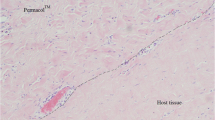
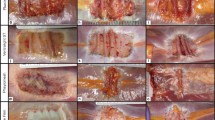
Abstract
Background
The purpose of this study was to evaluate the associations between patient characteristics or surgical site classifications and the histologic remodeling scores of synthetic meshes biopsied from their abdominal wall repair sites in the first attempt to generate a multivariable risk prediction model of non-constructive remodeling.
Methods
Biopsies of the synthetic meshes were obtained from the abdominal wall repair sites of 51 patients during a subsequent abdominal re-exploration. Biopsies were stained with hematoxylin and eosin, and evaluated according to a semi-quantitative scoring system for remodeling characteristics (cell infiltration, cell types, extracellular matrix deposition, inflammation, fibrous encapsulation, and neovascularization) and a mean composite score (CR). Biopsies were also stained with Sirius Red and Fast Green, and analyzed to determine the collagen I:III ratio. Based on univariate analyses between subject clinical characteristics or surgical site classification and the histologic remodeling scores, cohort variables were selected for multivariable regression models using a threshold p value of ≤0.200.
Results
The model selection process for the extracellular matrix score yielded two variables: subject age at time of mesh implantation, and mesh classification (c-statistic = 0.842). For CR score, the model selection process yielded two variables: subject age at time of mesh implantation and mesh classification (r 2 = 0.464). The model selection process for the collagen III area yielded a model with two variables: subject body mass index at time of mesh explantation and pack-year history (r 2 = 0.244).
Conclusion
Host characteristics and surgical site assessments may predict degree of remodeling for synthetic meshes used to reinforce abdominal wall repair sites. These preliminary results constitute the first steps in generating a risk prediction model that predicts the patients and clinical circumstances for which non-constructive remodeling of an abdominal wall repair site with synthetic mesh reinforcement is most likely to occur.
Similar content being viewed by others
References
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D et al (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183
Millennium Research Group (2006) US markets for soft tissue repair devices 2006. Millennium Research Group Inc., Toronto
Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240(4):578–583
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, Ijzermans JN et al (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343(6):393–398
Klinge U, Klosterhalfen B, Muller M, Schumpelick V (1999) Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165(7):665–673
Klosterhalfen B, Klinge U, Hermanns B, Schumpelick V (2000) Pathology of traditional surgical nets for hernia repair after long-term implantation in humans. Chirurg 71(1):43–51
Schachtrupp A, Klinge U, Junge K, Rosch R, Bhardwaj RS, Schumpelick V (2003) Individual inflammatory response of human blood monocytes to mesh biomaterials. Br J Surg 90(1):114–120
Bellows CF, Alder A, Helton WS (2006) Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Devices 3(5):657–675
Ratner B, Hoffman AS, Shoen FJ, Lemons JE (1996) Biomaterials science. Academic Press, San Diego, pp 243–254
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM (2008) Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14(11):1835–1842
Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B (2000) Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res 32(1):43–48
Klinge U, Si ZY, Zheng H, Schumpelick V, Bhardwaj RS, Klosterhalfen B (2001) Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg 14(1):47–54
El-Gazzaz GH, Farag SH, El-Sayd MA, Mohamed HH (2012) The use of synthetic mesh in patients undergoing ventral hernia repair during colorectal resection: risk of infection and recurrence. Asian J Surg 35(4):149–153
Matthews BD, Mostafa G, Carbonell AM, Joels CS, Kercher KW, Austin C et al (2005) Evaluation of adhesion formation and host tissue response to intra-abdominal polytetrafluoroethylene mesh and composite prosthetic mesh. J Surg Res 123(2):227–234
Harrell AG, Novitsky YW, Cristiano JA, Gersin KS, Norton HJ, Kercher KW et al (2007) Prospective histologic evaluation of intra-abdominal prosthetics four months after implantation in a rabbit model. Surg Endosc 21(7):1170–1174
Novitsky YW, Harrell AG, Cristiano JA, Paton BL, Norton HJ, Peindl RD et al (2007) Comparative evaluation of adhesion formation, strength of ingrowth, and textile properties of prosthetic meshes after long-term intra-abdominal implantation in a rabbit. J Surg Res 140(1):6–11
Novitsky YW, Cristiano JA, Harrell AG, Newcomb W, Norton JH, Kercher KW et al (2008) Immunohistochemical analysis of host reaction to heavyweight-, reduced-weight-, and expanded polytetrafluoroethylene (ePTFE)-based meshes after short-term and long-term intraabdominal implantations. Surg Endosc 22(4):1070–1076
