Abstract
Background
Transforming growth factor-beta 1 (TGF-β1) is a growth factor involved in various biologic processes, including peritoneal wound healing and dissemination of malignancies. Laparoscopic surgery is evolving rapidly, and indications are increasing. The peritoneal TGF-β1 expression during laparoscopic surgery is unknown.
Methods
For this study, 50 patients scheduled for laparoscopic cholecystectomy were randomized into five groups, then surgically treated with various pressures, light intensities, and dissection devices. Peritoneal biopsies were taken at the beginning and end of surgery. Tissue concentrations of total and active TGF-β1 were measured using enzyme-linked immunosorbent assay (ELISA) techniques.
Results
There was no significant difference in either total or active TGF-β1 concentration between peritoneal biopsies taken at the start of surgery and samples taken at the end of the procedure. Patients who underwent surgery with the ultrasonic scalpel had significant lower levels of both active (p < 0.005) and total (p < 0.01) TGF-β1 at the end of surgery than patients treated with electrocautery. Patients who had surgery with a high light intensity had significantly lower levels of total TGF-β1 levels (p < 0.005) with an unchanged active part than patients who had surgery with low light intensity.
Conclusion
The choice of dissection device and the light intensity used in laparoscopic surgery affect peritoneal TGF-β1 concentrations, indicating that peritoneal biology can be affected by laparoscopic surgery. Because TGF-β1 is involved in various biologic processes in the peritoneal cavity, this observation may have important clinical consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Transforming growth factor-beta 1 (TGF-β1) is a naturally occurring growth factor involved in various biologic processes, including peritoneal wound healing and dissemination of malignancies [1]. Peritoneal wound healing and subsequent adhesion formation are regulated by a complex mechanism of molecular processes. Alterations in the local concentrations of cytokines, growth factors, and proteases all may contribute to the process of peritoneum healing.
Findings suggest that TGF- β is a major stimulator of peritoneal adhesion formation [2], mainly by increasing the peritoneal production of plasminogen activator inhibitor-1 (PAI-1), which is the main inhibitor of fibrinolysis and a key factor in adhesiogenesis [3]. Furthermore, TGF-β is a major stimulator of extracellular matrix deposition by inducing the production of collagen, fibronectin, and integrins [4, 5]. Increased TGF-β concentrations have been found in the peritoneal fluid of patients with adhesions and in adhesion tissue itself [6]. Moreover, postoperative peritoneal administration of TGF-β increased adhesion formation in mice, whereas its inactivation is reported to reduce the incidence of adhesions [7].
By regulating chemotaxis, mitogenisis, and angiogenesis, TGF-β is involved in dissemination processes [8]. Secretion of TGF-β and activation of TGF-β signaling pathways have been associated with increased aggressiveness of several tumor types including pancreas, colon, stomach, lung, endometrium, prostate, breast, brain, and bone tumor [9, 10]. Literature on the relation of laparoscopic surgery to peritoneal dissemination and port-site metastasis is controversial.
Endoscopic surgery has developed rapidly in recent decades. It minimizes the surgical trauma, thereby reducing recovery time and the incidence of postoperative complications. Few studies have suggested that this strategy also may reduce the incidence of peritoneal adhesion formation [11]. The effect of laparoscopy on peritoneal TGF-β1 expression is unknown. Endoscopic surgery induces new entities in the abdominal cavity including intense illumination of the peritoneal cavity and increased intraabdominal pressure. Moreover, the use of new dissection devices, including the ultrasonic scalpel, is progressively advocated.
The current study was conducted to evaluate the hypothesis that peritoneal biology, specifically peritoneal TGF-β1 expression, could be affected by laparoscopic surgical techniques. The effects of illumination, intraabdominal pressure, and the choice of dissection devices were studied in patients undergoing laparoscopic cholecystectomy.
Patients and methods
Design of study
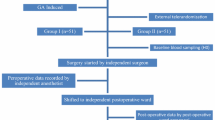
For this study, 50 patients with a diagnosis of symptomatic gallbladder stone disease and scheduled for elective laparoscopic cholecystectomy were randomized into five groups (Table 1). To evaluate the effect of intraabdominal pressure, three groups of 10 patients each underwent surgery with intraabdominal pressures of 10 mmHg (group A), 13 mmHg (group B), and 16 mmHg (group C). All three groups had surgery with the same light intensity and using electrocautery. The effect of light intensity was studied by comparing a group that had surgery under a high light intensity (group D) with a group that had surgery under a low light intensity (group B), using an intraabdominal pressure of 13 mmHg and electrocautery for both groups. The influence of the dissection device was assessed by comparing a group that had surgery using electrocautery (group B) with a group that had surgery using an ultrasonic scalpel (group E) under equal light intensities and intraabdominal pressures.
