Abstract
Background
Pneumoperitoneum (PP), as used for laparoscopic procedures, impairs stroke volume, renal blood flow, glomerular filtration rate and urine output. This study investigated whether perioperative fluid management can abolish these negative effects of PP on hemodynamics.
Methods
Twenty-one patients undergoing laparoscopic donor nephrectomy (LDN) were randomized into three groups: group 1 received overnight infusion and received a bolus of colloid before induction of anesthesia, followed by a bolus just before PP; group 2 received overnight infusion and a colloid bolus before anesthesia; group 3 served as controls and received only infusion during operation. All three groups received the same total amount of crystalloids and colloids until nephrectomy. Data analysis of the donor included; mean arterial pressure (MAP), stroke volume (SV), left ventricular ejection time (LVETc), perioperative urine output and renal function measured as the creatinine clearance (CrCl) until one-year post-operative.
Results
SV was significantly higher in group 1 compared to controls for all measurements. In the control group SV significantly decreased after changing from the supine to lateral position whereas there was no change in SV in both pre-hydrated groups. In all groups, MAP decreased after induction of anesthesia, and restored to pre-anesthetic values during PP. CrCl decreased in the control group during PP, but not in the other groups. From two days postoperative, CrCl was comparable between the three study groups.
Conclusion
Overnight infusion and a bolus of colloid just before PP attenuate hemodynamic compromise from PP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic donor nephrectomy (LDN) has become the method of choice to procure kidneys from living donors, mainly because of the reduced procedure-related morbidity and faster convalescence period [1–3]. Despite the benefits to the donor, there are concerns over the transient deterioration of renal function in the recipient of the kidney procured by the laparoscopic technique, compared with open donor nephrectomy (ODN) [2, 4–6]. The exact mechanism of delayed graft function after LDN is not fully understood.
Pneumoperitoneum (PP) elevates intra-abdominal pressure (IAP), causing a decrease in renal blood flow (RBF) and glomerular filtration rate (GFR) resulting in oliguria [7–10]. In an animal model, London et al. have shown that PP resulted in a decrease in RBF during normal saline infusion, whereas RBF did not decrease if volume expansion was given [11]. From these results vigorous hydration up to 2 l/h of crystalloids during LDN in patients has nowadays been advocated [1, 12–15].
In 52 patients, Bergman et al. [16] found, however, no difference in graft function after LDN between aggressive (>10ml/kg/h) and conservative (<10 ml/kg/h) intraoperative fluid management. Volume loading after establishment of PP is perhaps too late to counterbalance the collapsed venal system. Biancofiore et al. [17] studied the effect of volume loading on graft function with a crystalloid infusion starting the night before surgery. Early graft function did not differ between ODN and LDN, although the serum creatinine declined earlier, but not significantly, in those receiving kidneys from ODN procedure.
Fasting before operation and induction of anesthesia leads to relative hypovolemia and the goal is therefore to compensate this before PP is started. In this study, we compared three different fluid regimes in LDN patients, in which the effect of pre-hydration together with a bolus of colloids given just before induction of anesthesia and a second one just before inflation of PP on hemodynamics was of special interest.
Methods
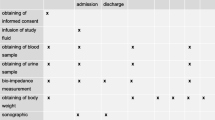
Patients undergoing LDN from June 2001 to November 2001 (N = 21) were included in the study. The anesthetic procedure was performed according to a strict protocol for medication, ventilation and fluid regimen. In our hospital the donor patients are admitted the day before the operation, they are fasted during the night from 00:00 and operated on at 08.00 the next morning. Patients were randomized the day before operation by sealed envelopes by the responsible anesthetist to three different fluid regimens (Table 1): in group 1 fluid administration was started at 22:00 the day before operation with 3 ml/ideal body weight (IBW)/h Ringers lactate (RL) until operation. Before induction of anesthesia, the patients received 6 ml/IBW of colloid (6% HES 130/0.4); thereafter 13 ml/IBW/h RL was started until nephrectomy, before installation of PP another bolus of 6 ml/IBW colloid was given. Group 2 received overnight infusion in the same way as in group 1 with a bolus of 6 ml/IBW colloid just before induction. During operation, an infusion was started with 13 ml/IBW/h RL and 2 ml/IBW/h of colloid was given for three hours. Group 3 was fasted from 00:00 on the day of operation and received only an infusion during operation with 13 ml/IBW/h RL and 4 ml/IBW/h of colloid for three hours. After nephrectomy the infusion protocol was adjusted, so that exactly six hours after start of operation all the patients had received in total 9 ml/IBW/h RL. Patients were fitted with anti-thrombosis stockings.
