with longer operative times and a higher rate of some perioperative outcome measures. It has been shown that prolonged operative duration is associated with increased complications. In a recent meta-analysis, the authors found that the likelihood of complications approximately doubles with operative time thresholds exceeding 2 [25]. Moreover, peri-operative complications [26], hospital length of stay [27, 28], 30-day adverse outcomes, such as reoperation, readmission, and intervention [29] have all been reported to be associated with increased costs. Therefore, outcome measures that were higher in the robotic-assisted gastric bypass (operative duration, aggregate bleeding and aggregate leak) and robotic-assisted sleeve gastrectomy cohorts (operative duration and rates of conversion, 30-day reoperation, 30-day readmission, 30-day intervention, anticoagulation for presumed or confirmed VTE, transfusion requirement, organ space SSI, aggregate leak, aggregate venous thromboembolism and aggregate SSI), can serve as proxies for higher cost associated with robotic-assisted metabolic and bariatric surgery. While revisional cases have been reported to be a safe and effective way to treat patients who have significant weight recidivism and relapse of comorbid conditions post-bariatric surgery [6, 7, 18, 19], there are no clear patient benefits to utilizing robotic assistance for these cases. Because of the large initial investment, consumables, annual maintenance, and other reusable equipment also associated with the robotic platforms [30, 31], health systems must be cognizant of the fact that some peri-operative outcomes may favor the use of conventional laparoscopy over the robotic-assisted approach for revisional bariatric procedures. These differences can contribute to higher healthcare expenditures with little effect on patient safety outcomes when the robotic platform is utilized in this patient cohort.
Conclusion
Conventional laparoscopic and robotic-assisted revision/conversion metabolic and bariatric procedures are both safe and effective surgical approaches. However, we found that robotic-assisted revision/conversion gastric bypass and sleeve gastrectomy is associated with longer operative times. Robotic-assisted and conventional laparoscopic gastric bypass were similar in outcomes, except a non-significantly higher rate of aggregate leak and bleeding. Outcomes between robotic-assisted and conventional laparoscopic sleeve gastrectomy were also statistically similar; however, the robotic-assisted cohort had numerous 30-day adverse outcomes, complications, and aggregate complications that were higher. These findings suggest less cost-effectiveness and no clear patient safety benefit with use of the robotic platform, particularly for revisional sleeve gastrectomy cases. Larger revisional cohorts are needed to validate our finding, given the limited sample size included in our analysis following our procedure-specific matching.
References
Hjorth S, Naslund I, Andersson-Assarsson JC et al (2019) Reoperations after bariatric surgery in 26 years of follow-up of the Swedish Obese Subjects study. JAMA Surg. https://doi.org/10.1001/jamasurg.2018.5084
Pinto-Bastos A, Conceição EM, Machado PPP (2017) Re-operative bariatric surgery: a systematic review of the reasons for surgery, medical and weight loss outcomes, relevant behavioral factors. Obes Surg 27(10):2707–2715
Mor A, Keenan E, Portenier D, Torquati A (2013) Case-matched analysis comparing outcomes of revisional versus primary laparoscopic roux-en-y gastric bypass. Surg Endosc 27(2):548–552
Kuzminov A, Palmer AJ, Wilkinson S, Khatsiev B, Venn AJ (2016) Re-operations after secondary bariatric surgery: a systematic review. Obes Surg 26(9):2237–2247
Bindal V, Gonzalez-Heredia R, Elli EF (2015) Outcomes of robot-assisted roux-en-y gastric bypass as a reoperative bariatric procedure. Obes Surg 25(10):1810–1815
Shimizu H, Annaberdyev S, Motamarry I et al (2013) Revisional bariatric surgery for unsuccessful weight loss and complications. Obes Surg 23:1766–1773
Brethauer SA, Kothari S, Sudan R et al (2014) Systematic review on reoperative bariatric surgery American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis 10:1–21
de Oliveira VLP, Martins GP, Mottin CC, Rizzolli J, Friedman R (2018) Predictors of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass in severely obese patients. Obes Surg 28(1):195–203
Switzer NJ, Karmali S, Gill RS, Sherman V (2016) Revisional bariatric surgery. Surg Clin North Am 96(4):827–842
Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW (2013) Weight recidivism post-bariatric surgery: a systematic review. Obes Surg 23(11):1922–1933
Goettler A, Grosse A, Sonntag D (2017) Productivity loss due to overweight and obesity: a systematic review of indirect costs. BMJ Open 7:e014632
Abraham et al (2016) Trends in bariatric surgery: procedure selection, revisional surgeries, and readmissions. Obes Surg 26(7):1371–1377
Varela JE, Nguyen NT (2015) Laparoscopic sleeve gastrectomy leads the US utilization of bariatric surgery at academic medical centers. Surg Obes Relat Dis 11:987–990
Hallowell PT, Stellato TA, Yao DA et al (2009) Should bariatric revisional surgery be avoided secondary to increased morbidity and mortality? Am J Surg 197(3):391–396
Dakin GF, Eid G, Mikami D, Pryor A, Chand B, Emerging Technology and Procedures Committee (2013) Endoluminal revision of gastric bypass for weight regain—a systematic review. Surg Obes Relat Dis 9:335–342
Buchs NC, Pugin F, Azagury DE, Huber O, Chassot G, Morel P (2013) Robotic revisional bariatric surgery: a comparative study with laparoscopic and open surgery. Int J Med Robot Comput Assist Surg 10(2):213–217
Snyder B, Wilson T, Woodruff V, Wilson E (2013) Robotically assisted revision of bariatric surgeries is safe and effective to achieve further weight loss. World J Surg 37(11):2569–2573
Westling A, Ohrvall M, Gustavsson S (2002) Roux-en-Y gastric bypass after previous unsuccessful gastric restrictive surgery. Gastrointest Surg 6:206–211
te Riele WW, Sze YK, Wiezer MJ, van Ramshorst B (2008) Conversion of failed laparoscopic gastric banding to gastric bypass as safe and effective as primary gastric bypass in morbidly obese patients. Surg Obes Relat Dis 4:735–739
Morales MP, Wheeler AA, Ramaswamy A et al (2010) Laparoscopic revisional surgery after Roux-en-Y gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis 6:485–490
Berger ER et al (2016) The impact of different surgical techniques on outcomes in Laparoscopic sleeve gastrectomies: the first report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP). Ann Surg 264(3):464–473
ASMBS (2018) Estimate of bariatric surgery numbers, 2011–2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 16 Jan 2019
Smeenk RM, Van’t Hof G, Elsten E, Feskens PGBM (2016) The results of 100 robotic versus 100 laparoscopic gastric bypass procedures: a single high volume centre experience. Obes Surg 26:1266–1273
Moon RC et al (2016) Robotic roux-en-y gastric bypass, is it safer than laparoscopic bypass? Obes Surg 26:1016–1020
Cheng H, Clymer JW, Chen B, Sadeghirad B, Ferko NC, Cameron CG, Hinoul P (2018) Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res 229:134–144
Shah N, Greenberg JA, Leverson G, Funk LM (2016) Predictors of high cost after bariatric surgery: a single institution review. Surgery 160(4):877–884
Khorgami Z et al (2017) Cost of bariatric surgery and factors associated with increased cost: an analysis of national inpatient sample. Surg Obes Relat Dis 13:1284–1289
Khorgami Z, Aminian A, Shoar S, Andalib A, Saber AA, Schauer PR, Brethauer SA, Sclabas GM (2017) Cost of bariatric surgery and factors associated with increased cost: an analysis of national inpatient sample. Surg Obes Relat Dis 13(8):1284–1289
Encinosa WE, Bernard D, Chen C, Steiner CA (2006) Healthcare utilization and outcomes after bariatric surgery. Med Care 44(8):706–712
Ho C, Tsakonas E, Tran K, et al (2011) Robot-assisted surgery compared with open surgery and laparoscopic surgery: clinical effectiveness and economic analyses. Canadian Agency for Drugs and Technologies in Health (CADTH Technology Report, No. 137). Table 16, Capital and Operating Costs of da Vinci Surgical System
Bailey JG et al (2014) Robotic versus laparoscopic roux-en-y gastric bypass in obese adults ages 18 to 65 years: a systematic review and economic analysis. Surg Endosc 28:414–426
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Edwin Acevedo, Jr., Michael Mazzei, Huaqing Zhao, Michael A. Edwards, and Mr. Xiaoning Lu have no conflicts of interests or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acevedo, E., Mazzei, M., Zhao, H. et al. Outcomes in conventional laparoscopic versus robotic-assisted revisional bariatric surgery: a retrospective, case–controlled study of the MBSAQIP database. Surg Endosc 34, 1573–1584 (2020). https://doi.org/10.1007/s00464-019-06917-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06917-5