Randomization by envelope was done immediately before the operation. Institutional review board approval was obtained, and written informed consent was given by the patients before enrollment.
Operative procedure
A uniform technique of videolaparocopic cholecystectomy was applied, including the use of four trocar ports in the “American” position and a 0° optic scope. The gallbladder hilum and Calot’s triangle were dissected, and metal clips for the cystic duct and artery were used. Biopsies of the parietal peritoneum were taken with forceps and scissors immediately after carbon dioxide (CO2) insufflation and after 45 min of surgery without the use of electrocautery or ultrasonic scalpel. Within 45 min after the procedure was completed and just before desufflation, the second biopsy was taken.
Tissue sampling and processing
The peritoneum was carefully dissected, with care taken not to include the underlying muscle. The tissue specimens were snap frozen in liquid nitrogen and stored at –70°C until further processing. Before homogenization, a sample of thawing peritoneal tissue was cut off before being blotted and weighed. Each biopsy was rinsed in phosphate-buffered saline (PBS) with 0.5 M of sodium chloride (pH 7.4), cut into small pieces, and placed into ice-cold homogenization buffer (PBS with 0.01% Triton X-100 (Sigma, St. Louis, MO, USA) in a final tissue concentration of 40-mg/ml of buffer. The tissue was homogenized for 60 s on ice using a Polytron homogenizer (Ultra Thurrax IKA T-25; Janke & Kunkel, Staufen, Germany) and centrifuged at 10,000 g for 4 min at 4°C. The supernatant then was stored at –70°C until further analysis. Tissue processing and assays were done in batches.
Biochemical assays
Concentrations of active and total TGF-β1 were measured using commercially available enzyme-linked immunosorbent assays (ELISA) (Promega, Madison, WI, USA). Both the active and total forms of TGF-β1 were measured because TGF-β is inactive when produced and must be activated to become an active cytokine. The active and total amounts of TGF-β1 were measured in separate steps. First, the active fraction of TGF-β1 was assayed directly in the ELISA plate. Next, the total amount of TGF-βb1 was assayed by acidifying the samples with 1 mol/l of hydrogenchloride to pH 3, followed by a 15 min incubation at 22°C, resulting in an activation of TGF-β1. To neutralize samples, 1 mol/l of NaOH was supplemented before addition to the ELISA plate, according to the instructions from the manufacturer. The lower detection limit for the TGF-β1 assay was 32 pg/ml. The intraassay variation was 3.3% to 4.5% (CV%), and the interassay variation was 7.6% to 19.1%.
Statistical analysis
Values are given as mean ± standard deviation. Analysis of differences between groups was performed using the Kruskal–Wallis test and the Mann–Whitney U test. All tests were two-tailed.
Results
Clinical results
There was no difference in sex (men, 22%; women, 78%) or age 51 ± 16 years between the groups. The overall incidence of previous laparotomies was 30%, without differences between the groups. Moreover, there was no difference in the occurrence of intraperitoneal adhesions or the incidence of bile leakage between the groups. Histologic studies of the removed gallbladders demonstrated no significant difference in the incidence of chronic inflammation between the groups. The timing of the second biopsy was equal in all the groups (38 ± 9.2 min).
Biochemical results
Light intensity
The patients who underwent surgery with a high light intensity had significantly lower total TGF-β1 levels (p < 0.005) at the end of surgery than the patients who had surgery at a low light intensity, as shown by biopsies. The active TGF-β-1 concentrations were similar in between the two groups. There were no differences between the groups at the start of the procedure (Fig. 1a and b).
Intraabdominal pressure
There was no difference in the measured total and active TGF-β1 levels in specimens from patients who had surgery with intraabdominal pressures of 10, 13, and 16 mmHg (Fig. 2a and b).
Total (a) and active (b) transforming growth factor beta 1 (TGF-β1) concentrations in peritoneal samples from the groups with intraabdominal pressures of 10 mmHg (group A), 13 mmHg (group B), and 16 mmHg (group C). Values are median (horizontal line), interquartile range (boxes), and 10th and 90th percentiles (error bars).