Induction of anesthesia was performed with propofol (2 mg/kg) after a bolus of sufentanil (0.3 μg/kg). Muscle relaxation was achieved with rocuronium (0.8 mg/kg) and monitored by train-of-four (TOF) guard, a bolus of rocuronium (0.3 mg/kg) was given for three or more twitches. Anesthesia was maintained with propofol by continuous infusion (4–11 mg/kg/h), aiming at a bispectral index between 45 and 55 (BIS monitor; Aspect Medical Systems, Newton, MA, USA), and analgesia was achieved by continuous infusion of sufentanil (0.4 μg/kg/h) until nephrectomy. One hour after the start of operation 20 mg mannitol was given intravenously.
After intubation all patients were ventilated in a pressure-controlled mode using a closed-loop ventilator (Physioflex®, Dräger, Lübeck, Germany) with the following initial settings: FiO2 of 0.4, positive end-expiratory pressure (PEEP) of 7 cm H2O and peak inspiratory pressure (PIP) of 22 cm H2O. Ventilation frequency was adjusted to keep PetCO2 between 4 and 5.5 kPa. After induction of anesthesia and before positioning of the patient, an esophageal Doppler probe (HemoSonicTM 100, Arrow International Inc., Reading, PA, USA) was positioned for measuring stroke volume (SV) and left ventricular ejection time, corrected for heart rate (LVETc) [18–20].
After positioning the patient in full lateral nephrectomy position, PP was installed with an IAP of 12 mmHg, which was constantly maintained at this level. All operations were done by the same team of anesthesiologists and surgeons. The surgical techniques have been described in detail elsewhere [21].
Mean arterial pressure (MAP) and SV (available after induction of anesthesia) were monitored noninvasively every five minutes. Urine output was measured from 22:00 the day before until the introduction of PP (T0), and was then measured every hour up to six hours thereafter (T1–6). Blood samples of the donors were collected to determine creatinine levels the day before operation, after induction of anesthesia, six hours after installation of PP, two days, one month, and one year after operation. Creatinine clearance (CrCl) was determined using the Cockcroft-Gault formula [22].
Statistical analysis
Data analysis was performed using SPSS for Windows (version 14.0, SPSS Inc., Chicago, USA). Data are presented as means with standard deviation (SD). Differences between the groups were analyzed using the independent t-test, depending on Levene’s test, pooled or unpooled. Repeated measures with a general linear model from SPSS were used to assess significance for CrCl. A (two-sided) p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics are shown in Table 2 and were comparable between the three groups. After induction of anesthesia, SV was significantly higher in both pre-hydrated groups compared to the control group (Fig. 1). After repositioning from supine to lateral, SV decreased significantly in the control group but not in groups 1 and 2 (Fig. 1). After installation of PP, SV remained stable in group 1 but not in groups 2 and 3 (Fig. 1).
After induction, LVETc was higher in group 1 compared to the control group during the whole procedure and remained stable (Table 3). In all groups MAP decreased after induction of anesthesia; in the control group MAP decreased significantly more compared to group 1 (p = 0.03). HR was comparable between the three groups (Table 3).
Urine output, measured from the start of operation until the moment of kidney extraction, was 1.9 ml/kg/h (range 1.2–3.2) for group 1, 1.4 ml/kg/h (range 0.8–2.3) for group 2, and 1.1 ml/kg/h (range 0.6–1.6) for group 3. In controls, the urine production was significantly lower compared to group 1 (p = 0.01). CrCl decreased in the control group directly after PP, but not in the other groups (Table 4). From two days postoperative, CrCl was comparable between the three study groups (Table 4).