Pascual G, Rodriguez M, Gomez-Gil V, Garcia-Honduvilla N, Bujan J, Bellon JM (2008) Early tissue incorporation and collagen deposition in lightweight polypropylene meshes: bioassay in an experimental model of ventral hernia. Surgery 144(3):427–435
Bellon JM, Rodriguez M, Garcia-Honduvilla N, Gomez-Gil V, Pascual G, Bujan J (2008) Postimplant behavior of lightweight polypropylene meshes in an experimental model of abdominal hernia. J Invest Surg 21(5):280–287
Bellon JM, Rodriguez M, Garcia-Honduvilla N, Gomez-Gil V, Pascual G, Bujan J (2009) Comparing the behavior of different polypropylene meshes (heavy and lightweight) in an experimental model of ventral hernia repair. J Biomed Mater Res B 89(2):448–455
Orenstein SB, Saberski ER, Kreutzer DL, Novitsky YW (2012) Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res 176(2):423–429
Pascual G, Rodriguez M, Sotomayor S, Perez-Kohler B, Bellon JM (2012) Inflammatory reaction and neotissue maturation in the early host tissue incorporation of polypropylene prostheses. Hernia 16(6):697–707
Pascual G, Hernandez-Gascon B, Rodriguez M, Sotomayor S, Pena E, Calvo B et al (2012) The long-term behavior of lightweight and heavyweight meshes used to repair abdominal wall defects is determined by the host tissue repair process provoked by the mesh. Surgery 152(5):886–895
Pascual G, Hernandez-Gascon B, Sotomayor S, Pena E, Calvo B, Bujan J et al (2013) Short-term behavior of different polymer structure lightweight meshes used to repair abdominal wall defects. Histol Histopathol 28(5):611–621
Costello CR, Bachman SL, Grant SA, Cleveland DS, Loy TS, Ramshaw BJ (2007) Characterization of heavyweight and lightweight polypropylene prosthetic mesh explants from a single patient. Surg Innov 14(3):168–176
Wood AJ, Cozad MJ, Grant DA, Ostdiek AM, Bachman SL, Grant SA (2013) Materials characterization and histological analysis of explanted polypropylene, PTFE, and PET hernia meshes from an individual patient. J Mater Sci Mater Med 24(4):1113–1122
Costello CR, Bachman SL, Ramshaw BJ, Grant SA (2007) Materials characterization of explanted polypropylene hernia meshes. J Biomed Mater Res B 83(1):44–49
Cozad MJ, Grant DA, Bachman SL, Grant DN, Ramshaw BJ, Grant SA (2010) Materials characterization of explanted polypropylene, polyethylene terephthalate, and expanded polytetrafluoroethylene composites: spectral and thermal analysis. J Biomed Mater Res B 94(2):455–462
Valentin JE, Badylak JS, McCabe GP, Badylak SF (2006) Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. J Bone Joint Surg Am 88(12):2673–2686
Jenkins ED, Melman L, Desai S, Brown SR, Frisella MM, Deeken CR et al (2011) Evaluation of intraperitoneal placement of absorbable and nonabsorbable barrier coated mesh secured with fibrin sealant in a New Zealand white rabbit model. Surg Endosc 25(2):604–612
Jenkins ED, Melman L, Desai S, Deeken CR, Greco SC, Frisella MM et al (2011) Histologic evaluation of absorbable and non-absorbable barrier coated mesh secured to the peritoneum with fibrin sealant in a New Zealand white rabbit model. Hernia 15(6):677–684
Brown SR, Melman L, Jenkins ED, Deeken CR, Frisella MM, Brunt LM et al (2011) Collagen type I:III ratio of the gastroesophageal junction in patients with paraesophageal hernias. Surg Endosc 25(5):1390–1394
Berard F, Gandon J (1964) Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various other factors. Ann Surg 160(Suppl 2):1–192
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13(10):606–608
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Firth D (1993) Bias reduction of maximum likelihood estimates. Biometrika 80(1):27–38
Heinze G, Schemper M (2002) A solution to the problem of separation in logistic regression. Stat Med 21(16):2409–2419
Heinze G, Ploner M (2003) Fixing the nonconvergence bug in logistic regression with SPLUS and SAS. Comput Methods Programs Biomed 71(2):181–187
Sugiura N (1978) Further analysis of the data by Akaike’s information and finite corrections. Comm Statist 7(1):13–26
Junqueira LC, Cossermelli W, Brentani R (1978) Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn 41(3):267–274
Junqueira LC, Bignolas G, Brentani RR (1979) Picosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11(4):447–455
Rich L, Whittaker P (2005) Collagen and picosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 22(2):97–104
Acknowledgments
This study utilized the REDCap® application for data maintenance, which is supported at the Washington University, St. Louis, by a Clinical and Translational Science Award (CTSA; UL1TR000448), and a National Cancer Institute (NCI) Cancer Center Support Grant to the Siteman Comprehensive Cancer Center (P30CA091842). Jaime A. Cavallo is supported by a KM1 Comparative Effectiveness Research (CER) Career Development Award (KM1CA156708) through the NCI of the National Institutes of Health (NIH), and the Washington University, St. Louis, CTSA program (UL1TR000448) through the National Center for Advancing Translational Sciences (NCATS) of the NIH. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCI, NCATS, or NIH.
