Abstract
Background
Videothoracoscopic thymectomy is an alternative surgical procedure for patients with nonthymomatous myasthenia gravis. The aim of this study is to present our experience and to analyze the factors contributing to the operative morbidity.
Methods
Ninety myasthenia gravis patients were operated through right-sided videothoracoscopy from June 2002 to September 2006. Prospective data recording was performed. Surgeon-related conversion to open surgery, length of the operation, chest tube duration time, duration of hospital stay, amount of drainage, pain score, and complications were evaluated. Factors contributing to longer operation time and longer postoperative stay were studied.
Results
The mean length of chest tube duration and postoperative hospital stay was 26.7 ± 18.6 hours and 2.2 days ± 1.1 days respectively. Visual analogue scale (VAS) values for pain evaluation were 2.0 ± 1.4. Surgeon-related open conversion occured in two patients (2.2%). Body mass index (BMI) was the sole significant factor for longer operation time. (23.04 ± 2.93 versus 25.61 ± 2.70 (p = 0.001). The amount of pyridostigmine was the only significant factor for longer hospital stay (213.3 ± 101.5 mg versus 270. 0 ± 122.6 mg (p = 0.044).
Conclusions
This study demonstrates the right-sided videothoracoscopy is a safe procedure. The only contributing factors were: BMI >25.61 for longer operation time, and pyridostigmine level >270 mg for duration of postoperative stay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Currently, the most common surgical approach to the thymus gland for myasthenia gravis (MG) is a median sternotomy, either partial or total. Other techniques, namely the transcervical approach and the more-radical approach that combines a median sternotomy with a transcervical incision also have their advocates [1, 2]. Surgical technique of thoracoscopic thymectomy has been well described [3]. However, the videothoracoscopic thymectomy literature still does not have enough articles describing a large number of patients and the experience of high-volume centers. In this article, we present our experience on 90 MG patients and analyze the factors contributing to longer operation time and longer postoperative hospital stay.
Material and methods
Patient characteristics
The perioperative and long-term results of the two thymectomy techniques (videothoracoscopic thymectomy and thymectomy through partial upper sternal splitting) were explained to all patients undergoing videothoracoscopic thymectomy at the Istanbul Medical Faculty between June 2002 and September 2006. Ninety patients who chose videothoracoscopic thymectomy technique were included in this study. Patient age, gender, duration of disease, body mass index (BMI), medication, length of the operation, chest tube duration time, duration of hospital stay, the amount of drainage, pain score, and complications were recorded prospectively. Patient myasthenic condition was graded according to modified Osserman classification as 0: asymptomatic, 1: ocular sign and symptoms, 2: mild generalized weakness, 3: moderate generalized weakness, bulbar dysfunction or both and, 4: severe generalized weakness, respiratory dysfunction, or both. Reasons for conversion to open surgery were noted. Conversion to open surgery occurred due to two main factors: surgeon-related conversion due to hemorrhage (two patients) and patient-related factors (nine patients). These patients were excluded from the study. Indepentent sample t tests were used in the analyses.
Diagnosis, preoperative care, and referral
The diagnosis of MG was based on the history of fatiguable weakness, a positive response to anticholinestherase test, and abnormal repetitive nerve stimulation or single-fiber electromyography studies. Detection of AChR antibodies by radioimmune assay was performed routinely. After the diagnosis was confirmed and MG symptoms were medically controlled, patients were referred for surgery. A course of five intravenous immunoglobulin treatment was started in patients with severe bulbar symptoms and severe generalized weakness. All patients were evaluated by a single surgeon (AT) and operations were performed by the same surgeon. Age was not considered as a factor for limiting the operation for MG unless the patient was a candidate for video-assisted thoracic surgery (VATS). For others, we limited the upper age limit to 60 years. We excluded patients with thymoma from the study group, although the presence of thymoma smaller than 2 cm on computed tomography was not considered a contraindication for VATS.
Exclusion criteria
Nine patients who had patient-related conversions and seven patients who were opererated with VATS but had thymomas were excluded from the study. Patient-related conversions included an unexpected thymoma with invasion to surrounding structures (three patients), inability to have a single-lung ventilation (one patient), intolerance to single-lung ventilation (four patients) and natural pleurodesis (one patient).