Discussion
This study showed that during LDN preoperative hydration together with a bolus of colloid given before induction of anesthesia and before installation of PP resulted in higher SV and higher urine output compared to a fluid regimen with only an intraoperative aggressive fluid infusion. The second group, which received no bolus of colloid before PP in contrast to group 1, showed a significant reduction in SV after installation of PP. In the control group, LVETc and urine output at the moment of kidney extraction showed significantly lower values compared to both pre-hydration groups. CrCl values six hours after the start of the operation was significantly reduced in the control group compared to preoperative values but not in the two pre-hydrated groups; this difference was reduced two days postoperatively.
Clinical studies yield conflicting data concerning the effect of LDN on recipient graft function compared to ODN. The largest study to date compared more than 5,000 kidney transplants from a database and found that LDN was associated with slower early graft function compared to ODN. However, renal function and graft survival at one year was similar between both groups. This was confirmed by retrospective analysis of 120 LDN and 100 ODN in our own institution in which serum creatinine in the recipients was significantly higher in the LDN group only in the first week after transplantation [4]. One very important discriminating factor between ODN and LDN is the pneumoperitoneum. From experimental studies it has become clear that PP decreases RBF and that the magnitude of this decrease is affected by the IAP used, the volume status, and positioning. To counterbalance the increased IAP, vigorous intravenous hydration during LDN is nowadays recommended in an attempt to optimize preload and promote diuresis, but randomized clinical data are missing. In a porcine model, Demyttenaere et al. [23] showed that the decrease in SV and renal cortical perfusion could be prevented by a simple hydration of 15 ml/kg/h saline combined with a bolus 20 ml/kg saline, in accordance with the findings of London et al. [11]. This was also seen in the present study in which pre-hydration with a normal infusion with crystalloids during operation combined with a bolus of colloids just before PP did not decrease SV but improved diuresis. Besides PP, the kidney lateral decubitus position, which is an anti-Trendelenburg position, contributes to hemodynamic alterations by decreasing preload through the effect of gravity on venous return [24]. Yokoyama et al. [25] found no significant change in hemodynamic values after postural change of their patients from supine to lateral but a significant reduction in SV after postural change to kidney position; these patients received a fluid regime of 20 ml/kg/h of crystalloids. This was confirmed by our study in which the control group showed a significant reduction in SV after postural change from supine to kidney position whereas there was no reduction in the two pre-hydrated groups, which received a bolus of colloid just before induction (Fig. 1).
After pre-hydration with crystalloids we infused colloids to achieve optimal plasma expansion just before installation of PP [26]. In our hospital we use 6% HES 130/0.4 for fluid expansion, because the rate for anaphylactic reactions is considerably lower than for gelatin products [27]. However, there are concerns that infusion of certain HES types may influence kidney function [28]. As long as adequate hydration using sufficient amounts of crystalloids are used, the latest generation of HES products (6% HES 130/0.4) do not increase the risk for renal dysfunction even when used in large amounts [29, 30]. Lang et al. [31] even demonstrated that 6% HES 130/0.4 improved tissue oxygenation during and after major surgical procedures compared with a crystalloid-based volume strategy.
In this study, we used the HemoSonicTM, a transoesophageal Doppler ultrasonography (TOD) device, to measure blood flow in the descending aorta. Several studies have confirmed good correlation with cardiac output measured by the thermodilution technique [18, 32]. It has been shown that the accuracy of the device is somewhat operator-dependent [20] and therefore the same two people did all the measurements with this device in the present study. Feldman et al. [33] used LVET to guide their fluid management in LDN patients. In the present study it was shown that LVETc was significantly lower in the control group that did not received pre-hydration and increased over time (Table 2). It should, however, be taken into account that the blood flow with this device is measured in the descending aorta, which is around 70% of the total cardiac output. This could influence our measurements if redistribution of flow away from the descending aorta occurs because of elevated IAP and this is more pronounced in hypovolemic patients.
Some other limitations of this study should be noted. In only four patients a MAG3 scan was performed, which provides the distribution of the function from the two kidneys of the donor. In the four measured patients the harvested kidney contributed 43–48% of the total kidney function, these four patients were divided over all three study groups. However because we do not have the data on the other patients, this could have biased our data on postoperative CrCl. Prehydration of the donor patients conform our protocol, started the night before operation, which contradicts fast-track surgery where kidney donor patients are admitted to hospital on the day of surgery. Also these patients can receive adequate pre-hydration, but further research should be done.