Author contributions
Study conception and design: Jaime A. Cavallo, Brent D. Matthews, and Corey R. Deeken; acquisition of data: Jaime A. Cavallo, Andres A. Roma, Jenny Ousley, Jennifer Creamer, Matthew D. Pichert, Sara Baalman, and Margaret M. Frisella; analysis and interpretation of data: Jaime A. Cavallo, Mateusz S. Jasielec, Brent D. Matthews, and Corey R. Deeken; drafting of manuscript: Jaime A. Cavallo, Mateusz S. Jasielec, Brent D. Matthews, and Corey R. Deeken; critical revision: Jaime A. Cavallo, Andres A. Roma, Mateusz S. Jasielec, Jenny Ousley, Jennifer Creamer, Matthew D. Pichert, Sara Baalman, Margaret M. Frisella, Brent D. Matthews, and Corey R. Deeken.
Disclosure
Dr. Cavallo has received research grant funding for unrelated studies from the NIH, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), and the American Hernia Society in collaboration with Davol® Incorporated. Ms. Frisella has received funding from Atrium Medical Corporation® and W. L. Gore and Associates® Incorporated for unrelated service contracts, as well as research grant funding for unrelated studies from the Foundation for Barnes-Jewish Hospital. Dr. Matthews has served on advisory boards for the Musculoskeletal Transplant Foundation, Covidien® Incorporated, and Synthes® Incorporated; served as a consultant for Atrium Medical Corporation®; received speaking fees or honoraria from Atrium Medical Corporation®, Davol® Incorporated, Ethicon® Incorporated, and W. L. Gore and Associates® Incorporated; received payments for authorship of an unrelated publication from McMahon Group® Incorporated; received research grant funding for unrelated research studies from Covidien® Incorporated, Ethicon® Incorporated, Karl Storz Endoscopy America® Incorporated, Kensey Nash Corporation®, Musculoskeletal Transplant Foundation, Synovis Surgical Innovations®, SAGES, NIH, and the Foundation for Barnes-Jewish Hospital. Dr. Deeken has served as a consultant for Atrium Medical Corporation® and Davol® Incorporated; received speaking fees or honoraria from Covidien® Incorporated and the Musculoskeletal Transplant Foundation; received research grant funding for unrelated research studies from Atrium Medical Corporation®, Covidien® Incorporated, Ethicon® Incorporated, Kensey Nash Corporation®, Musculoskeletal Transplant Foundation, OBI Biologics Incorporated®, and SAGES. Dr. Roma, Mr. Jasielec, Ms. Ousley, Dr. Creamer, Mr. Pichert, and Ms. Baalman have no conflicts of interest or financial ties to disclose.
Funding
Funding for this project was provided by the Department of Surgery, Washington University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cavallo, J.A., Roma, A.A., Jasielec, M.S. et al. Remodeling characteristics and collagen distribution in synthetic mesh materials explanted from human subjects after abdominal wall reconstruction: an analysis of remodeling characteristics by patient risk factors and surgical site classifications. Surg Endosc 28, 1852–1865 (2014). https://doi.org/10.1007/s00464-013-3405-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3405-6