Perioperative management
Anesthetic and final neurological assessments were performed at the ward on the day before surgery by specialists accustomed to myasthenic patients. Patients took their morning doses of pyridostigmine and/or corticosteroids perorally with a small amount of water. If surgery was delayed until the afternoon further doses of pyridostigmine were given. No other premedication was used. Anesthesia was induced with propofol 2–2.5 mg/kg and fentanyl 1 μg/kg. Muscle relaxation was achieved with mivacurium 0.1 mg/kg (1 x ED95 dose). The trachea was intubated with a left double-lumen tube (Mallinckrodt, Ireland; no. 39 for male and no. 37 for female patients). The position of the tube was confirmed with both auscultation and fiberoptic bronchoscopy (Karl Storz Company, Germany). Anesthesia was maintained with propofol infusion and supplemental doses of fentanyl. One-lung ventilation was applied throughout the thoracoscopic procedure. Muscle relaxation was monitored by both the train-of-four monitoring and observation of diaphragmatic movement. Supplemental doses of mivacurium 2 mg were applied if the TOF ratio was >25% or a diaphragmatic movement was observed through the thoracoscope.
Operative technique
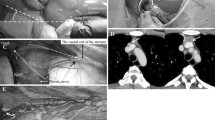
We prefer the right side because of the important landmarks of the right chest cavity (superior vena cava and left innominate vein) and because the space is larger in the right chest.
The patient is positioned supine with the table rotated 30° to the surgeon’s side. Additional support must be added so that the patient remains secure and supine on the table. Typically, three thoracoports of 5 mm, 10 mm and 11 or 15 mm are used above and middle around the mammary gland (Fig. 1). We prefer to use the ports according to the size and shape of the mammary gland. The surgeon corrects the place of the right mammary gland and opens the thoracoport holes around the mammary tissue without entering the gland. A combination of both blunt and sharp dissection is used to dissect out the gland without violating the capsule. One must pay careful attention to the phrenic nerve and avoid both direct and indirect injury. Superior vena cava and the phrenic nerve over it are the landmarks in the right-sided approach (Fig. 2).
Initially the right side of the mediastinal pleura is incised and the plane between the anterior gland and the sternum is fully developed. The contralateral pleura into the left hemithorax is then opened. At the time the left pleural space is entered, the endotracheal tube is temporarily disconnected to allow the left lung to collapse. With this maneuver incision along the left mediastinal pleura can be safely completed under the left side of the sternum and the left mediastinal pleura is taken into the specimen.
Next, the right inferior pole of the thymus is separated from the pericardium. By pushing the gland up towards the sternum, the plane is developed along the anterior pericardium, across the mediastinum and again entering the left pleural space. Next, two planes are connected through a combination of both blunt and sharp dissection and the entire inferior left pole is released.
One of the most difficult parts of the dissection is the venous tributaries to the brachiocephalic vein. The superior vena cava is mobilized away from the gland to expose the left brachiocephalic vein. Small thymic veins are dissected and clipped. These venous tributaries can be avulsed easily and can lead to troublesome bleeding. To facilitate the dissection, small gauses, peanuts, and conventional forceps are used. Retracting the thymus inferiorly allows exposure of the superior poles. The superior pole is retracted using a moderate amount of tension. Flexing the patient’s head at this juncture can augment the exposure. After releasing the right upper pole, the thymic vein can be seen and should be divided by clipping both sides (Fig. 3). We prefer to double clip the proximal side. The left upper pole is also dissected in a similar fashion. Once entirely free, the gland is placed in a specimen bag and removed through the superior port. The rest of the mediastinum is inspected for ectopic thymic tissue. The brachiocephalic vein is elevated and inspected to remove any posterior tissue. The brachiocephalic vein, superior vena cava, aorta, and pulmonary arteries should be clearly visualized.
Fatty tissue in the right cardiophrenic angle is dissected completely and left cardiophrenic angle partially. After careful hemostasis, both lungs are collapsed by disconnecting the ventilator and a Jackson Pratt drainage system is placed across the mediastinum so that both pleural spaces are drained. A positive end expiratory pressure of 7–8 cm H20 is applied to the endotracheal tube to resolve any residual atelectasis. Pain management is standardized as intraoperative intercostal blockage with 5 ml of bupivacaine 0.5% (Marcaine, AstraZeneca, Istanbul) to all port sides and additional nonsteroid anti-inflammatory agents.