Dissection device
Peritoneal biopsies of the patients in whom the dissection was performed using an ultrasonic scalpel showed significantly lower levels of both total (p < 0.005) and active (p < 0.01) TGF-β1 at the end of surgery than the biopsies of the patients who had surgery with electrocautery. There were no differences at the start of the procedure between the groups (Fig. 3a and b).
Total (a) and active (b) transforming growth factor beta 1 (TGF-β1) concentrations in peritoneal samples from the groups that had surgery with either electrocautery (group B) or ultrasonic scalpel (group E). Values are median (horizontal line), interquartile range (boxes), and 10th and 90th percentiles (error bars). *p < 0.005, ± p < 0.01.
There was no difference among the groups in either total or active TGF-β1 concentration, as shown by comparison between peritoneal biopsies taken at the beginning of the procedure and samples taken at the end of surgery. When the surgical variation arms were eliminated and the overall tissue levels before and after the intervention were compared, no significant differences were found in peritoneal levels between total and active TGF-β1. There was no difference in peritoneal TGF-β1 expression between patients with intraabdominal adhesions and those without adhesions.
Discussion
The current study demonstrated that peritoneal biology can be affected by laparoscopic surgery, and that various surgical techniques may have different effects. A high light intensity and the use of an ultrasonic scalpel reduced the peritoneal expression of total TGF-β1, whereas the ultrasonic scalpel also decreased the active part of TGF-β1. Within clinically applicable variances, the intraabdominal pressure used had no influence on the peritoneal TGF-β1 expression during short-term laparoscopy.
The use of an ultrasonic scalpel in laparoscopic surgery is progressively advocated for several reasons [12–15]. Coagulation of vascular structures is easy and safe with the ultrasonic scalpel, and there is no effect on pacemaker function, which is a major drawback of electrocautery. Most importantly, ultrasonic scalpel is described as reducing the incidence of gallbladder injury and iatrogenic bowel perforation, and the operating time is significant shorter [14]. We have demonstrated a decreased peritoneal expression of both active and total TGF-β1 when the patient undergoes surgery with the ultrasonic scalpel. Because TGF-β is a main stimulator of adhesion formation, the use of an ultrasonic scalpel may affect the peritoneal healing process by decreasing expression of type-1 plasminogen activator inhibitor (PAI-1). In a previous study, however, we did not find any effect of the ultrasonic scalpel on tissue plasminogen activator (t-PA), urokinase-type plasminogen activator (u-PA), or PAI-1 levels expressed in the peritoneum of patients undergoing a laparoscopic cholecystecomy [16]. Schemmel et al. [17] found no differences in peritoneal adhesion formation whether traditional incision, electrosurgery, or the ultrasonic scalpel was used in a rabbit uterine horn model. Similar observations were described by Tulandi et al. [18] in a rat uterine horn model. At this writing, clinical data are lacking.
The decreased TGF-β1 expression with the use of the ultrasonic scalpel may have other important clinical consequences. For one, TGF-β is involved in various biologic processes including chemotaxis, mitogenesis, and angiogenesis, all important in oncologic processes [19–21]. An increasing proportion of laparoscopic procedures is performed for oncologic pathology such as laparoscopic colectomy, nefrectomy, and hysterectomy. Experimental studies have demonstrated that laparoscopy is associated with less intraperitoneal tumor growth than laparotomy, whereas insufflation of CO2 may promote peritoneal tumor growth as compared with gasless laparoscopy [22, 23]. Lecuru et al. [24], however, did not find any deleterious effect of CO2 insufflation on ovarian tumor growth as compared with gasless laparoscopy or midline laparotomy in a rat model. Only a few clinical data exist to allow assessment of whether these experimental concerns may be translated into clinical problems. Velanovich [25] found no effect of laparoscopy on the occurrence of trocar-site disease or peritoneal progression of pancreatic cancer. The observation that the ultrasonic scalpel decreases TGF-β levels may suggest that this could be the dissection device of choice for this type of operation. Our results warrant further studies focused on this topic.
The effects of light on peritoneal biology are merely unknown. High temperatures have been described at the end of a fiberoptic bundle of light cables and endoscopes with both halogen and xenon light sources [26]. This heat generation is largely attributable to the radiated power in the visible light spectrum. Increased local temperature may affect mesothelial cell function, including the production and release of TGF-β1.