In this study we focused on intraoperative hemodynamic changes. Our data show that preoperative hydration together with a colloid bolus given before induction of anesthesia and before installation of PP resulted in higher SV and higher urine output during LDN, compared to controls that received only an aggressive intraoperative infusion. While under-hydration may contribute to renal dysfunction, perioperative fluid excess can also cause problems, such as pulmonary edema, ileus and increased risk of cardiopulmonary and wound healing complications, which might result in longer hospital stay [34]. However, there is a need to ensure adequate hydration status during PP without being overaggressive. First, our fluid regime will be tested in a large prospective study in order to prevent the negative effect of PP on early graft function in the recipient, and to study possible side-effects in the donor.
References
Bajorat J, Hofmockel R, Vagts DA, Janda M, Pohl B, Beck C, Noeldge-Schomburg G (2006) Comparison of invasive and less-invasive techniques of cardiac output measurement under different haemodynamic conditions in a pig model. Eur J Anaesthesiol 23: 23–30
Barron ME, Wilkes MM, Navickis RJ (2004) A systematic review of the comparative safety of colloids. Arch Surg 139: 552–63
Bergman S, Feldman LS, Carli F, Anidjar M, Vassiliou MC, Andrew CG, Stanbridge DD, Fried GM (2004) Intraoperative fluid management in laparoscopic live-donor nephrectomy: challenging the dogma. Surg Endosc 18: 1625–30
Biancofiore G, Amorose G, Lugli D, Bindi L, Esposito M, Pasquini C, Bellissima G, Fossati N, Meacci L, Pieri M, Vistoli F, Boggi U, Pietrabissa A, Mosca F (2004) Perioperative anesthetic management for laparoscopic kidney donation. Transplant Proc 36: 464–6
Chiu AW, Chang LS, Birkett DH, Babayan RK (1995) The impact of pneumoperitoneum, pneumoretroperitoneum, and gasless laparoscopy on the systemic and renal hemodynamics. J Am Coll Surg 181: 397–406
Cisek LJ, Gobet RM, Peters CA (1998) Pneumoperitoneum produces reversible renal dysfunction in animals with normal and chronically reduced renal function. J Endourol 12: 95–100
Cittanova ML, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P (1996) Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet 348: 1620–2
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41
Dehne MG, Muhling J, Sablotzki A, Dehne K, Sucke N, Hempelmann G (2001) Hydroxyethyl starch (HES) does not directly affect renal function in patients with no prior renal impairment. J Clin Anesth 13: 103–11
Demyttenaere SV, Feldman LS, Bergman S, Gholoum S, Moriello C, Unikowsky B, Fraser S, Carli F, Fried GM (2006) Does aggressive hydration reverse the effects of pneumoperitoneum on renal perfusion? Surg Endosc 20: 274–80
Fabrizio MD, Ratner LE, Montgomery RA, Kavoussi LR (1999) Laparoscopic live donor nephrectomy. The Urol Clin North Am 26: 247–56 xi
Feldman LS, Anidjar M, Metrakos P, Stanbridge D, Fried GM, Carli F (2004) Optimization of cardiac preload during laparoscopic donor nephrectomy: a preliminary study of central venous pressure versus esophageal Doppler monitoring. Surg Endosc 18: 412–6
Hawasli A, Oh H, Schervish E, Frontera R, Gonsherova I, Khoury H (2003) The effect of pneumoperitoneum on kidney function in laparoscopic donor nephrectomy. Am Surg 69: 300–3; discussion 303
Hazebroek EJ, Gommers D, Schreve MA, van_Gelder T, Roodnat JI, Weimar W, Bonjer HJ, IJzermans JN (2002) Impact of intraoperative donor management on short-term renal function after laparoscopic donor nephrectomy. Ann Surg 236: 127–32
Holte K, Sharrock NE, Kehlet H (2002) Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth 89: 622–32
Iwase K, Takenaka H, Ishizaka T, Ohata T, Oshima S, Sakaguchi K (1993) Serial changes in renal function during laparoscopic cholecystectomy. Eur Surg Res 25: 203–12