Results
Perioperative period
The study group consisted of 76 (84.4 %) female and 14 (15.5 %) male patients with a mean age of 30.9 years (standard deviation 13.7 years). Preoperative medications consisted of pyridostigmine bromide and corticosteroids with an average of 229.89 ± 110.5 mg and 15.89 ± 21.19 mg, respectively. Mean operation time was 64.3 ± 27.4 minutes. The mean amount of chest tube drainage was 146 ± 138 ml. The mean length of chest tube duration and postoperative hospital stay were 26.7 ± 18.6 hours and 2.2 days ± 1.1 days, respectively. VAS for pain evaluation revealed values of 2.0 ± 1.4. Complications were noticed in two patients (2.2%). Two patients experienced intensive care unit stay with 18 hours and 48 hours. There was no in-hospital or 30-day mortality.
Factors contributing to longer operation time and longer hospital stay
Age, BMI, Osserman stage, gender, age, medications (corticosteroids/day, pyridostigmine bromide/day), and duration of disease were analyzed to evaluate their effects on longer opeation time (>64 minutes) and longer hospital stay (>2.2 days).
The factor affecting longer operation time was BMI p = 0.001 (23.04 ± 2.93 versus 25.61 ± 2.70).
The factor affecting longer hospital stay was the amount of pyridostigmine (p = 0.044) (213.3 ± 101.5 mg versus 270. 0 ± 122.6 mg)
Discussion
In this study, we observed a mean operation time of 64.3 minutes and 146 ml of chest tube drainage. Chest tube duration time was 26.7 hours and postoperative hospital stay was 2.2 days. VAS for pain scored 2.0. Complications were seldomly noticed.
The factor affecting longer operation time was BMI (p = 0.001). The factor affecting longer postoperative hospital stay was the amount of pyridostigmine (p = 0.044).
During our initial surgical experience with 32 VATS patients, we performed a study to compare the immediate postoperative results after thymectomies performed through partial upper splitting and VATS [4]. This study demonstrated that videothoracoscopic thymectomy patients had a shorter duration of chest tube drainage (48.8 versus 29.8 hours, p < 0.001), less amount of drainage (264.4 vs. 178.6 mL, p = 0.001), shorter hospital stay (5.6 versus 2.3 days, p = 0.000) and lower visual analogue scale score (4.8 versus 3.1, p < 0.001) [4]. Other reports seemed to support our data with even better results [3, 5, 6]. In the past few years, the longest median postoperative stay and chest tube duration in VAT thymectomy were presented as four days (range 2–6 days) and two days (range 1–3 days) [7], respectively. In a large retrospective series presented lately, mortality was 0% and morbidity was 9.34 %. The mean operative time was found to be 90 ± 45 minutes [16]. The mean length of hospitalization was 2.3 days (range 2–6 days) [8]. In another smaller series that included 18 patients, the average chest tube drainage time was 2.1 days, and the mean hospitalization time was 6.3 days. Three of the 18 patients needed temporary mechanical ventilation for less than 72 hours, and two needed re-intubation due to either myasthenic or cholinergic crises [9]. As could be noted from these studies, greater experience shortens postoperative stay and decreases the number of complications. We believe these benefits are a positive impetus for earlier thymectomy.
We noticed that some operations took longer time and some operations were easier even through the same surgeon carried out the procedures. No variable except BMI affected operation time. Length of hospital stay was directly correlated with pyridostigmine dose, which reflected the severity of MG. We did not notice a similar study in the literature that studied the factors affecting the perioperative period during thymectomy with VATS.
We did not present our data concerning post-thymectomy results because we wanted to present a large data set with longer follow-up times; our aim was to present our results at least three years after the last procedure studied in the group. Although the rate of complete remission in the long term is certainly the most important result of surgery to determine the efficiency of the procedure, it is difficult to compare the series. The complete remission rate in patients who were followed up with a mean of 3.3 to 5.4 years showed the crude complete remission rate to be around 45% [10–12].
Videothoracoscopic thymectomy allowed better preservation of lung functions perioperatively when compared to median sternotomy [13]. We noticed a very fast and smooth recovery period. Although we did not have specific data for postoperative pulmonary functions, it is obvious that VATS had a pulmonary function-sparing effect. The cosmetic benefit supplied by VATS thymectomy is currently superior to other techniques since the incisions are around the mammary gland. The cosmetic benefit is also thought to be a positive impetus for earlier thymectomy, which has been shown to allow rapid and durable clinical benefit and may prevent the use of aggressive immunosuppressive treatment [14–17].