Surprisingly, in the current study, the use of high light intensity also decreased the levels of total TGF-β1 without affecting its active part. It might have been expected that high light intensity would lead to increased damage of the peritoneum and thus to an increase in TFG-β1. The opposite, however, was true. Laparoscopy is accompanied by insufflation of the peritoneal cavity with CO2, leading to cooling of the peritoneum and peritoneal injury. The high light intensity may have decreased the temperature shift in the peritoneal cavity due to a higher energy transmission. Additionally, specific frequencies of the light may change the biologic behavior of mesothelial cells. The relation of light and its specific frequencies to mesothelial cell biology should be subjected to further experimental studies.
In the current study, we could not demonstrate an effect of the used intraabdominal pressure on the peritoneal TGF-β1 expression during short-term laparoscopy. The variations in intraabdominal pressure, however, may have been too small for any differences to be detected because we were limited to clinically applicable variances in intraabdominal pressure.
Molinas et al. [27] demonstrated the effect of pressure on peritoneal wound healing processes. In an experimental study, they showed that the incidence of adhesions increases with the pressure of insufflation. Further experimental studies are indicated to elucidate this subject.
Overall measurements have shown no significant difference in TGF-β expression between specimens taken at the start of surgery and biopsies taken at the end of surgery. The latter, however, may have been too short for any differences to be detected, at least at the protein level. The majority of all endoscopic procedures remain short-term procedures (<1 hour), including diagnostic laparoscopy, appendectomy and cholecystectomy, indicating the clinical relevance of the current study. Additional studies on prolonged endoscopic procedures are required for further elucidation of the effect that endoscopic surgery has on peritoneal TGF-β1 expression.
The timing of the first biopsy is another point of interest. In the current study, the first biopsy was taken as soon as possible after initiation of the pneumoperitoneum. At that time point, however, the peritoneal layer may already have been damaged by insufflation, which results in increased abdominal pressure, intense illumination, and cooling of the peritoneal cavity. This hypothesis is supported by the observation of Bergstrom et al. [28], who described higher peritoneal PAI-1 levels immediately after initiation of a laparoscopic cholecystectomy than during conventional cholecystectomy. Additional experimental studies are needed to clarify this subject.
In conclusion, the current study suggests that peritoneal biology can be modulated by laparoscopic surgery, and that various surgical techniques may have different effects. The use of an ultrasonic scalpel and the use of higher light intensity decrease peritoneal TGF-β1 levels. The involvement of TGF-β1 in oncologic and peritoneal repair processes highlights the need for further clinical trials.
References
Zeamari S, Roos E, Stewart FA (2004) Tumor seeding in peritoneal wound sites in relation to growth-factor expression in early granulation tissue. Eur J Cancer 40: 1431–1440
Holmdahl L, Kotseos K, Bergström M, Falk P, Ivarsson ML, Chegini N (2001) Overproduction of transforming growth factor-beta 1 (TGF-beta 1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery 129: 626–632
Reijnen MM, Bleichrodt RP, van Goor H (2003) Pathophysiology of intraabdominal adhesion and abscess formation and the effect of hyaluronan. Br J Surg 90: 533–541
Kagami S, Kuhara T, Yasutomo K, Okada K, Loster K, Reutter W, et al. (1996) Transforming growth factor-beta (TGF-beta) stimulates the expression of beta1 integrins and adhesion by rat mesangial cells. Exp Cell Res 229: 1–6
Ignotz RA, Massague J (1987) Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell 51: 189–197
Hobson KG, DeWing M, Ho HS, Wolfe BM, Cho K, Greenhalgh DG, et al. (2003) Expression of transforming growth factor beta 1 in patients with and without previous abdominal surgery. Arch Surg 138: 1249–1252
Chegini N (1997) The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl 577:17–23