Junghans T, Bohm B, Grundel K, Schwenk W, Muller JM (1997) Does pneumoperitoneum with different gases, body positions, and intraperitoneal pressures influence renal and hepatic blood flow? Surgery 121: 206–11
Junghans T, Modersohn D, Dorner F, Neudecker J, Haase O, Schwenk W (2006) Systematic evaluation of different approaches for minimizing hemodynamic changes during pneumoperitoneum. Surg Endosc 20: 763–9
Kok NF, Alwayn IP, Lind MY, Tran KT, Weimar W, IJzermans JN (2006) Donor nephrectomy: mini-incision muscle-splitting open approach versus laparoscopy. Transplantation 81: 881–7
Lang K, Boldt J, Suttner S, Haisch G (2001) Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg 93: 405–9
Lind MY, Liem YS, Bemelman WA, Dooper PM, Hop WC, Weimar W, IJzermans JN (2003) Live donor nephrectomy and return to work: does the operative technique matter? Surg Endosc 17: 591–5
Lind MY, Mertens zur Borg IRAM, Hazebroek EJ, Hop WC, Alwayn IP, Weimar W, IJzermans JN (2005) The effect of laparoscopic and open donor nephrectomy on the long-term renal function in donor and recipient: a retrospective study. Transplantation 80: 700–3
London ET, Ho HS, Neuhaus AM, Wolfe BM, Rudich SM, Perez RV (2000) Effect of intravascular volume expansion on renal function during prolonged CO2 pneumoperitoneum. Ann Surg 231: 195–201
Mertens zur Borg IRAM, Lim A, Verbrugge SJC, IJzermans JN, Klein J (2004) Effect of intraabdominal pressure elevation and positioning on hemodynamic responses during carbon dioxide pneumoperitoneum for laparoscopic donor nephrectomy: a prospective controlled clinical study. Surg Endosc 18: 919–23
Moxon D, Pinder M, van Heerden PV, Parsons RW (2003) Clinical evaluation of the HemoSonic monitor in cardiac surgical patients in the ICU. Anaesth Intensive Care 31: 408–11
Neff TA, Doelberg M, Jungheinrich C, Sauerland A, Spahn DR, Stocker R (2003) Repetitive large-dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg 96: 1453–9
Nogueira JM, Cangro CB, Fink JC, Schweitzer E, Wiland A, Klassen DK, Gardner J, Flowers J, Jacobs S, Cho E, Philosophe B, Bartlett ST, Weir MR (1999) A comparison of recipient renal outcomes with laparoscopic versus open live donor nephrectomy. Transplantation 67: 722–8
Ratner LE, Montgomery RA, Kavoussi LR (1999) Laparoscopic live donor nephrectomy: the four year Johns Hopkins University experience. Nephrol Dial Transplant 14: 2090–3
Sasaki TM, Finelli F, Bugarin E, Fowlkes D, Trollinger J, Barhyte DY, Light JA (2000) Is laparoscopic donor nephrectomy the new criterion standard? Arch Surg 135: 943–7
Singer M, Bennett ED (1991) Noninvasive optimization of left ventricular filling using esophageal Doppler. Crit Care Med 19: 1132–7
Tournadre JP, Muchada R, Lansiaux S, Chassard D (1999) Measurements of systolic time intervals using a transoesophageal pulsed echo-Doppler. Br J Anaesth 83: 630–6
Troppmann C, Ormond DB, Perez RV (2003) Laparoscopic (vs open) live donor nephrectomy: a UNOS database analysis of early graft function and survival. Am J Transplant 3: 1295–301
Verheij J, van Lingen A, Beishuizen A, Christiaans HM, de Jong JR, Girbes AR, Wisselink W, Rauwerda JA, Huybregts MA, Groeneveld AB (2006) Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Med 32(7):1030–8
Yokoyama M, Ueda W, Hirakawa M (2000) Haemodynamic effects of the lateral decubitus position and the kidney rest lateral decubitus position during anaesthesia. Br J Anaesth 84: 753–7
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed at the Department of Anesthesiology, Erasmus University Medical Center Rotterdam and was financially supported by this Department
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mertens zur Borg, I.R.A.M., Di Biase, M., Verbrugge, S. et al. Comparison of three perioperative fluid regimes for laparoscopic donor nephrectomy. Surg Endosc 22, 146–150 (2008). https://doi.org/10.1007/s00464-007-9391-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-007-9391-9