The VATS approach to the thymic gland has also been described as: left thoracoscopy, bilateral thoracoscopy, and bilateral thoracoscopy combined with cervicotomy. We believe the endoscopic choice of the surgeon should fit with the type of thymectomy procedure advocated. If the team performs maximal thymectomy, endoscopic procedure should be cervicotomy plus bilateral thoracoscopy, which could be considered a more-aggressive approach compared to unilateral techniques. Bilateral thoracoscopy should be the only choice for extended thymectomy because it cannot be employed by unilateral intervention. If the procedure of choice for the thymic gland is simple thymectomy, unilateral interventions should be advocated. In these circumstences we prefer the right side because the right hemithoracic cavity is larger and the superior vena cava with the phrenic nerve is an important landmark.
We conclude that this surgical technique, which was not proven to be the standard method of treatment based on randomized studies, should still be used along with techniques that do not interfere with the original disease and its consequences. This means safer and minimally invasive operation with maximal tissue extraction.
References
Cooper J, Al-Jilaihawa A, Pearson FG, Humphrey JG, Humphrey HE (1988) An improved technique to fascilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 45:252–247
Jaretzki A III, Wolf M (1988) Maximal thymectomy for myasthenia gravis: surgical anatomy and operative technique. J Thorac Cardiovasc Surg 96:711–716
Mack JM (2001) Videoassisted thoracoscopic thymectomy for myasthenia gravis. Chest Surg Clin N Am 11:389–405
Toker A, Eroğlu O, Ziyade S, Tanju S, Senturk M, Dilege S, Kalayci G (2005) Comparison of early postoperative results of thymectomy:partial sternotomy vs videothoracoscopy. Thorac Cardiovasc Surg 53(2):110–113
Savcenko M, Wendt KG, Prince SL, Mack JM (2002) Video-assisted thymectomy for myasthenia gravis: an update of a single institution experience. Eur J CardioThorac Surg 22:978–983
Mineo TC, Pompe E, Lerut TE, Bernardi G, Coosemans W, Nofroni I (2000) Thoracoscopic thymectomy in autoimmune myasthenia: Results of left sided approach. Ann Thorac Surg 69:1537–1541
Wright GM, Barnett S, Clarke CP (2002) Video-assisted thoracoscopic thymectomy for myasthenia gravis. Intern Med J 32:367–371
Tomulescu V, Ion V, Kosa A, Sgarbura O, Popescu I. (2006) Thoracoscopic thymectomy mid term results. Ann Thorac Surg 82:1003–7
Li JF, Li JR, Yang F, Jiang GC, Wang J (2006). Long term effects of video-assissted thoracoscopic thymectomy for myasthean gravis: 5 years follow up of 18 cases. Zhonghua Yi Xue Za Zhi 86:2312–2314
Jaretzki A (1997) Thymectomy for myasthenia gravis: Analysis of the controversies regarding technique and results. Neurology 48:52–63
Detterbeck FC, Scott WW, Howard Jr JF, Eagen TM, Keagy PA, Starek JK, Mill MR, Wilcox BR (1996) Onehundred consecutive thymectomies for myasthenia gravis. Ann Thorac Surg 62:242–245
Calhoun RF, Ritter JH, Guthrie TJ, Pestronk A, Meyers BF, Patterson GA, Pohl MS, Cooper JD (1999) Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients. Ann Surg 230:555–561
Ruckert JC, Walter M, Muller JM (2000) Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 70:1656–1661
Genkins G, Papatestas AE, Horowitz SH, Kornfield P (1975) Studies in myasthenia gravis : Early thymectomy: Electrophysiologic and pathological correction. Am J Med 58:517–524
Bril V, Kojic J, Ilse WK, Cooper JD (1998) Long term clinical outcome after transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 65:1520–1522
Olanow CW, Wechsler AS, Roses AD (1982) A prospective study of thymectomy and serum acetylcholine receptor antibodies in myasthenia gravis. Ann Surg 196:113–121
Hazellrigg SR (2004) Thoracoscopic or Video-Assisted (VATS) thymectomy. Operative techniques in thoracic and cardiovascular Surgery. 9:184–192
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toker, A., Tanju, S., Sungur, Z. et al. Videothoracoscopic thymectomy for nonthymomatous myasthenia gravis: Results of 90 patients. Surg Endosc 22, 912–916 (2008). https://doi.org/10.1007/s00464-007-9507-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9507-2