Kane S, Prentice MA, Mariano JM, Cuttitta F, Jakowlew SB (2002) Differential induction of early response genes by adrenomedullin and transforming growth factor-beta 1 in human lung cancer cells. Anticancer Res 22: 1433–1444
Kaklamani VG, Pasche B (2004) Role of TGF-beta in cancer and the potential for therapy and prevention. Expert Rev Anticancer Ther 4: 649–661
Gold LI (1999) The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog 10: 303–360
Gutt CN, Oniu T, Schemmer P, Mehrabi A, Buchler MW (2004) Fewer adhesions induced by laparoscopic surgery? Surg Endosc 18: 898–906
Westervelt J (2004) Clipless cholecystectomy: broadening the role of the harmonic scalpel. JSLS 8: 283–285
Swank DJ, Jeekel H (2004) Laparoscopic adhesiolysis in patients with chronic abdominal pain. Curr Opin Obstet Gynecol 16: 313–318
Janssen IM, Swank DJ, Boonstra O, Knipscheer BC, Klinkenbijl JH, van Goor H (2003) Randomized clinical trial of ultrasonic versus electrocautery dissection of the gallbladder in laparoscopic cholecystectomy. Br J Surg 90: 799–803
Underwood RA, Dunnegan DL, Soper NJ (1999) Prospective, randomized trial of bipolar electrosurgery vs ultrasonic coagulation for division of short gastric vessels during laparoscopic Nissen fundoplication. Surg Endosc 13: 763–768
Brokelman WJA, Holmdahl L, Bergström M, Falk P, Klinkenbijl JHG, Reijnen MMP (2006) Peritoneal fibrinolytic respons to various aspects of laparoscopic surgery, a randomised clinical trial. J Surg Res 136:309–313
Schemmel M, Haefner HK, Selvaggi SM, Warren JS, Termin CS, Hurd WW (1997) Comparison of the ultrasonic scalpel to CO2 laser and electrosurgery in terms of tissue injury and adhesion formation in a rabbit model. Fertil Steril 67: 382–386
Tulandi T, Chan KL, Arseneau J (1994) Histopathological and adhesion formation after incision using ultrasonic vibrating scalpel and regular scalpel in the rat. Fertil Steril 61: 548–550
Xiong B, Gong LL, Zhang F, Hu MB, Yuan HY (2002) TGF-beta 1 expression and angiogenesis in colorectal cancer tissue. World J Gastroenterol 8: 496–498
Fibbi G, Pucci M, D’Alessio S, Grappone C, Pellegrini G, Salzano R, et al. (2001) Transforming growth factor beta-1 stimulates invasivity of hepatic stellate cells by engagement of the cell-associated fibrinolytic system. Growth Factors 19: 87–100
Ito H, Miyazaki M, Nishimura F, Nakajima N (2000) Secretion of extracellular matrix (fibronectin), growth factor (transforming growth factor beta), and protease (cathepsin D) by hepatoma cells. Oncology 58:261–270
Bouvy ND, Giuffrida MC, Tseng LN, Steyerberg EW, Marquet RL, Jeekel H, Bonjer HJ (1998) Effects of carbon dioxide pneumoperitoneum, air pneumoperitoneum, and gasless laparoscopy on body weight and tumor growth. Arch Surg 133: 652–656
Canis M, Botchorishvili R, Wattiez A, Pouly JL, Mage G, Manhes H, Bruhat MH (2000) Cancer and laparoscopy, experimental studies: a review. Eur J Obstet Gynecol Reprod Biol 91: 1–9
Lecuru F, Agostini A, Camatte S, Robin F, Aggerbeck M , Jaiss JP, Vilde F, Taurelle R (2001) Impact of pneumoperitoneum on visceral metastasis rate and survival: results in two ovarian cancer models in rats. British Journal of Obstetrics and Gynecology 108: 733–737
Velanovich V (2004) The effects of staging laparoscopy on trocar site and peritoneal recurrence of pancreatic cancer. Surg Endosc 18: 310–313
Hensman C, Hanna GB, Drew T, Moseley H, Cuschieri A (1998) Total radiated power, infrared output, and heat generation by cold light sources at the distal end of endoscopes and fiber optic bundle of light cables. Surg Endosc 12: 335–337
Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR (2001) Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril 76: 560–567
Bergstrom M, Ivarsson ML, Holmdahl L (2002) Peritoneal response to pneumoperitoneum and laparoscopic surgery. Br J Surg 89: 1465–1469
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00464-007-9484-5
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Brokelman, W.J., Holmdahl, L., Bergström, M. et al. Peritoneal transforming growth factor beta-1 expression during laparoscopic surgery: a clinical trial. Surg Endosc 21, 1537–1541 (2007). https://doi.org/10.1007/s00464-006-9164-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-006-9164-x